
Long-term survival following postoperative myocardial infraction after coronary artery bypass surgery
Introduction
Despite rapid advances in percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG) remains the gold standard in the treatment of multivessel coronary artery disease (CAD) (1). Unfortunately, variations in the coronary anatomy, extreme calcifications, stenotic lesions or complete coronary occlusions, and the clinical status of the patient may avert complete revascularization (CR) (2). A consequence of incomplete revascularization (IR) is the risk of postoperative myocardial injury (PMI), defined as an isolated elevation in cardiac biomarkers greater than the upper limit of normal, in the 48-h post-operative period (3). PMI is associated with increased hospital morbidity and mortality (4,5) with an estimated incidence of 1–10% (6,7). However, elevation of cardiac biomarkers occurs in virtually all patients undergoing CABG surgery, and there is no clear consensus on the degree of elevation of cardiac biomarkers at which it becomes either clinically relevant or prognostically significant. Therefore, the subtle and difficult presentations of PMI often require rapid and effective diagnostic and therapeutic intervention to prevent the complication of postoperative myocardial infarction (MI) and ensure preserved cardiac function. Should postoperative MI occur, emergent coronary angiography (ECA) has been shown to be an effective tool in eventually restoring hemodynamic instability following CABG (4,8-11). Data on the impact of postoperative MI on long-term outcomes after CABG are, however, surprisingly sparse.
Data from randomized controlled trials, like widely disputed EXCEL Study, support the notion that postoperative myocardial ischemia negatively affects the post-CABG outcomes, including all-cause mortality (12). However, real-life data outside randomized trials are not common. The inclusion and exclusion criteria in randomized trials might be fraught with selection bias, and this does not represent the cases seen in everyday practice in a wholesome manner. This prompted us to investigate the registry data from our institution, where all-inclusive analysis of postoperative MI would be placed in the setting of real-life patient characteristics. In this study, we determine the impact of postoperative myocardial infraction (MI) on early and late survival after CABG, with particular attention to the impact on long-term mortality, in a large cohort of real-life patients.
We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1279/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committee of Medical Chambers in Krakow (No.: L.dz.OIL/KBL/OIL/11/216) and individual consent for this retrospective analysis was waived. Data was collected retrospectively from the KROK registry (Polish National Registry from Cardiac Surgery Procedures) (available at https://www.krok.csioz.gov.pl). The registry is an ongoing, nationwide, multi-institutional registry for all general cardiac surgery in Poland that collects in-hospital data on patients and outcomes; details of the registry design have been described in other papers (13-16). Long-term survival data are transmitted directly to the registry from follow-up mortality data obtained from National Health Fund—the nationwide, mandatory, public health insurance institution in Poland—and included in the registry (13).
Patient selection
We performed a retrospective review of 4,642 consecutive patients who underwent isolated CABG at our institution over a four-year period (01/2012 to 12/2016). We included all patients diagnosed with postoperative MI in the early postoperative period, defined as 48 hours after CABG.
CABG surgery
If a patient received aspirin preoperatively, it was continued throughout the perioperative period. Clopidogrel was discontinued 5 days preoperatively, unless the surgery was performed emergently. All patients underwent surgery through a median sternotomy with cardio-pulmonary bypass (CPB) performed in moderate hypothermia (esophageal temperature, 32 °C) or normothermia, depending on the surgeon’s preference, using a nonpulsatile roller pump and a 40 µm arterial blood filter (both, Jostra Medizintechnik AG, Hirrlingen, Germany) with a blood flow of 2.0–2.4 L/min/m2 and a mean arterial pressure of 40–60 mmHg. Anticoagulation was achieved by administration of heparin (300 IU/kg) before the start of CPB and monitored by activated clotting time (ACT; 480 seconds during CPB, and normal after reversal by protamine at the end of CPB). Heparin was also included in the initial CPB solution (10,000 IU).
Completeness of revascularization was assessed according to the anatomic definition in which all diseased arterial systems with a vessel size ≥1.5 with at least one significant stenosis (>50%) receive a graft (17).
Postoperative MI definition
Because there is currently no clear definition for prognostically significant postmyocardial injury in terms of the level of post-operative cardiac biomarker elevation, we decided to define postoperative MI following the guidelines of the European Society of Cardiology (ESC) Joint Working Groups on Cardiovascular Surgery and the Cellular Biology of the Heart (18) with the definition of type 5 of myocardial infarction published by the fourth Global MI task force (3). Postoperative MI was diagnosed when there was an elevation in cardiac troponin levels (cTn) values >10×99th percentile upper reference limit (URL) during the first 48 h after CABG surgery. In patients with normal baseline cardiac cTn values (<99th percentile URL) along with either: (I) new pathological Q waves or new left bundle branch block (LBBB), or (II) angiographically documented new graft or new native coronary artery occlusion, or (III) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality (RWMA). Routine testing of myocardial necrosis markers [cTn and myocardial muscle creatine kinase (CK-MB)] were tested in a serial fashion upon intensive care unit (ICU) admission, at 6 and 12 h postoperatively, and later if clinically indicated.
Postoperative MI in patients presenting with acute coronary syndrome or urgent surgery was diagnosed according to the definition of type 5 of myocardial infarction (3). If a recurrence of infarction was suspected based on clinical signs or symptoms after the first MI, immediate measurement of cTn was recommended. A second sample was obtained 3–6 hours later or earlier with more sensitive cTn assays. If the cTn concentration was elevated but stable or decreasing at the time of suspected reinfarction, a >20% increase in cTn was required for a diagnosis of reinfarction. If the original cTn concentration was normal, the criteria for a new acute MI apply (3).
Emergency coronary angiography (ECA) was performed in the early postoperative period if the patient had any of the following criteria: new-onset unexplained hemodynamic collapse, malignant ventricular arrhythmias, sudden cardiac arrest, persistent ST -segment changes on ECG, elevated cTn/CK-MB, or new RWMA on 2D-TTE, or other clinical findings suggestive of myocardial ischemia. ECA was deferred if acceptable hemodynamic stability could not be achieved or maintained despite maximal medical therapy. ECAs were performed in a standardized manner by our cardiology colleagues at the same hospital.
Baseline characteristics
For each patient included in the study, demographic data (age, sex, weight, height), comorbidities (hypertension, hyperlipidemia, diabetes mellitus, tobacco use, previously known CAD, previous myocardial infarction, previous PCI, valvular heart disease (VHD), heart failure, cerebrovascular accident, atrial fibrillation, chronic obstructive pulmonary disease, renal insufficiency) and functional status at the time of cardiac surgery [Canadian Cardiovascular Society (CCS) class, New York Heart Association (NYHA) class, creatinine clearance and EuroSCORE II] were collected. Information on the type of revascularization (CR over IR), circumstances of the procedure (elective vs. non-elective) were also collected.
Statistical analysis
Normal distribution was tested using the Kolmogorov-Smirnov test. Continuous, normally distributed variables were summarized as mean ± standard deviation and compared with standard t-test; variables with non-normal distributions were summarized as median (interquartile range; IQR) and compared with the Mann–Whitney U test. Categorical variables were expressed as number (%) and compared with the Chi-square test or Fisher exact test, when appropriate.
Univariate analysis for remodeling function was performed, using Kaplan-Meier survival plots and log-rank tests, to compare 1- and 5-year survival between two groups (PMI and no-PMI).
Univariate cox proportional-hazard models were constructed to calculate hazard ratios (HRs) of selected parameters for death within 1- and 5-year follow-up after CABG (Tables S1,S2). The multivariate stepwise forward Cox proportional-hazards model was used to assess the standardized impact of PMI on hazard ratio of death within 1 year and 5 years after CABG. Parameters which were statistically significant in univariate models and parameters which were determined clinically significant (i.e., gender) were included in the final model. In case of high interdependence between parameters (i.e., acute coronary syndrome and non-elective surgery), the parameter with higher impact on the outcome was selected for the final model. The ensuing statistical models were used to define the HRs point estimate and 95% CI.
Sensitivity analysis of obtained results was performed using inverse probability of treatment weighting (IPTW) and general estimating equations (Tables S3,S4). Propensity scores were calculated using a multivariable logistic regression (Hosmer Lemeshow test: χ2=10.454, df =8; P=0.235), where the occurrence of PMI was considered the dependent variable. Clinically relevant variables from baseline characteristics were included [age, sex, diabetes, preoperative creatinine level, acute coronary syndrome (ACS), left ejection ventricular fraction (LVEF), non-elective surgery, EuroSCORE II]. The adjusted ORs of PMI for in-hospital mortality, 30-day mortality, 1-year mortality and 5-year mortality were calculated.
Univariate and multivariate stepwise forward regression models were constructed to determine risk factors for PMI. Parameters which were statistically significant in univariate models and parameters which were determined clinically significant (i.e., EuroSCORE II, age, LVEF) were included in the final model. In case of high interdependence between parameters (i.e., acute coronary syndrome and non-elective surgery), the parameter with higher impact on the outcome was selected for the final model. The ensuing statistical models were used to define the odds ratios (ORs) point estimate and 95% CI. Results of univariate Cox regression analysis for death within 1 year and 5 years of CABG are reported in Tables S1,S2.
A two-sided P value <0.05 was considered statistically significant for all calculations. The statistical analysis was performed with IBM SPSS Statistics for Windows, Version 26.0. (IBM Corp. Armonk, NY, USA).
Results
Between January 2012 and December 2016, 4,642 patients underwent isolated CABG at our institution (mostly hypertensive men with a mean age of 66 years with left main disease (53.1%) (Table 1), of whom 81.7% underwent elective revascularization. Of the 4,642 consecutive isolated CABG surgeries performed at our institution over a four-year period, 141 patients (3.04%) were diagnosed with postoperative MI in the early postoperative period (Figure 1). In patients with postoperative MI, the highest observed T troponin levels within the first 24 hours was 3.20±3.79 ng/mL and CK-MB was 182.36±190.17 U/L. The mean troponin T within the first 48 hours after CABG was 3.63±3.46 ng/mL and CK- MB was 166.55±178.10 U/L. There were significant differences in pre- and postoperative LVEF in patients with PMI, with a preoperative EF of 50.0%±11.2% and a postoperative EF of 38.2%±11.9% (P=0.0001). No cases of acute mitral regurgitation at postoperative TEE were observed. In patients with postoperative infarction, rescue PCI was the most common therapeutic approach (55.3%), conservative treatment was recommended in 43.2%, and redo CABG was performed in 2 cases (1.4%).
Table 1
| Variables | No post-operative MI (n=4501) | Postoperative MI (n=141) | P |
|---|---|---|---|
| Age (years) | 66.00±9.01 | 67.01±9.34 | 0.068* |
| Sex (female) | 22% (n=990) | 29.8% (n=42) | 0.028# |
| CCS class | 0.015# | ||
| 1 | 14.5% (n=652) | 20.6% (n=29) | |
| 2 | 35.9% (n=1,616) | 26.2% (n=37) | |
| 3 | 36.2% (n=1,629) | 34.0% (n=48) | |
| 4 | 13.4% (n=604) | 19.1% (n=27) | |
| NYHA class | 0.129# | ||
| 1 | 38.1% (n=1,714) | 34.0% (n=48) | |
| 2 | 48.0% (n=2,160) | 46.1% (n=65) | |
| 3 | 11.3% (n=510) | 17.7% (n=25) | |
| 4 | 2.6% (n=117) | 2.1% (n=3) | |
| Myocardial infarction in the past | 54.8% (n=2,466) | 58.2% (n=82) | 0.429# |
| Current smoking | 15.5% (n=699) | 14.2% (n=20) | 0.664# |
| Diabetes | 34.1% (n=1,537) | 35.5% (n=50) | 0.746# |
| Hypertension | 91.9% (n=4,137) | 94.3% (n=133) | 0.299# |
| Hyperlipidemia | 35.5% (n=1,597) | 39.7% (n=56) | 0.301# |
| Heart rate | 0.334# | ||
| Sinus rhythm | 94.4% (n=4,247) | 95.7% (n=135) | |
| Atrial fibrillation | 4.9% (n=222) | 2.8% (n=4) | |
| Different | 0.7% (n=32) | 1.4% (n=2) | |
| Renal function | 0.672# | ||
| Normal | 63.3% (n=2,592) | 62.1% (n=82) | |
| Moderate | 63.3% (n=1,243) | 33.3% (n=44) | |
| Severe | 5.7% (n=234) | 4.5% (n=6) | |
| Dialysis | 0.6% (n=26) | 0 | |
| Chronic lung disease | 3.8% (n=171) | 4.3% (n=6) | 0.781# |
| Acute coronary syndrome | 22.8% (n=1,026) | 31.9% (n=45) | 0.011# |
| Left main coronary artery stenosis | 53.1% (n=2,388) | 56.0% (n=79) | 0.488# |
| CABG urgency | 0.003# | ||
| NON-elective | 18.4% (n=828) | 28.4% (n=40) | |
| Elective | 81.6% (n=3,673) | 71.6% (n=101) | |
| Incomplete revascularization | 29.4% (n=1,323) | 46.8% (n=66) | 0.001# |
| EuroSCORE II | 2.18±1.02 | 2.78±1.21 | 0.005* |
*, Mann-Whitney U test; #, Chi2 test. Renal function is defined by calculated creatinine clearance as estimated using the Cockcroft-Gault formula as follows: normal (>85 mL/min); moderately impaired (50–85 mL/min); severely impaired (<50 mL/min); on dialysis (virtually absent). MI, myocardial infraction; CCS, Canadian Cardiovascular Society; CABG, coronary artery bypass graft; NYHA, New York Heart Association.
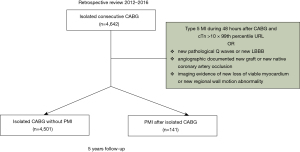
The mean follow-up time was 5.1±2.07 years (range, 4.4–6.9 year). Baseline characteristics, clinical and surgical data of the entire study group are shown in Table 1. In-hospital mortality in the postoperative MI group was 4.3%, and 3.6% in the group of patients without postoperative MI (P=0.135). Postoperative MI was more common in patients with recent acute coronary syndrome (P=0.011) and with non-elective surgery (P=0.003, Table 1). Patients with postoperative MI after CABG had higher rate of postoperative complications including cardiac tamponade and mediastinal re-exploration for bleeding (Table 2).
Table 2
| Variables | No post-operative MI (n=4,501) | Postoperative MI (n=141) | P |
|---|---|---|---|
| Prolonged hospital stay | 10.1% (314/3,109) | 29.3% (36/123) | 0.001# |
| Resuturing of the sternum | 1.6% (72/4,441) | 3.7% (5/135) | 0.064# |
| Retoracotomy/tamponade | 3.7% (165/4,441) | 10.4% (14/135) | 0.001# |
| In-hospital mortality | 3.6% (162/4,501) | 4.3% (6/141) | 0.168# |
#, Chi2 test. MI, myocardial infraction.
Impaired 1 year (log rank test, P<0.001) and 5-year (log rank test, P<0.044) survival was observed in the PMI group (Figure 2).

Based on the multivariate cox-regression models, the standardized risk of death within 1 year and 5 years after CABG was higher in patients with PMI (HR 1.942, 95% CI: 1.277–3.073, P=0.005; HR 1.451, 95% CI: 1.010–2.083, P=0.044, respectively) (Figure 3).

Based on the sensitivity analysis with IPTW, the adjusted OR of PMI on 1-year mortality equaled 2.602, 95% CI: 1.563–4.331, P=0.000 and for 5-years mortality equaled 1.512, 95% CI: 0.987–2.314, P=0.057. Detailed data on IPTW can be found in supplementary tables.
Based on the multivariate logistic regression, the main risk factors for postoperative MI were IR [OR (95% CI), 2.25 (1.59–3.12), P=0.001], non-elective surgery [OR (95% CI), 1.68 (1.10–2.54), P=0.015] and female gender [OR (95% CI), 1.48 (1.01–2.18), P=0.045]. The results of the multivariate logistic regression for postoperative MI are shown in Figure 4.

Discussion
Postoperative MI is a serious complication associated with poor short- and long-term outcomes after CABG. Our analyses were based on 4,642 consecutive real-life patients who underwent isolated, first-time CABG surgery. We present one of the most comprehensive analyses on the impact of postoperative MI on outcome after primary CABG in real-life patients.
The early postoperative period is crucial to the long-term outcome of cardiac surgery. It is the most vulnerable time for the development of many cardiac complications (11,19). PMI is defined as an isolated elevation of cardiac biomarkers (>10× URL) in the 48-hour postoperative period. However, this level of elevation of cardiac biomarkers occurs in virtually all patients undergoing CABG surgery. According to the ESC Working Groups diagnostic criteria for type 5 MI have been proposed, but there is currently no clear definition of a prognostically significant PMI associated with worsening clinical outcomes (18). Moreover, previous studies showing an association between PMI after CABG and clinical outcomes were based on different types of cardiac biomarkers, without perfusion imaging or echocardiographic analysis of regional wall motion abnormalities due to the lack of a clear definition for PMI. This may lead to misinterpretation and can be ambiguous for further clinical evaluation of postoperative MI (4,20). Therefore, there is no clear consensus on the degree of elevation of cardiac biomarkers at which it becomes either clinically relevant or prognostically significant PMI after CABG surgery. Moreover, long-term data on the effect on postoperative MI after CABG are currently lacking.
In our study we defined postoperative MI associated with CABG, as defined by the fourth Global MI task force (3,4,8). It should be emphasized that the incorrect definition of postoperative MI after CABG was the cause of a methodological error from the highly controversial EXCEL trial (12,21), where CABG related MI was defined by an elevation of troponin >5 times the 99 the percentile URL instead of >10 times the 99th percentile (3). The discussion following the EXCEL trial has led to revision of the ESC/EACTS Guidelines on Myocardial Revascularization (22) and the subanalysis of the EXCEL trial reported by Ben-Yehuda et al. (12) showed that only extensive myonecrosis (CK-MB ≥10× URL) present in clinically relevant MI is associated with increased 3-year all-cause and cardiac mortality after revascularization.
Therefore, the most important finding of the current analysis is the reduced survival rate in patients with postoperative MI after CABG surgery based on the real-life registry data. Increased mortality was observed throughout the 5-year follow-up period, with the highest mortality rate occurring in the first year after surgery. Although the groups with and without PMI differed in baseline characteristics, multivariate cox regression model allowed to standardize the impact of PMI on the risk of death and identified PMI as an independent parameter increasing the risk within 1 and 5 years after CABG.
The incidence of late bleeding complications, defined by the need for re-exploration for bleeding and cardiac tamponade, was relatively high in the PMI group, when compared to the rates reported in the literature (23). One might speculate that this is related to the higher prevalence of dual antiplatelet therapy in patients with CAD during life-saving CABG procedures.
There are several risk stratification models to determine mortality risk in patients undergoing CABG surgery based on preoperative risk factors such as EuroSCORE, EuroSCORE II and STS score. However, none of these risk stratification models estimates the risk of PMI (18). This is somehow supported by our study results, as neither of the common risk factors for worse outcome after CABG (i.e., EuroSCORE, age, LVEF, and left main stenosis) were related to the increased risk of PMI. Based on the calculated multivariate logistic regression model, IR was the most crucial parameter (OR 2.249, 95% CI: 1.589–3.184). Other significant risk factors for PMI were female sex (OR 1.485, 95% CI: 1.009–2.184) and non-elective surgery (OR 1.676, 95% CI: 1.105–2.540).
Complete revascularization is the ultimate goal of CABG surgery but may not be achieved in some clinical scenarios. Patient-specific coronary pathology, i.e., small targets, severely calcified artery, undetectable vessel, coronary injury, or other unexpected findings during surgery such as limited availability of conduits are some of the reasons for IR (24). Nevertheless, IR in CABG surgery is still one of the main factors increasing the risk of early and late survival (24) and based on our study results also the risk of PMI. IR is more commonly observed in patients with diabetes, hyperlipidemia and arterial hypertension. IR was found to be more common in female patients than in males perhaps due to higher incidence of small coronaries (25). Nevertheless, the independent impact of female sex on PMI occurrence is a very important observation that could only arose from a registry data as the issue of females underrepresentation in previous coronary surgery RCTs is well known.
Emergent surgery is a well-established risk factor for mortality and morbidity, especially in patients undergoing CABG for acute coronary syndrome (26). Similarly, in our study non-elective cardiac surgery, usually due to ACS, was associated with higher incidence of PMI and higher risk of 1-year mortality. Nevertheless, according to the ESC guidelines for the management of ACS in patients presenting without persistent ST segment elevation, emergent surgery should be performed in patients eligible for CABG who have persistent ischemia or hemodynamic instability and should not be postponed because of recent administration of antiplatelet therapy (27). Patients who qualify for life-saving CABG have worse characteristics, including Killip class II, anterior wall infarction, greater left ventricular dysfunction, and more frequent use of mechanical ventilation and intra-aortic balloon pump (28). Hypothetically, this can also be explained by certain human factors such as surgeon fatigue and all-night operations, which can affect the results of emergent CABG. However, we did not analyze these factors.
Limitations of the study
The retrospective nature of our study is an obvious limitation. The data presented in this study are based on the experience of a single center. Limitations of the KROK registry have been described previously (14-16,29). The KROK registry does not capture rates of long-term stroke and other MACCE complications. Importantly, the KROK registry also does not capture the exact causes of death (cardiovascular vs. noncardiovascular). Furthermore, the completeness of revascularization index (CRI) was calculated from the difference between the number of coronary grafts and the number of diseased coronary artery systems as reported in the KROK database. Therefore, CRIs may be underestimated as they do not represent the ratio of planned to performed anastomoses. The possible differences in this respect could further influence the remote results (29). A number of data was not available in the analyzed registry (baseline cardiac enzyme level, antiplatelet treatment), however we have presented the ultimate clinical endpoints in the overall analyzed population.
Conclusions
Postoperative myocardial MI after CABG defined by the 4th Universal Definition is associated with reduced short- and long-term survival. The main risk factors for postoperative MI are incomplete revascularization, female gender, and non-elective surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1279/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1279/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1279/coif). Dr. MK serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2020 to August 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committee of Medical Chambers in Krakow (No.: L.dz.OIL/KBL/OIL/11/216) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Gössl M, Faxon DP, Bell MR, et al. Complete versus incomplete revascularization with coronary artery bypass graft or percutaneous intervention in stable coronary artery disease. Circ Cardiovasc Interv 2012;5:597-604. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2018;40:237-69. [Crossref] [PubMed]
- Steuer J, Hörte LG, Lindahl B, et al. Impact of perioperative myocardial injury on early and long-term outcome after coronary artery bypass grafting. Eur Heart J 2002;23:1219-27. [Crossref] [PubMed]
- Namay DL, Hammermeister KE, Zia MS, et al. Effect of perioperative myocardial infarction on late survival in patients undergoing coronary artery bypass surgery. Circulation 1982;65:1066-71. [Crossref] [PubMed]
- Yau JM, Alexander JH, Hafley G, et al. Impact of perioperative myocardial infarction on angiographic and clinical outcomes following coronary artery bypass grafting (from PRoject of Ex-vivo Vein graft ENgineering via Transfection [PREVENT] IV). Am J Cardiol 2008;102:546-51. [Crossref] [PubMed]
- Li Z, Anderson I, Amsterdam EA, et al. Effect of coronary artery disease extent on contemporary outcomes of combined aortic valve replacement and coronary artery bypass graft surgery. Ann Thorac Surg 2013;96:2075-82. [Crossref] [PubMed]
- Costachescu T, Denault A, Guimond JG, et al. The hemodynamically unstable patient in the intensive care unit: hemodynamic vs. transesophageal echocardiographic monitoring. Crit Care Med 2002;30:1214-23. [Crossref] [PubMed]
- Thielmann M, Massoudy P, Jaeger BR, et al. Emergency re-revascularization with percutaneous coronary intervention, reoperation, or conservative treatment in patients with acute perioperative graft failure following coronary artery bypass surgery. Eur J Cardiothorac Surg 2006;30:117-25. [Crossref] [PubMed]
- Rasmussen C, Thiis JJ, Clemmensen P, et al. Significance and management of early graft failure after coronary artery bypass grafting: feasibility and results of acute angiography and re-re-vascularization. Eur J Cardiothorac Surg 1997;12:847-52. [Crossref] [PubMed]
- Piana RN, Adams MR, Orford JL, et al. Rescue percutaneous coronary intervention immediately following coronary artery bypass grafting. Chest 2001;120:1417-20. [Crossref] [PubMed]
- Ben-Yehuda O, Chen S, Redfors B, et al. Impact of large periprocedural myocardial infarction on mortality after percutaneous coronary intervention and coronary artery bypass grafting for left main disease: an analysis from the EXCEL trial. Eur Heart J 2019;40:1930-41. [Crossref] [PubMed]
- Suwalski P, Kowalewski M, Jasiński M, et al. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J Thorac Cardiovasc Surg 2019;157:1007-1018.e4. [Crossref] [PubMed]
- Filip G, Litwinowicz R, Kapelak B, et al. Trends in isolated aortic valve replacement in middle-aged patients over the last 10 years: epidemiology, risk factors, valve pathology, valve types, and outcomes. Kardiol Pol 2019;77:688-95. [Crossref] [PubMed]
- Bartus K, Sadowski J, Litwinowicz R, et al. Changing trends in aortic valve procedures over the past ten years-from mechanical prosthesis via stented bioprosthesis to TAVI procedures-analysis of 50,846 aortic valve cases based on a Polish National Cardiac Surgery Database. J Thorac Dis 2019;11:2340-9. [Crossref] [PubMed]
- Bartus K, Litwinowicz R, Sadowski J, et al. Bioprosthetic or mechanical heart valves: prosthesis choice for borderline patients?-Results from 9,616 cases recorded in Polish national cardiac surgery registry. J Thorac Dis 2020;12:5869-78. [Crossref] [PubMed]
- Sandoval Y, Brilakis ES, Garcia S. Completeness of revascularization in multivessel coronary artery disease. J Thorac Dis 2016;8:E1493-6. [Crossref] [PubMed]
- Thielmann M, Sharma V, Al-Attar N, et al. ESC Joint Working Groups on Cardiovascular Surgery and the Cellular Biology of the Heart Position Paper: Perioperative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur Heart J 2017;38:2392-407. [Crossref] [PubMed]
- Litwinowicz R, Filip G, Bryndza M, et al. Outcomes of emergency coronary angiography after cardiac surgery. Eur J Prev Cardiol 2020;27:1339-42. [Crossref] [PubMed]
- Søraas CL, Friis C, Engebretsen KV, et al. Troponin T is a better predictor than creatine kinase-MB of long-term mortality after coronary artery bypass graft surgery. Am Heart J 2012;164:779-85. [Crossref] [PubMed]
- Stone GW, Kappetein AP, Sabik JF, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381:1820-30. Erratum in: N Engl J Med 2020;382:1078. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Mazur P, Litwinowicz R, Tchantchaleishvili V, et al. Left Internal Mammary Artery Skeletonization Reduces Bleeding-A Randomized Controlled Trial. Ann Thorac Surg 2021;112:794-801. [Crossref] [PubMed]
- Benedetto U, Gaudino M, Di Franco A, et al. Incomplete revascularization and long-term survival after coronary artery bypass surgery. Int J Cardiol 2018;254:59-63. [Crossref] [PubMed]
- Ngaage DL, Hashmi I, Griffin S, et al. To graft or not to graft? Do coronary artery characteristics influence early outcomes of coronary artery bypass surgery? Analysis of coronary anastomoses of 5171 patients. J Thorac Cardiovasc Surg 2010;140:66-72, 72.e1.
- Ad N, Holmes SD, Patel J, et al. Comparison of EuroSCORE II, Original EuroSCORE, and The Society of Thoracic Surgeons Risk Score in Cardiac Surgery Patients. Ann Thorac Surg 2016;102:573-9. [Crossref] [PubMed]
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2021;42:1289-367. Erratum in: Eur Heart J 2021;42:1908 Erratum in: Eur Heart J. 2021;42:1925. Erratum in: Eur Heart J 2021 May 13. [Crossref] [PubMed]
- Klempfner R, Barac YD, Younis A, et al. Early Referral to Coronary Artery Bypass Grafting Following Acute Coronary Syndrome, Trends and Outcomes from the Acute Coronary Syndrome Israeli Survey (ACSIS) 2000-2010. Heart Lung Circ 2018;27:175-82. [Crossref] [PubMed]
- Kowalewski M, Jasiński M, Staromłyński J, et al. Long-Term Survival Following Surgical Ablation for Atrial Fibrillation Concomitant to Isolated and Combined Coronary Artery Bypass Surgery-Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J Clin Med 2020;9:1345. [Crossref] [PubMed]


