
Effect of left ventricular ejection fraction (LVEF) on mortality of total arch replacement in subacute/chronic type A aortic dissection
Introduction
Type A aortic dissection (TAAD) is a life-threatening condition. Without surgical intervention, acute TAAD patients have an hourly mortality rate of 1–2% after the appearance of symptoms (1), and the estimated mortality rate within the first 2 weeks of onset ranges from 57% to 74% (2). As most patients with TAAD receive surgical treatment in the acute phase, only a small portion of these patients progress to the subacute/chronic stage. The mortality of subacute/chronic TAAD patients is significantly lower than that of acute TAAD patients (1); however, adverse aortic events, such as rupture and sudden death, still threaten subacute/chronic TAAD patients who receive conservative treatment (3).
TAAD involving the aortic arch remains an inherently lethal condition and surgical treatment is needed. The surgical repair of TAAD, especially repair that involves total arch replacement (TAR), is a difficult procedure that is associated with high postoperative morbidity and mortality. The continuous improvement of surgical techniques has resulted in a significant decrease in the surgical mortality of TAAD patients; however, the mortality rate, which ranges from 3.09% to 30%, remains high (4,5). Risk for mortality was significantly increased when the patient presented with advanced age, malperfusion, hypotension or shock and neurologic symptoms (4,6,7). Patients with a low left ventricular ejection fraction (LVEF) are at a higher risk of postoperative complications and mortality following certain cardiac surgeries (8). Previous study has shown that acute TAAD patients with preoperative left ventricular dysfunction are at higher surgical risk for in-hospital mortality (9). However, no study has assessed the relationship between the LVEF and the postoperative outcomes of TAR in subacute/chronic TAAD. Thus, this single-center, retrospective cohort study sought to evaluate the effect of preoperative LVEF on the in-hospital and mid-term outcomes of TAR in patients with subacute/chronic TAAD. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1905/rc).
Methods
Patients selection and definitions
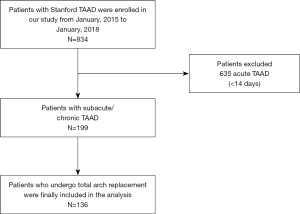
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Beijing Anzhen Hospital approved this study (Institutional Review Board File 2014019). The individual consent for this retrospective analysis was waived. From January 2015 to January 2018, 834 consecutive TAAD patients received surgery at our hospital. After excluding all acute TAAD patients, 199 patients with subacute/chronic TAAD remained. One hundred and thirty-six of these subacute/chronic TAAD patients received a TAR, and were included in the analysis (Figure 1). All the patients were diagnostically confirmed to have TAAD by computed tomography angiography (CTA). Experienced clinical radiologists evaluated CTA to define the presence and extent of the dissection flap. Subacute/chronic TAAD was defined as dissection involving the ascending aorta with symptoms for >14 days (10). The primary endpoint was death. Neurological complications were defined as cerebral hemorrhage, cerebral infarction, limb paralysis, and paraplegia. Respiratory complications were defined as lung infection, pleural effusion, and respiratory failure.
Data collection
The data were collected from the “A study of the prediction model for and interventions in Acute Aortic Syndrome (ChiCTR1900022637)” database. The data included patient demographic, history of disease, imaging examination, operative detail, and postoperative outcome data. GE (USA) Vivid 7 and E9 ultrasound systems (M3S) were used for the 2-dimensional and doppler echocardiographic studies in transthoracic echocardiography (Figure 2). The patients were examined in the supine position by echocardiography. The parameters obtained included aortic sinus diameter, ascending aortic diameter, left atrial diameter, left ventricular end-systolic diameter, left ventricular end-diastolic diameter, ejection fraction, pericardial effusion, and valvular regurgitation. The severity of aortic regurgitation was graded as mild [vena contract width (VCW) <0.3 cm, pressure-half time (PHT) >500 ms, effective regurgitant orifice area (EROA) <0.10 cm2, and regurgitant volume (RVol) <30 mL/beat], moderate (VCW 0.3–0.6 cm, PHT 200–500 ms, EROA 0.10–0.19 cm2, and RVol 30 to 44 mL), or severe (VCW >0.6 cm, PHT <200 ms, EROA ≥0.3 cm2, and RVol ≥60 mL/beat) (11). Follow-up data were obtained for all patients from records of clinical encounters or telephone calls after discharge. The end follow-up date was April 1, 2020.
Surgical techniques
The surgical techniques for TAR used at our hospital are similar to those reported in the literature (12-14). Briefly, after the induction of general anesthesia, the operation was performed through a full median sternotomy. Cardiopulmonary bypass with moderate hypothermic circulatory arrest at 25 °C, right axillary artery cannulation for cardiopulmonary bypass, and selective antegrade cerebral perfusion were performed using the TAR with the frozen elephant trunk (FET) technique. This procedure involves the implantation of a FET (Cronus; MicroPort Medical Co., Ltd., Shanghai, China) in the descending aorta. If the true lumen was too narrow and rigid to deploy the FET, part of the intimal flap closest to the anastomosis was excised to create a double-lumen blood supply. Next, the proximal FET was anastomosed with a tetrafurcate vascular graft, followed by anastomoses of the 3 arch vessels with sidearm grafts in the following sequence: the left common carotid artery, the ascending aorta, the left subclavian artery, and then the innominate artery.
Statistical analysis
Continuous variables with a normal distribution are expressed as mean ± standard deviation. Continuous variables without a normal distribution are expressed as median (interquartile range). Categorical variables are expressed as number (percentage). The t-test was used to determine whether the continuous variables followed a normal distribution. When the variables were not distributed normally, the Wilcoxon rank-sum test was applied. The chi-square test or Fisher test was used to compare categorical variables. Univariable and multivariable Cox proportional hazards regression was performed to assess the association between the LVEF and mortality. We calculated the survival rate using the Kaplan-Meier analytical method combined with the log-rank test. A P value <0.05 was considered statistically significant (2-sided). All the analyses were performed with the statistical software package R (http://www.R-project.org, The R Foundation).
Results
Patient characteristics
Table 1 summarizes the details of the clinical features of the subacute/chronic TAAD patients at hospital admission. The patients had an average age of 47.8±11.0 years, and 77.9% of the group were male; 61.8% of the participants had hypertension, 51.5% were smokers, and 5.9% had diabetes mellitus. The patients were divided into 4 groups according to the quartiles of the LVEF from 41% to 78%. The prevalence of significant medical comorbidities, such as a history of cerebrovascular disease, cardiovascular disease, and Marfan syndrome, were similar across the 4 groups. There were also no differences in relation to sex, body mass index (BMI), or the proportion of previous cardiac surgery or thoracic endovascular aortic repair among the groups. Patients with a LVEF in the lowest quartile had a larger left ventricular end diastolic diameter (P<0.01), left ventricular end systolic diameter (P<0.01), and ascending aorta diameter (P<0.01), and were more likely to have severe aortic regurgitation (P<0.02).
Table 1
| Patient demographics | Total N=136 | 1st quartile (N=29) | 2nd quartile (N=28) | 3rd quartile (N=35) | 4th quartile (N=44) | P value |
|---|---|---|---|---|---|---|
| LVEF, % | – | <55 | 55–58 | 59–62 | >62 | – |
| Age, years | 48.1±12.7 | 45.1±11.4 | 48.2±11.0 | 44.8±10.3 | 51.7±10.3 | 0.02* |
| BMI | 25.4±3.9 | 25.0±4.4 | 25.2±2.8 | 25.9±4.9 | 25.2±3.2 | 0.87 |
| Sex (male) | 106 (77.9%) | 23 (79.3%) | 20 (71.4%) | 26 (74.3%) | 37 (84.1%) | 0.58 |
| Time of onset, days | 30.0 (20.0–90.0) | 30.0 (30.0–90.0) | 30.0 (27.5–60.0) | 30.0 (19.5–90.0) | 55.0 (20.8–135.0) | 0.16 |
| Hypertension | 84 (61.8%) | 14 (48.3%) | 14 (50.0%) | 25 (71.4%) | 31 (70.5%) | 0.84 |
| Smoking | 70 (51.5%) | 16 (55.2%) | 15 (53.6%) | 14 (40.0%) | 25 (56.8%) | 0.21 |
| Diabetes | 8 (5.9%) | 2 (6.9%) | 1 (3.6%) | 2 (5.7%) | 3 (6.8%) | 0.94 |
| History of cerebrovascular disease | 5 (3.7%) | 0 (0.0%) | 0 (0.0%) | 2 (5.7%) | 3 (6.8%) | 0.28 |
| Marfan syndrome | 3 (2.2%) | 1 (3.4%) | 0 (0.0%) | 1 (2.9%) | 1 (2.3%) | 0.82 |
| History of cardiovascular disease | 28 (20.6%) | 4 (13.8%) | 3 (10.7%) | 10 (28.6%) | 11 (25.0%) | 0.22 |
| History of cardiac surgery | 12 (8.8%) | 3 (10.3%) | 0 (0.0%) | 4 (11.4%) | 5 (11.4%) | 0.33 |
| History of TEVAR | 8 (5.9%) | 0 (0.0%) | 1 (3.6%) | 3 (8.6%) | 4 (9.1%) | 0.34 |
| Coronary artery disease | 10 (7.4%) | 0 (0.0%) | 2 (7.1%) | 4 (11.4%) | 4 (9.1%) | 0.34 |
| Left ventricular end diastolic diameter, mm | 54.4±9.6 | 60.9±9.0 | 57.5±9.5 | 51.7±9.3 | 50.3±7.2 | <0.01* |
| Left ventricular end systolic diameter, mm | 37.5±9.4 | 46.6±10.1 | 40.2±8.6 | 34.7±7.1 | 31.9±4.9 | <0.01* |
| Ascending aorta diameter, mm | 52.5±12.3 | 54.9±14.2 | 54.1±14.8 | 46.8±8.8 | 45.1±9.1 | <0.01* |
| Aortic regurgitation | 0.02* | |||||
| Mild | 43 (31.6%) | 4 (13.8%) | 10 (35.7%) | 11 (31.4%) | 18 (40.9%) | |
| Moderate | 14 (10.3%) | 5 (17.2%) | 3 (10.7%) | 5 (14.3%) | 1 (2.3%) | |
| Severe | 39 (28.7%) | 13 (44.8%) | 11 (39.3%) | 7 (20.0%) | 8 (18.2%) | |
| Mitral regurgitation | 0.29 | |||||
| Mild | 50 (36.8%) | 11 (37.9%) | 11 (39.3%) | 13 (37.1%) | 15 (34.1%) | |
| Moderate | 5 (3.7%) | 3 (10.3%) | 1 (3.6%) | 1 (2.9%) | 0 (0.0%) | |
| Severe | 3 (2.2%) | 2 (6.9%) | 0 (0.0%) | 0 (0.0%) | 1 (2.3%) |
Results are expressed as n (%) or mean ± standard deviation or median interquartile range. *, P<0.05. BMI, body mass index; TEVAR, thoracic endovascular aortic repair; LVEF, left ventricular ejection fraction.
Intraoperative details
Table 2 sets out the intraoperative details for subacute/chronic TAAD. Intraoperative parameters, such as cardiopulmonary bypass time, aortic cross-clamp time, nasopharyngeal temperature, and hypothermic circulatory arrest time, were similar between the groups. The proportion of Bentall procedures was significantly higher in both the first and second quartiles than the third and fourth quartiles. We also observed a significantly higher proportion of mitral valve repair/replacements in patients with a LVEF in the lowest quartile.
Table 2
| Operative details | Total N=136 | 1st quartile (N=29) | 2nd quartile (N=28) | 3rd quartile (N=35) | 4th quartile (N=44) | P value |
|---|---|---|---|---|---|---|
| Bentall procedure | 60 (44.1%) | 17 (58.6%) | 17 (60.7%) | 12 (34.3%) | 14 (31.8%) | 0.02* |
| Coronary artery bypass grafting | 11 (8.1%) | 4 (13.8%) | 2 (7.1%) | 2 (5.7%) | 3 (6.8%) | 0.65 |
| Mitral valve repair/replacement | 5 (3.7%) | 5 (17.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | <0.01* |
| Tricuspid valve repair/replacement | 1 (0.7%) | 0 (0.0%) | 1 (3.6%) | 0 (0.0%) | 0 (0.0%) | 0.274 |
| Cardiopulmonary bypass time, min | 195.5±50.3 | 206.2±56.1 | 198.3±44.1 | 183.4±36.3 | 196.4±58.5 | 0.33 |
| Aortic cross-clamp time, min | 105.1±33.2 | 114.2±38.6 | 107.8±28.8 | 96.3±28.1 | 104.3±35.1 | 0.19 |
| Nasopharyngeal temperature, °C | 23.4±1.3 | 23.5±1.4 | 23.2±1.4 | 23.6±1.2 | 23.3±1.2 | 0.70 |
| Hypothermic circulatory arrest time, min | 25.2±9.7 | 24.0±8.0 | 26.5±10.8 | 25.7±10.4 | 24.8±9.7 | 0.76 |
| Postoperative outcomes | ||||||
| Ventilator time, hour | 40.0 (26.0–84.0) | 54.0 (38.0–132.0) | 40.5 (33.0–72.0) | 34.0 (25.0–82.0) | 38.0 (20.0–78.0) | 0.65 |
| Intensive care unit length of stay, days | 1.7 (1.0–3.1) | 2.1 (1.1–3.6) | 1.8 (1.1–3.2) | 1.4 (0.9–3.3) | 1.1 (0.9–3.0) | 0.95 |
| Hospital length of stay, days | 17.0 (12.0–23.0) | 17.0 (13.0–24.0) | 17.5 (13.8–22.2) | 14.0 (9.0–22.0) | 17.0 (13.8–22.2) | 0.34 |
| Neurological complications | 9 (6.6%) | 3 (10.3%) | 1 (3.6%) | 4 (11.4%) | 1 (2.3%) | 0.29 |
| Respiratory complications | 16 (11.8%) | 5 (17.2%) | 4 (14.3%) | 2 (5.7%) | 5 (11.4%) | 0.52 |
| Dialysis | 10 (7.4%) | 2 (6.9%) | 3 (10.7%) | 2 (5.7%) | 3 (6.8%) | 0.89 |
Results are expressed as n (%) or mean ± standard deviation or median interquartile range. *, P<0.05.
Postoperative outcome
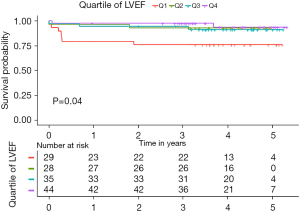
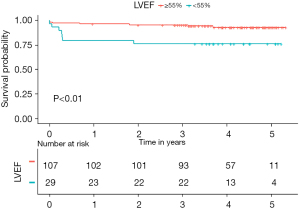
The in-hospital mortality was 4.4% (6/136). The rate of postoperative complications were similar among the groups (Table 2). Table S1 provides details of the 14 postoperative adverse events that occurred during the follow-up period. We used the Cox proportional hazards regression model to evaluate the association between the preoperative LVEF and mortality. A decreased LVEF was identified as an independent predictor of all-cause mortality (Table 3). When categorized into quartiles, the patients in the highest LVEF quartile had a significantly lower risk of mortality than those in the lowest LVEF quartile [hazards ratio (HR) 0.17, 95% CI: 0.04–0.84, P=0.03; Table S2). The median follow-up time was 3.97 years [interquartile range (IQR): 3.20–4.67 years]. The Kaplan-Meier survival analysis demonstrated an inverse relationship between the LVEF and total mortality (log-rank P=0.04; Figure 3), such that patients with LVEFs in the lowest quartile (LVEF <55%) had a significantly worse prognosis those with LVEFs in the upper 3 quartiles (LVEF ≥55%) (P<0.01, Figure 4).
Table 3
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 0.99 (0.95, 1.04) | 0.78 | – | – | |
| Gender (male) | 0.35 (0.12, 1.01) | 0.053 | – | – | |
| BMI | 0.91 (0.79, 1.06) | 0.23 | – | – | |
| Diabetes | 1.36 (0.18, 10.4) | 0.77 | – | – | |
| Hypertension | 1.15 (0.39, 3.44) | 0.80 | – | – | |
| History of cardiovascular disease | 1.08 (0.30, 3.88) | 0.90 | – | – | |
| History of heart surgery | 0.83 (0.11, 6.38) | 0.86 | – | – | |
| Aortic regurgitation | – | – | |||
| No | Ref | – | – | ||
| Mild | 1.37 (0.23, 8.19) | 0.73 | – | – | |
| Moderate | † | † | – | – | |
| Severe | 4.88 (1.05, 22.58) | 0.04* | 2.7 (0.54, 13.4) | 0.220 | |
| LVEF | 0.90 (0.84, 0.97) | <0.01* | 0.93 (0.86, 0.99) | 0.03* | |
| Cardiopulmonary bypass time, min | 1.01 (1.00, 1.02) | <0.01* | 1.01(1.001, 1.02) | 0.054 | |
| Hypothermic circulatory arrest time, min | 0.96 (0.91, 1.03) | 0.26 | – | – | |
| Nasopharyngeal temperature, °C | 1.39 (0.89, 2.16) | 0.15 | – | – | |
| Bentall procedure | 2.35 (0.79, 7.00) | 0.13 | – | – | |
| Coronary artery bypass grafting | 3.58 (1.00, 12.84) | 0.05 | – | – | |
†, the result failed because of the small sample size; *, P<0.05. CI, confidence interval; HR, hazards ratio; BMI, body mass index; LVEF, left ventricular ejection fraction.
Discussion
TAAD is classified according to the time of symptom onset as an acute (≤14 days) or subacute/chronic (>14 days) dissection. Due to the high mortality of early stage TAAD patients, timely diagnosis and treatment are crucial. Most studies on TAAD have examined acute cases with data from well-recognized databases, such as International Registry of Aortic Dissection (IRAD) (15) and the German Registry for Acute Aortic Dissection Type A (GERAADA) (16). Conversely, studies of subacute/chronic TAAD are not as widely reported in the literature. The purpose of this article is to aid surgeons to weigh the long-term benefits against potential surgical risks when considering elective surgical repair for subacute/chronic TAAD.
Our hospital had a larger proportion of patients with subacute/chronic TAAD (23.9%, 199/834) than those reported in earlier studies in other countries (3,17). This may be because the uneven distribution of aortic centers in China prevents patients in rural areas from receiving urgent diagnosis and treatment. Access to treatment is further affected by financial barriers under the present health insurance system (18). By the time TAAD patients are transferred to our center, many are already in the subacute/chronic stage. Further, most TAAD patients in China are younger than those in Western countries (15,19). In our cohort, the patients had a mean age of 47.8±11.0 years. Thus, TAR with or without FET was a priority for these patients to promote the remodeling of the dissected aorta. With a 30-day mortality rate of 3.7%, and mid-term survival rate of 89.7%, the outcome of TAR for subacute/chronic TAAD at our hospital was satisfactory.
Unlike acute TAAD, emergency surgery is not recommended for patients with subacute/chronic TAAD, especially for high-risk patients, such as those with organ ischemia or neurological complications. The indications for surgical intervention in these subacute/chronic patients remain controversial. According to guidelines by Erbel et al., for subacute/chronic TAAD patients who are asymptomatic, the maximal aortic diameter should be >55 mm before prophylactic thoracic aortic repair is considered (10). However, Kim et al. (3) report that the long-term results of non-surgical treatment for subacute/chronic TAAD had a substantial risk of adverse aortic events even in patients with an aortic diameter < the 55 mm surgical threshold. Thus, it may be reasonable for subacute/chronic TAAD patients with a lower aortic diameter to undergo surgery (3,20). At our hospital, we perform surgical repair on patients who do not meet the 55 mm threshold to avoid the potential adverse events related to non-surgical treatment. In the present study, the mean preoperative ascending aorta diameter of our subacute/chronic TAAD cohort was 52.5±12.3 mm; thus, only 37.5% (51/136) of patients met the guidelines for surgery. Notably, we found that patients in the mortality group presented with a larger preoperative ascending aorta diameter (60.5±15.0 vs. 51.5±11.6 mm, P<0.01; Table S3).
The key finding of the present study was that a reduced LVEF is independently related to the postoperative mortality of TAR in subacute/chronic TAAD. Left ventricular dysfunction is a complex condition with multiple potential etiologies. In our study, there was a relatively high prevalence of moderate to severe aortic valve dysfunction (39%), and the mortality group had a higher proportion of severe aortic insufficiency (64.3%), which suggests that the decreased LVEF was likely caused by severe aortic regurgitation. In patients with subacute/chronic TAAD, the progressive dilatation of the dissected proximal aorta exacerbates aortic insufficiency (17). Additionally, we found that the onset time was negatively correlated with the LVEF in patients with severe aortic insufficiency (P<0.05; Figure S1).
Severe aortic insufficiency may affect left ventricular function and increase the risk of surgery (8). Indeed, a low LVEF is known to increase surgical risk in routine cardiac surgery (21,22). Langer et al. reported that patients with a LVEF <50% who underwent aortic root replacement had significantly higher in-hospital, perioperative, and mid-term mortality rates than those with a LVEF >50% (23). Moreover, Cefarelli et al. found that low LVEF was a risk factor for the poor prognosis of patients with acute TAAD after aortic surgery, and preoperative severe left ventricular function (LVEF <35%) was a strong independent predictor of postoperative mortality (24). However, there has been no specific study on the effect of the LVEF on surgical outcomes for subacute/chronic TAAD. In the present study, only 12 (8.8%) patients had a LVEF <50%, which made it statistically difficult to divide patients into LVEF <50% and LVEF >50% groups as done in previous studies (19,25). Consequently, we grouped our cohort of subacute/chronic TAAD patients according to LVEF quartiles, and found that patients with LVEFs in the higher 3 quartiles (LVEF ≥55%) had significantly better surgical outcomes than those with LVEFs in the lowest quartile (LVEF <55%). Thus, even when the subacute/chronic dissected ascending aorta has not dilated to >55 mm, patients with aortic insufficiency should receive surgical repair before the LVEF further deteriorates to a cardiac function <55%.
Preoperative cardiac evaluation is important to enable the identification of patients at a higher risk for adverse cardiac events after cardiopulmonary bypass, and can provide valuable information for surgical planning in different risk populations. Improving survival is the goal of prophylactic surgical repair for subacute/chronic TAAD. Thus, it is important to consider the potential surgical risk caused by a decreased LVEF, especially for patients receiving TAR with FET, who experience much longer cardiopulmonary bypass times and hypothermic circulatory arrest. For some patients with a reduced LVEF, medical treatment to improve cardiac function could be used to bide time until surgery is suitable. Alternatively, clinicians could consider a simpler repair (e.g., an ascending aorta replacement only) for subacute/chronic TAAD patients with reduced LVEF. Moreover, the hybrid debranching thoracic endovascular aortic repair approach combining debranching of aortic arch vessels with thoracic endovascular aortic repair of the aortic arch is a way to extend the envelope of intervention in aortic arch pathologies, particularly in patients with reduced LVEF, who are suboptimal candidates for open surgery.
This study had some inherent limitations. First, the investigation was a retrospective observational study, and has possible biases due to the clinical characteristics of TAAD unique to the Asian population. Second, the sample size for subacute/chronic TAAD was small, which may have restricted the statistical power. Third, the absolute number of events may not have been sufficient to enable adequate multivariable competing analyses. Fourth, we did not address long-term outcomes in the present study; however, the follow-up of all patients is ongoing. Further multicenter prospective studies need to be conducted to determine a revised threshold indication for surgery.
Conclusions
Patients with subacute/chronic TAAD who received a TAR at our center had satisfactory mid-term outcomes. We found that subacute/chronic TAAD patients with a LVEF <55% had significantly higher mid-term mortality. Thus, surgeons should consider a LVEF <55% as a potential surgical risk when choosing to perform TAR for subacute/chronic TAAD. Future research needs to be conducted to elucidate whether this relationship exists for all variations of elective subacute/chronic TAAD repair.
Acknowledgments
The authors would like to thank AiMi Academic Services (www.aimieditor.com) for its English-language editing and review services. The abstract of this article has been shared in the Great Wall International Congress of Cardiology 2020/Asian Heart Society Congress 2020.
Funding: This study was supported by the National Natural Science Foundation of China (No. 82170487 and No. 82070483), the Foundation of Beijing Outstanding Young Talent Training Program (No. 2017000021469G254), Scientific Research Common Program of Beijing Municipal Commission of Education (No. KM202110025014), Beijing Hospitals Authority Clinical Medicine Development of special funding support (No. XMLX202107).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1905/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1905/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1905/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of Anzhen Hospital (Institutional Review Board File 2014019). The individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mészáros I, Mórocz J, Szlávi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest 2000;117:1271-8. [Crossref] [PubMed]
- Masuda Y, Yamada Z, Morooka N, et al. Prognosis of patients with medically treated aortic dissections. Circulation 1991;84:III7-13. [PubMed]
- Kim WK, Park SJ, Kim HJ, et al. The fate of unrepaired chronic type A aortic dissection. J Thorac Cardiovasc Surg 2019;158:996-1004.e3. [Crossref] [PubMed]
- Yang B, Norton EL, Rosati CM, et al. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: A 20-year experience. J Thorac Cardiovasc Surg 2019;158:675-87.e4. [Crossref] [PubMed]
- Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol 2011;58:2455-74. [Crossref] [PubMed]
- Oliver CL, Ernst W, Uwe M, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. [Crossref] [PubMed]
- Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002;105:200-6. [Crossref] [PubMed]
- Pieri M, Belletti A, Monaco F, et al. Outcome of cardiac surgery in patients with low preoperative ejection fraction. BMC Anesthesiol 2016;16:97. [Crossref] [PubMed]
- Lin CY, Lee KT, Ni MY, et al. Impact of reduced left ventricular function on repairing acute type A aortic dissection: Outcome and risk factors analysis from a single institutional experience. Medicine (Baltimore) 2018;97:e12165. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Mentias A, Feng K, Alashi A, et al. Long-Term Outcomes in Patients With Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol 2016;68:2144-53. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Liu ZG, Sun LZ, Chang Q, et al. Should the "elephant trunk" be skeletonized? Total arch replacement combined with stented elephant trunk implantation for Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2006;131:107-13. [Crossref] [PubMed]
- Ma WG, Zheng J, Dong SB, et al. Sun's procedure of total arch replacement using a tetrafurcated graft with stented elephant trunk implantation: analysis of early outcome in 398 patients with acute type A aortic dissection. Ann Cardiothorac Surg 2013;2:621-8. [PubMed]
- Berretta P, Patel HJ, Gleason TG, et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg 2016;5:346-51. [Crossref] [PubMed]
- Czerny M, Schoenhoff F, Etz C, et al. The Impact of Pre-Operative Malperfusion on Outcome in Acute Type A Aortic Dissection: Results From the GERAADA Registry. J Am Coll Cardiol 2015;65:2628-35. [Crossref] [PubMed]
- Rylski B, Milewski RK, Bavaria JE, et al. Outcomes of surgery for chronic type A aortic dissection. Ann Thorac Surg 2015;99:88-93. [Crossref] [PubMed]
- Yip W, Fu H, Chen AT, et al. 10 years of health-care reform in China: progress and gaps in Universal Health Coverage. Lancet 2019;394:1192-204. [Crossref] [PubMed]
- Wang W, Duan W, Xue Y, et al. Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg 2014;148:2995-3000. [Crossref] [PubMed]
- Dagenais F. Commentary: Chronic type A dissection: When to operate? J Thorac Cardiovasc Surg 2019;158:1005-6. [Crossref] [PubMed]
- McCarthy PM. Aortic valve surgery in patients with left ventricular dysfunction. Semin Thorac Cardiovasc Surg 2002;14:137-43. [Crossref] [PubMed]
- Chaliki HP, Mohty D, Avierinos JF, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 2002;106:2687-93. [Crossref] [PubMed]
- Langer NB, Ando M, Simpson M, et al. Influence of left ventricular ejection fraction on morbidity and mortality after aortic root replacement. J Thorac Cardiovasc Surg 2019;158:984-91.e1. [Crossref] [PubMed]
- Cefarelli M, Murana G, Surace GG, et al. Elective Aortic Arch Repair: Factors Influencing Neurologic Outcome in 791 Patients. Ann Thorac Surg 2017;104:2016-23. [Crossref] [PubMed]
- Li Y, Yang N, Duan W, et al. Acute aortic dissection in China. Am J Cardiol 2012;110:1056-61. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


