
Second and later-line erlotinib use in non-small cell lung cancer: real world outcomes and practice patterns overtime in Canada
Introduction
Treatment of advanced non-small cell lung cancer (NSCLC) prior to 2009 consisted of an algorithm that contained of one or two lines of chemotherapy followed by best supportive care or erlotinib, a targeted inhibitor that was developed for use in the general lung cancer population. Erlotinib is a reversible ERBB1 receptor inhibitor that is postulated to work in many cancers due to its anti-proliferative effects through the inhibition of the epidermal growth factor receptor (EGFR) pathway (1,2). In 2005, Shepherd et al. published a randomized controlled trial of erlotinib monotherapy after first or second-line chemotherapy in patients with stage IV NSCLC. Erlotinib treatment in this setting yielded a 2-month improvement in overall survival (OS) (hazard ratio, 0.70; P<0.001) and became the internationally recognized standard of care (3). At the same time, early clinical and preclinical data emerged suggesting that patients with a mutation in the EGFR gene had dramatic responses to EGFR targeted therapy, with improved overall response rate and duration of response than patients without (4-6). This was confirmed in 2009 with the publication of the Iressa Pan Asian Study (IPASS) by Mok et al. revealing that EGFR mutations are a predictive biomarker for response to EGFR kinase inhibitors (7). Since the IPASS study, multiple EGFR inhibitors (first, second and third-generation) have been developed and established as standard first-line therapy in patients whose tumors harbor an activating EGFR mutation (8-13).
The prevalence of EGFR mutations in advanced NSCLC is variable depending on the geographical region and ethnicity of the patient. In patients with adenocarcinoma histology and of Asian ethnicity, prevalence can be as high as 50%, compared 15–20% in Caucasians (14,15). The Canadian population is comprised of many different ethnicities and EGFR mutation rates occur in approximately 20.6% of non-squamous patients (16). Testing for the EGFR mutation has evolved overtime in Canada. In Ontario, Canada testing for the EGFR mutation began in 2010, and reflex testing at the level of the pathologist for EGFR mutations in non-squamous NSCLC has been implemented between 2011–2014 in most centers (17). In 2015, the prevalence of EGFR mutation testing was approximately 72% for advanced non-squamous NSCLC patients at one institution in Ontario, Canada (18).
Erlotinib is approved and continues to be funded in Ontario as second or later-line monotherapy in stage IV adenocarcinoma regardless of whether or not a predictive EGFR mutation is present (19). In Canada, other approvals in the wild-type population are for afatinib as second line treatment in squamous histology and erlotinib as maintenance therapy post platinum doublet (20,21). Neither of these two regimens are funded by the public payer in Ontario. The indication for erlotinib as second or third-line therapy in wild-type NSCLC has been de-listed by the U.S. Food and Drug Administration (FDA) after the IUNO trial failed to demonstrate improvement in OS or PFS when erlotinib was used as maintenance therapy post platinum-doublet chemotherapy in patients without tumor EGFR mutations (22,23). EGFR mutation testing became standard of care in Ontario by 2011 (24,25). Real-world data on the efficacy of this therapy in unselected patients and EGFR wild-type patients has yielded conflicting results (26-32). The uptake and patient outcomes of erlotinib as later-line treatment since EGFR testing has been in place in Ontario, Canada is unknown. The purpose of this study was to characterize the use of second or later-line erlotinib therapy in Ontario from 2007–2016, as well as evaluate the impact of erlotinib therapy on survival and emergency department (ED) visits in a real-world population.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-804).
Methods
Study design and data sources
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Research Ethics Board of the Princess Margaret Cancer Centre/University Health Network, Toronto, Canada (REB: 19-5286) and individual consent for this retrospective analysis was waived.
This was a retrospective cohort study designed to explore the effect of second or later-line erlotinib on survival in advanced NSCLC. The cohort was restricted to patients in Ontario, Canada, with a valid health card, over the age of 65. This cohort was derived at ICES (formerly known as the Institute for Clinical and Evaluative Sciences). ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. Ontario health care is delivered by a single payer and patients’ unique Ontario Health Insurance Plan (OHIP) numbers can be linked to various databases containing information on health system encounters, diagnoses, and (for patients over the age of 65) prescription drugs. Provincial datasets used for this study include Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), Ontario Cancer Registry (OCR), Registered Persons Database (RPDB), Ontario Disability Benefit (ODB), Ontario Health Insurance Plan Claims Database (OHIP), Continuing Care Reporting System (CCRS), Ontario Mental Health Reporting System (OMHRS), National Ambulatory Care Reporting System (NACRS), Local Health Integration Network (LHIN), Drugs List (DIN), Congestive Heart Failure (CHF), Chronic Obstructive Pulmonary Disease (COPD), Ontario Diabetes Dataset (ODD), New Drug Funding Program (NDFP), Surname Based Ethnicity Group (ETHNIC), Ontario Dementia Database (DEMENTIA). These datasets were linked using unique encoded identifiers and analyzed at ICES.
Cohort
Our cohort was comprised of all patients with valid public health insurance, aged 65 years or older and diagnosed with advanced NSCLC (defined using relevant diagnosis codes from the International Classification of Diseases, revision 10, with disease AJCC 8th edition stage IIIB/IV). Patients that completed first line chemotherapy in Ontario, Canada between 1/1/2007 and 12/31/2016 were included, and entered the cohort on the last date of first-line chemotherapy administration.
Patients were excluded if: first-line chemotherapy did not contain any of vinorelbine, gemcitabine, paclitaxel, or pemetrexed; second-line chemotherapy that did not contain any of docetaxel, pemetrexed, nivolumab, pembrolizumab, gemcitabine; another cancer diagnosis was made in the 5 years preceding their lung cancer diagnosis or during follow up; they received chemotherapy prior to their diagnosis of lung cancer; they were >105 years of age at the time of diagnosis (per standard protocol); died on the cohort entry date; received erlotinib prior to the cohort entry date; or switched chemotherapy drugs four or more times.
Primary exposure
The primary exposure in the model was a time varying covariate of erlotinib exposure, in order to isolate for the effect of erlotinib and mitigate immortal person time bias. A prescription filled for erlotinib was considered an exposure.
Primary and secondary outcomes
The primary outcome was the hazard ratio for mortality in the cohort. Data on patient status was collected until death or study end (12/31/2018); 2007 was chosen as the first inclusion year as this is the initial year staging information was available in the administrative database, and 2016 as the last inclusion year as this would allow for at least 2 years of follow up. If patients were alive at the time of study end, they were censored. Overall survival of patients in the model was calculated from day of lung cancer diagnosis to date of death or censorship.
All cause ED visits were also evaluated and treated as a count variable during the follow up period.
Other covariates
Variables collected as potential covariates in the model included age at diagnosis, chemotherapy history (time varying), duration of first line chemotherapy, histology, aggregated diagnosis groups (ADG) score (reflecting comorbidities), socioeconomic status (SES), sex, and area of residence (LHIN), place of residence (urban vs. rural), ethnicity (if available), second-line chemotherapy drugs received, duration of second-line therapy, history of comorbidities [CHF, dementia, COPD, type II diabetes mellitus (DM)], year of cohort entry, time on erlotinib, and ICES unique identifier (IKN). First-line chemotherapy was defined as initial use of “vinorelbine, gemcitabine, paclitaxel, or pemetrexed” after diagnosis of advanced NSCLC. Concomitant use of a platinum agent is not systematically collected in the NDFP data, and was not available to us.
SES was estimated using income quintiles which are generated based on conversion of subjects’ postal codes using Statistics Canada’s Postal Code Conversion File and linkage to census data (33). Patient comorbidity at the time of diagnosis was estimated using the Johns Hopkins ACG System Version XX (Baltimore, MD, USA) using diagnostic codes from CIHI-DAD, OHIP, RPDB, OMHRS (34,35).
Statistical methods
Descriptive statistics were used for baseline characteristics, and Chi-square analysis was performed to evaluate differences between groups. A Cox proportional hazards model was used to model the effect of erlotinib treatment on survival. To mitigate immortal person time bias, erlotinib therapy was treated as a two-level time varying covariate, and chemotherapy history was coded as three-level time varying covariate (post first-line, post second-line, post 3+ lines) within the model. Variables used in the model were initially chosen a priori. The final variables included erlotinib use (time dependent), age at diagnosis, sex, chemotherapy history (time dependent), duration of first-line chemotherapy, index year of entry into cohort, histological subtype, area of residence, SES, and ADG score. As EGFR mutation status is not captured in these databases, an analysis was performed to evaluate the hazard function for erlotinib use in the cohort of patients receiving erlotinib.
To assess ED visits for patients receiving erlotinib, a Poisson regression analysis was performed. Clustering within subjects was accounted for due to the recurring nature of ED visits for an individual. After univariable screening, variables included in the final Poisson model include erlotinib use (time dependent), age, year of cohort entry, area of residence, SES, and ADG score.
Missing data was dealt with using stepwise deletion. Missing data was only present for income quintile, all other data were complete. Patients were censored in the analysis if they were alive at the end of the follow up period, or if lost to follow up.
Results
Participants
From 1/1/2007–12/31/2016, 30,208 patients were diagnosed with stage IIIB or IV NSCLC in Ontario, Canada. Only 30.4% of patients received chemotherapy with pemetrexed, gemcitabine, paclitaxel or vinorelbine. These numbers are consistent with previously published data on rates of chemotherapy use in advanced NSCLC in Ontario (36). The final cohort consisted of 3,846 patients (Figure 1).

Of patients that received at least one dose of first-line chemotherapy, 19.7% received second or later-line erlotinib. Patients that received erlotinib were more likely to be female (46.5% vs. 42%), have no history of COPD (50.4% vs. 45.8%; Table 1). Gemcitabine (as a single agent or in combination with platinum) was the most commonly used agent in first-line in all groups. Use of pemetrexed in the first-line was more common in the non-erlotinib exposed group (18.7% vs. 10.8%), likely reflecting the increased use of pemetrexed and decreased use of erlotinib in later years. Patients treated with erlotinib were more likely to have received second-line chemotherapy (51.1% vs. 21.9%), and to have received 3 or more lines of chemotherapy (3.5% vs. 1.1%; Table 1). Median follow-up time for the entire cohort was 347 days (IQR 213–603 days).
Table 1
| Cohort characteristics | No erlotinib, % (n) (n=3,087) | Erlotinib, % (n) (n=759) |
|---|---|---|
| Sex | ||
| Female | 42 [1,296] | 46.5 [353] |
| Male | 58 [1,791] | 53.5 [406] |
| Age at diagnosis (mean) | 72 SD 5.2 | 72 SD 5.2 |
| Stage | ||
| IIIB | 16.8 [520] | 15.5 [118] |
| IV | 83.2 [2,567] | 84.5 [641] |
| Ethnicity | ||
| Chinese | 3.6 [110] | 5.9 [45] |
| Unknown | 95.8 [2,958] | 93 [706] |
| South Asian | 0.6 [19] | 1.1 [8] |
| Histology | ||
| Adenocarcinoma | 55.9 [1,726] | 58.5 [444] |
| Squamous | 18.7 [578] | 13.6 [103] |
| Large Cell | 2.1 [64] | 2.1 [16] |
| Other | 23.3 [719] | 25.8 [196] |
| Income quintile | ||
| 1 | 20.4 [628] | 17.0 [129] |
| 2 | 21.3 [656] | 21.6 [164] |
| 3 | 21 [646] | 20.7 [157] |
| 4 | 19.6 [601] | 22.6 [170] |
| 5 | 17.8 [544] | 18.1 [137] |
| Missing | 0.4 [14] | |
| Place of residence | ||
| Urban | 85.6 [2,640] | 87.8 [661] |
| Rural | 14.4 [446] | 12.2 [93] |
| CHF | 14.3 [442] | 11.6 [88] |
| Dementia | 2.2 [67] | 1.6 [12] |
| COPD | 54.2 [1,675] | 49.6 [376] |
| Type II DM | 30 [923] | 31.7 [241] |
| ADG score | ||
| 0-4 | 4.4 [137] | 4.3 [33] |
| 5-9 | 24.7 [762] | 25.8 [196] |
| 10-14 | 46.8 [1,146] | 48 [364] |
| 15-19 | 22.4 [691] | 20.1[157] |
| 20+ | 1.7 [51] | 1.3 [10] |
| Year of cohort entry | ||
| 2007 | 5.1 [157] | 6.2 [47] |
| 2008 | 8.9 [275] | 13.2 [101] |
| 2009 | 9.3 [287] | 12.6 [95] |
| 2010 | 10.4 [321] | 13.4 [102] |
| 2011 | 9.7 [300] | 13.8 [105] |
| 2012 | 9.7 [300] | 11.3 [86] |
| 2013 | 9.7[300] | 10.3 [78] |
| 2014 | 10.6 [328] | 9.2 [70] |
| 2015 | 13.3 [410] | 5.4 [41] |
| 2016 | 13.2 [409] | 4.5 [34] |
| First-line chemotherapy | ||
| Pemetrexed | 18.7 [576] | 10.8 [82] |
| Paclitaxel | 15.7 [483] | 17.5[133] |
| Gemcitabine | 49.9 [1,540] | 55.5 [421] |
| Vinorelbine | 15.8 [488] | 16.2 [123] |
| Second-line chemotherapy | ||
| Total | 21.9 [677] | 51.1 [404] |
| Pemetrexed | 60 [407] | 69.8[282] |
| Pembrolizumab | <1.6 [<5] | <1.6 [<5] |
| Docetaxel | 30.8 [208] | 26.7 [108] |
| Gemcitabine | <8 [<25] | <1.4 [<11] |
| Nivolumab | 1.2 [36] | <0.7 [<5] |
| Prior lines of chemotherapy | ||
| 1st line only | 78.1 [2,410] | 46.8 [355] |
| 1st and 2nd line | 20.8 [643] | 49.6 [377] |
| 3+ lines | 1.1 [34] | 3.6 [27] |
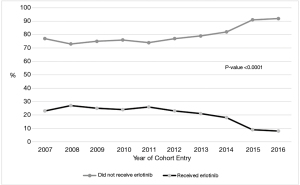
Uptake of erlotinib therapy over time
The proportion of patients that were prescribed second or third-line erlotinib decreased over time (P<0.0001; Figure 2). In patients treated with erlotinib, the median time on erlotinib was 58 days (IQR 30–113 days) and the number of patients that received erlotinib for ≥90 days was 231 (30%), ≥180 days 98 (13%), and ≥365 days 42 (5%).
Primary outcome
Our primary outcome was to assess the effect of erlotinib use on survival in all patients with advanced NSCLC that received second or later-line erlotinib therapy. 96.5% of patients experienced the event of interest (death) during follow up, with 3.5% patients censored (3.3% at time of maximal follow-up, 0.2% at the time of lost OHIP). Unadjusted median overall survival in the entire cohort was 325 days (95% CI: 314–337 days). In the erlotinib and non-erlotinib cohort, the unadjusted median overall survival was 513 days (95% CI: 485–539 days) and 282 days (95% CI: 270–291 days) respectively.
Given the issue with immortal person time bias in our retrospective survival model, we included two time-dependent covariates (chemotherapy history and erlotinib use) to compare the hazard ratio for death of patients while on erlotinib therapy to those not on erlotinib therapy. This was performed so that the hazard ratio for death for patients on erlotinib could be interpreted in the context of those who have experienced the same lines of chemotherapy at the initiation of erlotinib. Due to the time-dependent nature of the covariates, only the adjusted and unadjusted hazard ratio for death for patients on erlotinib, and not the adjusted median overall survival, could be reported.
The unadjusted HR for death for patients on erlotinib therapy, conditional of having the same prior number of lines of chemotherapy was 1.86 (95% CI: 1.71–2.03, P<0.0001) and the adjusted HR for death was 1.89 (95% CI: 1.73–2.07, P<0.0001). Patients on erlotinib were 1.89-fold more likely to die than those not on erlotinib.
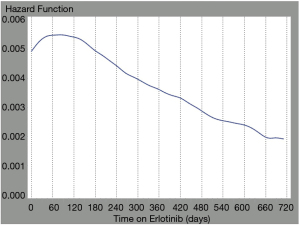
We examined the hazard of mortality over time for patients that were treated with erlotinib during erlotinib therapy. We suspected a non-constant hazard function as the population exposed to erlotinib would be mixed, i.e., composed of EGFR positive (often untested) and wild type patients (as testing for EGFR was not funded in Ontario until 2014). In a mixed population, we would expect the hazard function to change over time. The hazard function for erlotinib treatment in our analysis changed over time, confirming the mixed population (Figure 3). For the first 89 days of erlotinib therapy, patients had an increasing hazard function, indicating worsening survival on erlotinib. However, if patients remained on erlotinib for longer than 89 days, their hazard function began to decrease, indicating improving survival on erlotinib, likely associated with EGFR mutant lung cancer.
Secondary outcome
In our cohort patients had a mean number of ED visits of 1.9 (SD 2.25) with a range of 0–42 visits. We examined the number of ED visits for patients on and off of erlotinib using a Poisson model with all-cause ED visits as the outcome. Erlotinib was treated as a time-dependent covariate, and other covariates in the model included age, year of cohort entry, area of residence, SES, and ADG score. During erlotinib treatment, patients receiving erlotinib had a marginally higher relative risk of ED visits. The unadjusted relative risk was 1.14 (95% CI: 1.05–1.23, P=0.0008) and the adjusted relative risk was 1.10 (95% CI: 1.02–1.19, P=0.013), compared to patients receiving either best supportive care or chemotherapy.
Discussion
To our knowledge, this is the largest retrospective study of real-world second or later-line erlotinib use in unselected advanced NSCLC patients. Despite the longer crude survival difference in patients treated with erlotinib, the hazard for death suggests this difference was not attributable to erlotinib treatment, and rather the increased survival seen in the erlotinib group is reflective of the fact that these patients simply lived long enough to receive erlotinib. Review of the literature suggests marginal benefit of erlotinib therapy in the unselected or EGFR wild-type population. Many of those studies excluded patients who would typically be treated with erlotinib in the real world (e.g., those with brain metastases, poor performance status, organ dysfunction, recent radiation). However, these real-world patients are included in our analysis, which may account for the differences seen. Patients treated with erlotinib in our study also had higher relative risk of visiting the ED. This likely reflects increased healthcare utilization by advanced cancer patients receiving ongoing active medical management at end of life, rather than erlotinib toxicity.
The landmark NCIC CTG BR.21 trial was designed and conducted in an era where EGFR targeted therapies were believed to benefit patients with EGFR protein expression, and activating EGFR mutations had not yet been discovered. This randomized trial of unselected patients receiving second or third-line erlotinib demonstrated an OS of 6.7 months compared to 4.7 months with placebo, respectively (hazard ratio, 0.70; P<0.001) (3). This important finding was reproduced in a phase IV registrational study of second-line erlotinib in unselected patients with a median OS Of 7.2 months (30). Other real-world studies of erlotinib in wild-type or unselected, pre-treated advanced NSCLC have not been as promising. A study of 54 patients with EGFR wild-type NSCLC treated with erlotinib after failure of second-line pemetrexed yielded a median survival of 5.8 months (95% CI: 3.3–8.6 months), with no responses seen (response rate 0%, 97.5% CI: 0.0–6.8%) (26). A French retrospective study demonstrated a survival benefit of 4.2 months (95% CI: 3.5–5.4 months) with erlotinib in unselected NSCLC patients, and 1.3 months (95% CI: 1.0–1.8) with supportive care, but this analysis did not account for immortal person time bias (32).
This study highlights the importance of using targeted therapy in a targeted fashion. The EGFR status was unknown in this patient population, and prior to 2011 was not routinely tested in Ontario, Canada. The study population therefore includes those with and without EGFR gene mutations. This is evidenced by the changing hazard function for erlotinib therapy based on duration of treatment. Initially the hazard function is positive, likely reflecting EGFR wild-type patients that are not deriving benefit. For patients receiving erlotinib for greater than 89 days, the hazard function decreases, likely accounting for the subset of patients with undiagnosed EGFR mutations experiencing substantial benefit (13% of cohort received erlotinib for >180 days).
Patients that received erlotinib therapy were more likely to be female, have adenocarcinoma histology, have received multiple lines of therapy, and were less likely to have COPD (often associated with smoking). This is an anticipated finding, as many of these factors portend a better prognosis in advanced NSCLC and these patients would be more likely to live to receive second or later-line therapy. The historical context of this study is important as during the study period, EGFR mutations were emerging as a predictive biomarker for EGFR targeted therapy. This is reflected in the decreasing proportion of patients receiving erlotinib as second- or third-line therapy over time, starting in 2011 after the publication of the IPASS trial (Figure 1). This is encouraging as it indicates physicians in Ontario rapidly change practice based on published high-level evidence and guidelines.
There are important limitations to this study. We are unable to identify which patients had an EGFR mutation as initially this was not tested, and in later years, although tested, is not captured in our databases. Ideally, we would be able to exclude those patients with EGFR mutations to understand the clinical outcomes of second or later-line erlotinib therapy in the EGFR wild-type population, which is representative of the current group for whom this indication is still funded. We were also unable to verify whether patients received platinum, or the specific agent. We expect that most patients would have received a platinum agent in the first-line, as funding for pemetrexed, vinorelbine, paclitaxel in our study cohort would have required concomitant platinum administration. Imbalances in the use of cisplatin versus carboplatin could not be detected in our model, but we do not anticipate that this would impact on our findings. We used time varying covariates (chemotherapy and erlotinib use) in our models, however patient comorbidity score was not incorporated as a time varying covariate. Thus, the score at cohort entry was employed as a fixed value for simplicity in the analysis. Comorbidity is by nature time varying and changes in comorbidity over time were not accounted for. However, given the short life expectancy in this advanced lung cancer population, we do not anticipate changes from cohort entry nor impact on patient survival.
Our study suggests that treatment with erlotinib therapy is unlikely to benefit unselected patients with advanced NSCLC. While our study showed potential harm in this group, this finding must be tempered with the positive results of the phase III NCIC CTG BR.21 and the LUX-Lung 8 randomized trials (3,21). Also, the importance of under-genotyped patients must not be ignored – even today many advanced lung cancer patients in Canada have not been adequately genotyped for optimal therapy (16,37). Notwithstanding, our study highlights the importance of using targeted therapy in a targeted fashion, and the value of EGFR kinase inhibitor therapy in those with EGFR wild-type NSCLC is minimal at best.
Acknowledgments
Parts of this study are based on data and information compiled and provided by: MOHLTC, Cancer Care Ontario (CCO), Canadian Institute for Health Information (CIHI). No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. The authors thank IQVIA Solutions Canada Inc for use of their Drug Information File.
Funding: This study received funding from the Princess Margaret Cancer Foundation (OSI Pharmaceuticals Foundation Chair) and the University of Toronto (Ontario Student Opportunity Trust Fund). This study was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-804
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-804
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-804
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-804). KP has received travel funding from Roche, continuing medical education speaker honorarium from Merck, Pfizer and Astra Zeneca, and consulting fees from Amgen. NBL has received continuing medical education speaker honoraria from Merck and Bristol Myers Squibb as well as institutional research funding from Array, Guardant Health, Pfizer, MSD, Roche, Astra Zeneca, Lilly, EMD Serono, Takeda, and Bayer. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Research Ethics Board of the Princess Margaret Cancer Centre/University Health Network, Toronto, Canada (REB: 19-5286) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol 2005;23:3227-34. [Crossref] [PubMed]
- Metro G, Finocchiaro G, Toschi L, et al. Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC). Rev Recent Clin Trials 2006;1:1-13. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Sequist LV, Bell DW, Lynch TJ, et al. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol 2007;25:587-95. [Crossref] [PubMed]
- Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:3908-14. [Crossref] [PubMed]
- Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol 2005;23:2513-20. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- John T, Liu G, Tsao MS. Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene 2009;28:S14-23. [Crossref] [PubMed]
- Shiau CJ, Babwah JP, da Cunha Santos G, et al. Sample features associated with success rates in population-based EGFR mutation testing. J Thorac Oncol 2014;9:947-56. [Crossref] [PubMed]
- Cheema PK, Raphael S, El-Maraghi R, et al. Rate of EGFR mutation testing for patients with nonsquamous non-small-cell lung cancer with implementation of reflex testing by pathologists. Curr Oncol 2017;24:16-22. [Crossref] [PubMed]
- Lim C, Tsao MS, Le LW, et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall-cell lung cancer. Ann Oncol 2015;26:1415-21. [Crossref] [PubMed]
- Ministry of Health Ontario. Exceptional Access Program Reimbursement Criteria for Frequently Requested Drugs. 2020. [Accessed November 01 2020]. Available online: http://www.health.gov.on.ca/en/pro/programs/drugs/docs/frequently_requested_drugs.pdf
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [Crossref] [PubMed]
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897-907. [Crossref] [PubMed]
- Cicènas S, Geater SL, Petrov P, et al. Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (IUNO study). Lung Cancer 2016;102:30-7. [Crossref] [PubMed]
- FDA. Erlotinib (Tarceva). 2016 [Accessed 2020 Aug 24]. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/erlotinib-tarceva#:~:text=On October 18%2C 2016%2C the,factor receptor (EGFR) mutations
- Vincent MD, Kuruvilla MS, Leighl NB, et al. Biomarkers that currently affect clinical practice: EGFR, ALK, MET, KRAS. Curr Oncol 2012;19:S33-44. [Crossref] [PubMed]
- Ellis PM, Blais N, Soulieres D, et al. A systematic review and Canadian consensus recommendations on the use of biomarkers in the treatment of non-small cell lung cancer. J Thorac Oncol 2011;6:1379-91. [Crossref] [PubMed]
- Germonpré P, Van den Wyngaert T. Second-line erlotinib after failure of pemetrexed-containing chemotherapy in advanced non-small cell lung cancer (NSCLC): Real-world effectiveness, safety and tolerability. PLoS One 2019;14:e0215135 [Crossref] [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [Crossref] [PubMed]
- Vergnenegre A, Smit EF, Toy E, et al. Second-line therapy for non-small cell lung cancer in clinical practice: final results and treatment pathways from the SELECTTION observational study. Curr Med Res Opin 2012;28:1253-62. [Crossref] [PubMed]
- Zietemann V, Duell T. Prevalence and effectiveness of first-, second-, and third-line systemic therapy in a cohort of unselected patients with advanced non-small cell lung cancer. Lung Cancer 2011;73:70-7. [Crossref] [PubMed]
- Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol 2010;5:1616-22. [Crossref] [PubMed]
- Wu FZ, Song JJ, Zhao ZW, et al. The efficacy and safety of erlotinib compared with chemotherapy in previously treated NSCLC: A meta-analysis. Math Biosci Eng 2019;16:7921-33. [Crossref] [PubMed]
- Debieuvre D, Moreau L, Coudert M, et al. Second- or third-line treatment with erlotinib in EGFR wild-type non-small cell lung cancer: Real-life data. Rev Mal Respir 2019;36:649-63. [Crossref] [PubMed]
- Postal CodeOM Conversion File (PCCF), Reference Guide, 2017. Statistics Canada Catalogue no. 92-154-G. [Accessed 2020 Nov 9]. Available online: https://www150.statcan.gc.ca/n1/pub/92-154-g/92-154-g2017001-eng.htm
- Austin PC, Walraven Cv. The mortality risk score and the ADG score: two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortality in a general adult population cohort in Ontario, Canada. Med Care 2011;49:940-7. [Crossref] [PubMed]
- Weiner JP, Starfield BH, Steinwachs DM, et al. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care 1991;29:452-72. [Crossref] [PubMed]
- Sacher AG, Le LW, Lau A, et al. Real-world chemotherapy treatment patterns in metastatic non-small cell lung cancer: Are patients undertreated? Cancer 2015;121:2562-9. [Crossref] [PubMed]
- Nadjafi M, Sung MR, Santos GDC, et al. Diagnostic patterns of non-small-cell lung cancer at Princess Margaret Cancer Centre. Curr Oncol 2020;27:244-9. [Crossref] [PubMed]


