
Translation and validation of the Chinese version of Patient-completed Asthma Knowledge Questionnaire and its implementation in patient education
Introduction
Asthma is a common and chronic respiratory disease, which has affected 1–18% of the population in different countries and led to a growing socio-economic burden (1). According to a national health study completed in 2019, the overall prevalence of asthma in China was 4.2%, representing 45.7 million Chinese adults (2).
For asthma treatment, the ultimate object is overall control of the disease which is defined by the Global Initiative for Asthma (GINA) (1). But from the multicenter, cross-sectional study published before, it was shown that only 28.5% Chinese patients achieved the complete control of asthma (3). The worse control of asthma results from many factors, including age, gender, unhealthy lifestyle, poor family environment, family history, allergies, poor medication compliance and lack of knowledge and self-management of the disease (4-7). It is necessary to assess the knowledge level and competencies of the patient before initiating patient education in order to carry out personalized asthma education effectively (8,9). However, there is no accepted instrument to evaluate knowledge of asthma in adult patients with asthma in China.
A high-quality and effective patient-completed knowledge questionnaire can be a powerful tool to identify the cognitive gap. As far as we know, currently, the only questionnaire recommended internationally to measure the level of disease-related knowledge in asthma patients is the Patient-completed Asthma Knowledge Questionnaire (PAKQ), which has been verified in French and English (10,11). Therefore, a top priority is to conduct additional validation research in different population subgroups and in more languages. So far, the Chinese version of the PAKQ questionnaire has not been acquired and validated in previously published studies.
In this study, we translated PAKQ into Chinese with a standard procedure for the first time. We explored the reliability and validity of the Chinese version of PAKQ among adult patients with asthma in China to help clinicians understand the current status of asthma knowledge of patients, promote asthma education, and improve patients’ treatment compliance. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1604/rc).
Methods
Participants
According to common criteria, analysis is acceptable when the quantity of respondents is five times as many as the quantity of analysis items; we considered the minimum effective sample size was 270 participants (12). The inclusion criteria are as follows: being 18 years of age or over; been diagnosed with asthma; being able to read Mandarin Chinese. The exclusion criteria are as follows: attacked with other chronic respiratory diseases; cognitive impaired. Asthma was diagnosed according to Global Initiative for Asthma Global Strategy for Asthma Management and Prevention recommendations (13) and subjects were consistent with variable airflow limitations and variable symptoms (14). Finally, a total of 464 patients from 16 clinical research centers in China were included in this study from April 2020 to September 2020. Detailed patient demographic information is shown in Table 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine (clinical trial ethics committee approval number: 2019YK061) and informed consents were taken from all the patients.
Table 1
| Characteristics | Values |
|---|---|
| Age, years | |
| Mean ± SD | 50.06±15.44 |
| Min–max | 15–79 |
| Gender | |
| Male | 188 (40.52%) |
| Female | 276 (59.48%) |
| Education level | |
| Primary school and below | 19 (4.09%) |
| Junior high school | 100 (21.55%) |
| High school | 110 (23.70%) |
| College | 92 (19.83%) |
| Bachelor | 118 (25.43%) |
| Master degree or above | 25 (5.39%) |
| BMI (kg/m2) | |
| Mean ± SD | 23.44±3.38 |
| Min–max | 15.5–34 |
| Smoking history | |
| Smoker | 30 (6.47%) |
| Occasional smoking (<1 cigarette per day) | 23 (4.96%) |
| Ex-smoker | 87 (18.75%) |
| Non-smoker | 324 (69.83%) |
| Duration of asthma (years) | |
| Mean ± SD | 12.16±15.61 |
| Min–max | 0–70 |
| Occupation | |
| Don’t work | 19 (4.09%) |
| Retired | 201 (43.32%) |
| Student | 12 (2.59%) |
| Full-time | 232 (50.00%) |
| Severity of asthma | |
| Mild | 224 (48.28%) |
| Moderate | 176 (37.93%) |
| Severe | 64 (13.79%) |
| Received any education about asthma | |
| Never | 153 (32.97%) |
| Ever | 311 (67.03%) |
| Asthma control level | |
| Uncontrolled | 76 (16.38%) |
| Partly controlled | 189 (40.73%) |
| Controlled | 199 (42.89%) |
| ACT score | |
| Mean ± SD | 20.10±4.16 |
| Min–max | 6–29 |
| The patient’s cognitive level of asthma (evaluated by a professional physician) | |
| Good | 255 (54.96%) |
| Bad | 209 (45.04%) |
BMI, body mass index; ACT, asthma control test.
Study design
We utilized an observational, multicenter and cross-sectional design in this study in adult patients with asthma. The study was conducted at 16 centers with one or two researchers per center. The researchers were responsible for patient recruitment, clinical information collection and guiding patients to complete the PAKQ questionnaire independently and watch the educational video. At baseline, basic information, including demographic data, education, history, asthma control test (ACT) score and asthma knowledge level assessed by investigators, was collected. All participants were asked to complete the translated PAKQ. After 14±4 days, the subjects completed PAKQ questionnaire again to assess the retest reliability. Then all participants were trained in the clinic via watching an asthma education video and completed the PAKQ questionnaire again immediately after the education. The education video, recorded based on the GINA report (13), is 40 minutes long and covers all aspects of the PAKQ construct. The study was carried out under the supervision of the researcher to ensure that the patient did not receive any assistance while filling the questionnaires.
PAKQ questionnaire
The PAKQ questionnaire contains 54 items which are divided into four visual categories (10): (I) pathophysiology of asthma; (II) triggers of asthma; (III) diagnosis and management; (IV) treatment; and all items relate to a single concept of asthma knowledge. Responses are rated by true, false, and unknown. The response was considered to be a dichotomous score of ‘correct answer’ (score =1) and incorrect answer’ (score =0), while ‘unknown’ is considered to be the incorrect answer. Total score ranges from 0 to 54 and the higher scores responses get, the better command of asthma knowledge they have. Missing data was also considered incorrect because the subject’s knowledge could not be assessed. Indeterminate test results, for example, not clear enough to recognize, would be considered invalid and discarded.
Translation and cross-cultural adaptation
The translation and cross-cultural adaptation of the original PAKQ questionnaire followed previous established guidelines (10,15,16). The procedure was conducted in five steps. Step 1: forward translation. Two Chinese qualified translators who were all knowledgeable about English-speaking culture but had Mandarin Chinese as their primary language independently translated the original English version of PAKQ into Chinese. Step 2: synthesis of the translations. Two translators and a respiratory physician with bilingual background compared and verified the two version, finally reach a consensus to complete a preliminary Chinese version. Step 3: back translation. The verified Chinese PAKQ questionnaire was translated into English by a native English speaker who had no contact with the original English version of PAKQ. Step 4: committee review. An expert committee composed of two bilingual asthma specialists and one psychometrics specialist proofread and compared the source English version with preliminary Chinese version and the back translation. Ensure that each item had the same meaning and the translation was fully comprehensible. A prefinal Chinese version of the PAKQ was established then. Step 5: pre-testing. Before the formal study, five Chinese native asthma patients were invited to test the pre-final Chinese version to evaluate the fluency of sentences and difficulty in understanding. According to pre-testing findings, the expert committee made appropriate adjustments to form the final Chinese version of the PAKQ (Table 2).
Table 2
| Item | Correct answer | Mean | SD | Discrimination | Item-domain correlation | Commonality | Factor loading | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cronbach’s α if deleted | Low-score group (n=128) | High-score group (n=150) | CR | |||||||
| About asthma | ||||||||||
| 1: People with asthma have inflamed (red and swollen) airways (breathing tubes) in their lungs | T | 0.69 | 0.465 | 0.886 | 0.48±0.50 | 0.85±0.35 | 6.965** | 0.474 | 0.571 | 0.412 |
| 2: Children who have asthmatic parents are at greater risk of getting asthma than children without asthmatic parents | T | 0.77 | 0.419 | 0.885 | 0.48±0.50 | 0.97±0.16 | 10.743** | 0.616 | 0.493 | 0.626 |
| 3: People with allergies are more likely to have asthma than people without allergies | T | 0.89 | 0.313 | 0.886 | 0.73±0.44 | 0.94±0.24 | 4.699** | 0.570 | 0.655 | 0.606 |
| 4: People over 50 years of age cannot develop asthma | F | 0.80 | 0.397 | 0.887 | 0.61±0.49 | 0.81±0.40 | 3.650** | 0.534 | 0.632 | 0.526 |
| 5: Most people with asthma can lead a normal life | T | 0.83 | 0.376 | 0.887 | 0.67±0.47 | 0.90±0.30 | 4.716** | 0.502 | 0.616 | 0.481 |
| 6: The flu vaccine (an injection protecting against influenza) is not recommended for people who suffer from asthma | F | 0.53 | 0.500 | 0.887 | 0.34±0.47 | 0.70±0.46 | 6.486** | 0.466 | 0.459 | 0.365 |
| 7: People with asthma cannot do as much physical exercise as other people | F | 0.60 | 0.491 | 0.887 | 0.39±0.49 | 0.77±0.42 | 6.929** | 0.442 | 0.533 | 0.347 |
| Poorly-controlled asthma may be associated with: | ||||||||||
| 8a: Worse quality of life | T | 0.89 | 0.313 | 0.886 | 0.74±0.44 | 0.97±0.16 | 5.638** | 0.531 | 0.586 | 0.574 |
| 8b: A higher risk of attending a hospital emergency department for asthma | T | 0.86 | 0.343 | 0.886 | 0.70±0.46 | 0.97±0.16 | 6.477** | 0.612 | 0.789 | 0.686 |
| 8c: A higher risk of being admitted to hospital for asthma | T | 0.86 | 0.343 | 0.886 | 0.67±0.47 | 0.99±0.08 | 7.619** | 0.584 | 0.752 | 0.642 |
| 8d: Faster reduction in lung capacity over time with increasing difficulty breathing out | T | 0.92 | 0.271 | 0.886 | 0.80±0.40 | 0.99±0.08 | 5.409** | 0.543 | 0.545 | 0.595 |
| 8e: Death | T | 0.82 | 0.384 | 0.886 | 0.63±0.48 | 0.95±0.23 | 6.740** | 0.608 | 0.636 | 0.626 |
| 9: The severity of asthma can vary with time | T | 0.78 | 0.412 | 0.886 | 0.59±0.49 | 0.89±0.31 | 6.087** | 0.505 | 0.604 | 0.493 |
| Asthma triggers | ||||||||||
| 10: People with allergies get asthma symptoms if they are exposed to things they are allergic to (e.g., cats, pollen, and dust mites) | T | 0.93 | 0.261 | 0.887 | 0.84±0.37 | 0.98±0.14 | 4.139** | 0.484 | 0.518 | 0.526 |
| 11: Tobacco smoking does not generally make asthma worse | F | 0.81 | 0.392 | 0.885 | 0.56±0.50 | 0.95±0.21 | 8.265** | 0.536 | 0.557 | 0.545 |
| The following factors can trigger asthma symptoms in asthmatic people: | ||||||||||
| 12a: Dust | T | 0.90 | 0.299 | 0.885 | 0.71±0.46 | 0.99±0.08 | 6.926** | 0.656 | 0.682 | 0.737 |
| 12b: Smoke | T | 0.90 | 0.305 | 0.885 | 0.71±0.46 | 0.99±0.08 | 6.926** | 0.672 | 0.646 | 0.759 |
| 12c: Air pollution | T | 0.93 | 0.257 | 0.886 | 0.79±0.41 | 0.99±0.08 | 5.549** | 0.619 | 0.636 | 0.701 |
| 12d: Cold air | T | 0.84 | 0.362 | 0.886 | 0.62±0.49 | 0.93±0.26 | 6.430** | 0.576 | 0.597 | 0.612 |
| 12e: Strong emotions or stress | T | 0.81 | 0.396 | 0.886 | 0.60±0.49 | 0.91±0.28 | 6.340** | 0.591 | 0.59 | 0.586 |
| 12f: Change in temperature | T | 0.87 | 0.334 | 0.886 | 0.66±0.47 | 0.94±0.24 | 5.972** | 0.588 | 0.595 | 0.639 |
| 12g: Strong smell | T | 0.88 | 0.329 | 0.886 | 0.70±0.46 | 0.98±0.14 | 6.571** | 0.585 | 0.647 | 0.63 |
| 12h: Laughter | T | 0.55 | 0.498 | 0.888 | 0.39±0.49 | 0.73±0.44 | 6.071** | 0.425 | 0.56 | 0.297 |
| 12i: Viruses (e.g., common cold) | T | 0.81 | 0.389 | 0.885 | 0.58±0.50 | 0.95±0.21 | 7.965** | 0.483 | 0.623 | 0.425 |
| 12j: Sunshine | F | 0.58 | 0.494 | 0.889 | 0.42±0.50 | 0.67±0.47 | 4.315** | 0.270 | 0.634 | 0.221 |
| 12k: Heartburn (acid reflux) | T | 0.43 | 0.495 | 0.888 | 0.24±0.43 | 0.58±0.50 | 6.087** | 0.460 | 0.548 | 0.348 |
| 13: In some workplaces there may be substances (dust, chemicals, etc.) that may cause the development of asthma | T | 0.95 | 0.213 | 0.887 | 0.88±0.33 | 0.98±0.14 | 3.332** | 0.432 | 0.55 | 0.454 |
| 14: Anti-inflammatory medication for arthritis or pain relief makes symptoms worse for certain people with asthma | T | 0.31 | 0.465 | 0.888 | 0.16±0.37 | 0.50±0.50 | 6.397** | 0.409 | 0.575 | 0.275 |
| Diagnosis and management | ||||||||||
| An asthma diagnosis can be confirmed (checked) by: | ||||||||||
| 15a: Questionnaire | F | 0.31 | 0.465 | 0.889 | 0.18±0.39 | 0.53±0.50 | 6.518** | 0.391 | 0.64 | 0.217 |
| 15b: Physical check-up (e.g., doctor listening to the lungs) | F | 0.25 | 0.436 | 0.888 | 0.14±0.35 | 0.46±0.50 | 6.241** | 0.345 | 0.575 | 0.133 |
| 15c: Breathing test (e.g., the patient blowing hard into a machine called a spirometer) | T | 0.8 | 0.397 | 0.884 | 0.42±0.50 | 0.93±0.25 | 10.578** | 0.613 | 0.646 | 0.76 |
| 15d: Chest X-ray | F | 0.19 | 0.391 | 0.888 | 0.07±0.26 | 0.35±0.48 | 6.126** | 0.330 | 0.531 | 0.221 |
| 15e: Allergy skin prick tests (e.g., allergen is gently pricked onto the skin | F | 0.24 | 0.426 | 0.888 | 0.13±0.34 | 0.44±0.50 | 6.071** | 0.441 | 0.587 | 0.279 |
| Asthma can cause: | ||||||||||
| 16a: Shortness of breath | T | 0.84 | 0.370 | 0.884 | 0.49±0.50 | 0.97±0.16 | 10.395** | 0.632 | 0.714 | 0.798 |
| 16b: Wheezing | T | 0.8 | 0.397 | 0.883 | 0.41±0.49 | 0.94±0.24 | 11.184** | 0.656 | 0.787 | 0.837 |
| 16c: Tightening of the chest | T | 0.85 | 0.354 | 0.884 | 0.50±0.50 | 0.99±0.12 | 10.731** | 0.619 | 0.661 | 0.761 |
| 16d: Sputum (phlegm or mucous) | T | 0.81 | 0.394 | 0.885 | 0.45±0.50 | 0.95±0.21 | 10.546** | 0.579 | 0.712 | 0.717 |
| 16e: Cough | T | 0.86 | 0.345 | 0.885 | 0.56±0.50 | 0.98±0.14 | 9.178** | 0.581 | 0.694 | 0.696 |
| 16f: Heartburn | F | 0.36 | 0.479 | 0.891 | 0.35±0.48 | 0.47±0.50 | 2.068* | 0.212 | 0.737 | −0.004 |
| 17: People can stop taking their controller medication (e.g., Pulmicort™, QVAR™, Alvesco™, Breo™, Flixotide™, Seretide™, Symbicort™ and Singulair™) if they do not have regular asthma symptoms | F | 0.58 | 0.494 | 0.884 | 0.21±0.41 | 0.87±0.33 | 14.619** | 0.585 | 0.482 | 0.538 |
| 18: There are small devices called peak-flow meters that patients can use to check if the airways in their lungs are narrowed | T | 0.48 | 0.500 | 0.885 | 0.15±0.36 | 0.77±0.42 | 13.193** | 0.482 | 0.504 | 0.44 |
| A person’s asthma is well controlled if: | ||||||||||
| 19a: They take reliever medication (e.g., Ventolin™, Bricanyl™, Airomir™ and Symbicort™) 5 to 7 times per week | F | 0.33 | 0.472 | 0.888 | 0.20±0.40 | 0.56±0.50 | 6.782** | 0.360 | 0.605 | 0.228 |
| 19b: Asthma wakes them up at night no more than twice a month | T | 0.56 | 0.497 | 0.885 | 0.18±0.39 | 0.75±0.43 | 11.691** | 0.462 | 0.606 | 0.502 |
| 19c: They can do normal daily activities, including exercise | T | 0.76 | 0.428 | 0.883 | 0.34±0.48 | 0.94±0.24 | 12.845** | 0.621 | 0.64 | 0.728 |
| 19d: They have asthma symptoms 5 to 7 times per week | F | 0.65 | 0.478 | 0.887 | 0.43±0.50 | 0.87±0.33 | 8.583** | 0.449 | 0.549 | 0.291 |
| 19e: They need to take reliever medication (e.g., Ventolin™, Bricanyl™, Airomir™ and Symbicort™) before exercise | F | 0.51 | 0.500 | 0.887 | 0.27±0.45 | 0.76±0.43 | 9.246** | 0.497 | 0.646 | 0.322 |
| 19f: Their breathing test result (e.g., expiratory flow) is 70% of their personal best | F | 0.22 | 0.416 | 0.889 | 0.18±0.39 | 0.39±0.49 | 3.945** | 0.250 | 0.611 | 0.026 |
| Treating asthma | ||||||||||
| 20: The goal of treating asthma is to keep the disease under control | T | 0.85 | 0.360 | 0.886 | 0.66±0.47 | 0.95±0.21 | 6.381** | 0.553 | 0.6 | 0.65 |
| 21: Reliever inhalers (e.g., Ventolin™, Bricanyl™, Airomir™ and Symbicort™) are the best medications for long-term control of asthma | F | 0.3 | 0.458 | 0.891 | 0.31±0.47 | 0.41±0.49 | 1.636 | 0.433 | 0.566 | 0.33 |
| 22: All people with asthma need a written action plan (a document that provides information on what to do if asthma worsens) | T | 0.68 | 0.467 | 0.887 | 0.49±0.50 | 0.87±0.34 | 7.149** | 0.531 | 0.653 | 0.533 |
| The following medications are controller medications and should be taken regularly every day: | ||||||||||
| 23a: Short-acting bronchodilators (e.g., Ventolin™, Bricanyl™ and Airomir™) | F | 0.43 | 0.495 | 0.886 | 0.20±0.40 | 0.68±0.47 | 9.331** | 0.521 | 0.665 | 0.499 |
| 23b: Inhaled corticosteroids (e.g., Pulmicort™, QVAR™, Alvesco™, Asmanex™, Breo™ and Flixotide™) | T | 0.47 | 0.499 | 0.887 | 0.20±0.40 | 0.69±0.47 | 9.274** | 0.533 | 0.673 | 0.532 |
| 23c: Combination inhalers (e.g., Symbicort™, Seretide™ and Flutiform™) | T | 0.68 | 0.467 | 0.885 | 0.29±0.46 | 0.89±0.31 | 12.717** | 0.631 | 0.608 | 0.706 |
| 23d: Leukotriene receptor antagonists (e.g., Singulair™) | T | 0.47 | 0.500 | 0.890 | 0.33±0.47 | 0.60±0.49 | 4.700** | 0.448 | 0.571 | 0.357 |
**, P<0.01; *, P<0.05. PAKQ, Patient-completed Asthma Knowledge Questionnaire; CR, critical ratio; T, true. F, false.
Patient and public involvement statement
No patients were involved in the research design and conception of this research study. The participants were not consulted to develop the relevant outcomes or interpret the results.
Statistical analysis
All data analysis was performed using SPSS Version 21.0 (IBM SPSS Statistics, Armonk, NY, USA) and SAS 9.4 (Cary, NC, USA). Descriptive statistics were used to summarize the demographic characteristics and presented as n (%) or mean ± SD. The data was presented as n (%) or mean ± SD. Two-sided test was used, P<0.05 was considered significant.
Scores for each subscale were calculated by taking the mean of the items. Reliability was evaluated in terms of internal consistency reliability and retest reliability. The internal consistency of the Chinese version of PAKQ was evaluated using Cronbach’s alpha coefficient (Cronbach’s α), with an alpha >0.70 considered to be acceptable reliability (17,18). Test-retest reliability was assessed by calculating the intraclass correlation coefficient (ICC) values and Pearson’s correlation coefficient (r) at the baseline and second pre-education entries with an interval of 14±4 days. An ICC value of ≥0.70 was considered acceptable for test-retest reliability and 0.81 or more indicated good agreement between both tests (19).
For the factor structure, construct validity was assessed using confirmatory factor analysis (CFA) (20). The Kaiser-Meyer-Olkin (KMO) statistic and Bartlett’s test of sphericity were used to check the adequacy of performing the factor analysis. KMO value >0.5 and significant Bartlett’s test P<0.05 were considered adequate. To investigate the factor structure of the PAKQ scale, model χ2/df and P values were obtained, along with comparative fit index (CFI), a goodness-of-fit index (GFI), standardized root mean square residual (SRMR), root mean squared error of approximation (RMSEA) and normed fit index (NFI).The following criterion was used for determining good model fit: RMSEA <0.08, GFI >0.90, CFI >0.95, SRMR <0.08, CMIN/DF <3, NFI >0.95 (20). A large modification indices (>10) was inspected in structure analysis to improve the model fit (21).
The responsiveness following education was assessed using paired-sample t-test by comparing the difference between PAKQ scores at pre-education and post-education test. The optimal cut-off point for identifying knowledge level of individuals was evaluated by receiver operator characteristic (ROC) curve analysis (22). The patients were divided into two groups according to their cognitive level (evaluation of patients’ performance during the visit by physicians): the good cognitive level group and the bad cognitive level group. The test results were not available to the physicians. The accuracy and the area under the ROC curve were calculated to explore the optimal cut-off point for distinguishing. Measures of diagnostic accuracy (sensitivity, specificity, positive and negative predictive values) with 95% CIs were also calculated.
Results
Demographic characteristics
A total of 464 participants with a mean age of 50.06±15.44 were included. 59.48% of the participants were women. All subjects eligible for the study responded to the questionnaire 3 times and completed the video education. According to the GINA and Chinese asthma guideline (13,14), 48.28% patients were considered with mild asthma, 37.93% with moderate asthma and 13.79% of patients were severe asthma. The education level of patients included in the study was relatively high, and more than 70% of the patients had received high school education or above. Detailed general characteristics of the study participants are presented in Table 1.
Item analysis
The items of the PAKQ were analyzed by the discrimination and the homogeneity test; results are shown in Table 2. The discrimination is based on summing the items and then the patients are divided 2 subgroups by their scores (bounded by 27% and 73% quantiles). The critical ratio (CR) was used to evaluate the discrimination of each item. In this study, the CR of all items, except item 21, were above 2, indicating a good ability to distinguish between good and bad cognition of asthma. The homogeneity of each item with the domain was tested by the correlation coefficient of the item and the score of its domain, Cronbach’s α, commonality and factor loading. In this study, the commonality of each item was above 0.20, conforming to the minimal criteria. With the Cronbach’s α of 0.888 at baseline test, the removal of any items would not improve internal consistency reliability. The factor loading showed that 17 of 54 items had an inadequate value (the minimal criteria 0.4). Of them, 7 items had an inadequate item-domain correlation value. According to international guidelines, an asthma knowledge questionnaire should be provided with a necessary content. Therefore, all the items were retained to balance the content validity and internal structure, like the original English version (10).
Reliability
For internal consistency reliability, Cronbach’s α was 0.888 for the baseline total PAKQ score (results shown in Table 3). In addition, the Cronbach’s α for total PAKQ score at pre-education and post-education test were 0.876 and 0.759, respectively. The Cronbach’s α coefficients were all above 0.7, indicating good internal consistency. For test-retest reliability, data was collected from initial evaluation and second pre-educational visit. The PAKQ scores for each scale of the two tests were obtained and analyzed to acquire the intraclass correlation coefficient (ICC) and Pearson’s correlation coefficient, and the results are shown in Table 4. The ICC values for total PAKQ score and each subscale were all above 0.7, demonstrating an acceptable retest reliability of the questionnaire.
Table 3
| Test | Cronbach’s α |
|---|---|
| Baseline | 0.888 |
| Pre-education | 0.876 |
| Post-education | 0.759 |
PAKQ, Patient-completed Asthma Knowledge Questionnaire.
Table 4
| Subscale | Item | No. of item | ICC | 95% CI | Pearson’s correlation | P value |
|---|---|---|---|---|---|---|
| About asthma | I1-I9 | 13 | 0.863 | 0.845–0.881 | 0.809 | <0.01 |
| Asthma triggers | I10-I14 | 15 | 0.870 | 0.853–0.887 | 0.796 | <0.01 |
| Diagnosis and management | I15-I19 | 19 | 0.892 | 0.877–0.905 | 0.877 | <0.01 |
| Treating asthma | I20-I23 | 7 | 0.704 | 0.663–0.742 | 0.616 | <0.01 |
| PAKQ total score | I1-I23 | 54 | 0.932 | 0.919–0.943 | 0.874 | <0.01 |
Pearson rank correlation coefficient was used to determine the correlations between the PAKQ baseline and pre-education test of total score and subscale scores. PAKQ, Patient-completed Asthma Knowledge Questionnaire; ICC, intraclass correlation coefficient.
Construct validity
To determine whether the questionnaire was suitable for factor analysis, kaiser-Meyer-Olkin sampling Adequacy Scale (KMO) was used to evaluate sample sufficiency, and Bartlett’s sphericity test was used to test whether the questionnaire items were independent of each other. The KMO value was 0.851 at the baseline visit and considered satisfactory (>0.8). In Bartlett’s test, a χ2=8,637.905 (P<0.001) was obtained, implying a suitable data and sufficient size for factor analysis. CFA was then conducted to evaluate the construct validity of the Chinese PAKQ. The results of CFA are shown in Table 5. The fit indices of the four-factor model were ideal, with good χ2/df (1.695), RMSEA (0.039), RMR (0.01), PNFI (0.71) and SRMR (0.058) values, the lowest AIC and BIC values, and acceptable GFI (0.856), CFI (0.885), NFI (0.764) and NNFI (0.869) values.
Table 5
| Model fit index | Standard model fit | Ideal value |
|---|---|---|
| χ2/df | 1.695 | <3 |
| GFI | 0.856 | >0.9 |
| RMSEA | 0.039 | <0.10 |
| RMR | 0.010 | <0.05 |
| CFI | 0.885 | >0.9 |
| NFI | 0.764 | >0.9 |
| NNFI | 0.869 | >0.9 |
| PNFI | 0.671 | >0.5 |
| SRMR | 0.058 | <0.1 |
| AIC | 18,665.207 | The lower the better |
| BIC | 19,613.241 | The lower the better |
PAKQ, Patient-completed Asthma Knowledge Questionnaire; χ2/df, Chi-square goodness-of-fit test; GFI, goodness of fit index; RMSEA, Root Mean square Error of Approximation; RMR, Root Mean square Residual; CFI, parsimony comparative of fit index; NFI, normal-of-fit index; NNFI, Non-Normed Fit Index; PNFI, parsimony normed of fit index; SRMR, Standardized Root Mean Square Residual; AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion.
Responsiveness
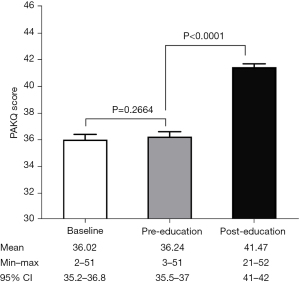
Figure 1 shows the comparison results of PAKQ score at baseline, pre-education and post-education. Paired-t test indicates a non-significant difference between baseline and pre-education (P=0.2664), which was consistent with the retest reliability. Post-education scores were significantly higher than pre-education scores, indicating a good responsiveness following the video education (P<0.0001).
Accuracy
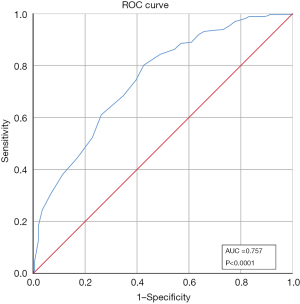
For diagnostic accuracy, we utilized baseline data of the PAKQ to identify if the tool could accurately identify those that demonstrated poor knowledge of the disease. The area under the ROC curve was calculated, representing the accuracy with which the PAKQ score distinguished between poor knowledge level and good knowledge level. Figure 2 shows the ROC curve of the baseline PAKQ total score which produced areas under the ROC curve value of 0.757 (95% CI: 71.38–80.11%), demonstrating that the baseline PAKQ score is of high diagnostic value for patients’ knowledge level of asthma. The optimum cut-off value for distinguishing knowledge level was >35 points, which also maximized the product and sum of the sensitivity and specificity. There were 296 individuals (63.79%) predicted with good asthma knowledge and 168 (36.21%) with poor level. The cut-off point of >35 had a sensitivity of 82.0% and a specificity of 58.4%, respectively. The positive predictive value (PPV) of being classed as good asthma knowledge level was 70.6%, and the negative predictive value (NPV) of being classed as poor asthma knowledge was 72.6%.
Discussion
The National standards for asthma self-management education, jointly issued by the American College of Allergy, Asthma, and Immunology (ACAAI) and American Academy of Allergy, Asthma, and Immunology (AAAAI) stated that improving patients’ asthma knowledge and self-management is an integral part of effective asthma control (8). The control level of asthma is positively correlated with personal literacy, and patients who are lacking in disease cognition usually show poor medication compliance (23). Abundant evidence has proven the effectiveness of an asthma education towards patients (24,25). Ideally, this education should provide a foundation of knowledge and should focus on topics that are closely pertinent to each patient (20,26-28). The clinicians should assess the patient’s cognitive level regularly. The development of tools has facilitated homogenized assessment and follow-up, which is urgently needed clinically in worldwide. Previous questionnaires used in studies from China were mostly self-made or lacked scientific validated data support, some even were simple inquiring (29-31).
To the best of our knowledge, the present study provides the first cross-culture adaption and validation data on the Chinese version of the Patient-completed Asthma Knowledge Questionnaire as the primary evaluation for patients’ knowledge of asthma. The French and English versions of PAKQ have been validated previously (10,11). Our results showed the Chinese version of PAKQ had good reliability and validity according to internal consistency, retest reliability and responsiveness assessment.
The internal consistency reliability mainly reflects the reliability relationship between the internal questions in the questionnaire, which examines whether the same content or characteristics were measured in the test (32). In this study, Cronbach’s α values of PAKQ ranged from 0.759 to 0.888 at three visits. ICC values ranged from 0.704 to 0.892 between baseline and pre-education test in the four subscales, and the ICC for the total score of PAKQ is as high as 0.932. The results showed both high internal consistency and test-retest reliability. Also, it was shown that deletion of any item hardly increases the overall Cronbach’s alpha value of the questionnaire, so we kept all the items as they were in the original version.
Construct validity was evaluated by confirmatory factor analysis and the modified model was good, indicating PAKQ’s data fits the theoretical model well. In three visits, the results showed the same responsiveness and reproducibility as the English and French version (10,11). Using the paired t-test, we found no statistically significant difference between the total score at baseline visit and the second visit, namely pre-education test. But after the video education, the participants’ mean PAKQ total scores improved significantly, as well as scores of each subscale. This result not only demonstrates the effectiveness of video education for patients, but also indicates the good responsiveness of the Chinese PAKQ. To find the best cut-off value for determining knowledge level of patients, we performed an ROC curve based on baseline PAKQ score and the patient’s cognitive level determined by the professional physician. And we found that 35 points was the best value for determining a patient’s knowledge level, which is not included in the prior two versions of questionnaire. This discovery may play a role of reference for other versions of PAKQ in clinical applications.
There are several limitations in this study. First, patients recruited in the study were all from hospitals in several certain cities. Chances are that the education level of the patients was relatively higher than those from rural and remote area. Therefore, the results may not reflect the asthma knowledge level of patients from other backgrounds. Further validation in a population with lower education level may be useful to obtain a full-scale and solid consequence. Second, since there is no Chinese asthma knowledge questionnaire previously verified for patients with asthma, for example, the Consumer Questionnaire (CQ) (33), our study did not compare the Chinese PAKQ with standard questionnaires to obtain the concurrent validity. But even so, we can get a relatively accurate estimate of the patient’s cognitive level from the doctors’ detailed questioning. We also found that there was a strong correlation between patients’ baseline PAKQ scores and physicians’ judgments of patients’ knowledge levels. Third, the item analysis of PAKQ, which indicated a good discrimination and commonality, showed 17 items with inadequate factor loading. The reason is that this questionnaire is not original. Namely, it is translated according to the English version of the questionnaire, without deleting or adding any items. Taking into account that there are also some problems in the original questionnaire(10), we reserved all items to act pursuant to international guidelines and clinical experts. We will further optimize the localization and strive for further progress in adaptation of the questionnaire in the future.
Conclusions
The study provided further support for the validity and reliability of the PAKQ and its usefulness as a tool to facilitate homogenized assessments in patients with asthma in China. This questionnaire can be used in scientific trials for primary evaluation in routine clinical practice. Further large-scale epidemiological investigation will be carried out to acquire data of knowledge level of patients all over China.
Acknowledgments
The authors thank the respiratory physicians and nurses from 16 hospitals who participated in the study. We gratefully thank Dr. Vanessa M. McDonald and Dr. Daniel Beaurivage for providing us the original English version of the PAKQ. We also thank Canghong Zhi for providing medical writing support.
Funding: This work was supported by the National Natural Science Foundation of China (81870021).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1604/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1604/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1604/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1604/coif). DZ is a current employee of Medical Affair of Joincare Pharmaceutical Group Industry Co., Ltd. and this work was supported by the company. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine (clinical trial ethics committee approval number: 2019YK061) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2020. Available online: www.ginasthma.org
- Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019;394:407-18. [Crossref] [PubMed]
- Lin JT, Wang WQ, Zhou X, et al. The level of asthma control in China from a national asthma control survey. Zhonghua Jie He He Hu Xi Za Zhi 2017;40:494-8. [PubMed]
- Al-Zahrani JM, Ahmad A, Al-Harbi A, et al. Factors associated with poor asthma control in the outpatient clinic setting. Ann Thorac Med 2015;10:100-4. [Crossref] [PubMed]
- Selberg S, Hedman L, Jansson SA, et al. Asthma control and acute healthcare visits among young adults with asthma-A population-based study. J Adv Nurs 2019;75:3525-34. [Crossref] [PubMed]
- Pinnock H, Parke HL, Panagioti M, et al. Systematic meta-review of supported self-management for asthma: a healthcare perspective. BMC Med 2017;15:64. [Crossref] [PubMed]
- Zhao J, He Q, Zhang G, et al. Status of asthma control in children and the effect of parents’ knowledge, attitude, and practice (KAP) in China: a multicenter study. Ann Allergy Asthma Immunol 2012;109:190-4. [Crossref] [PubMed]
- Gardner A, Kaplan B, Brown W, et al. National standards for asthma self-management education. Ann Allergy Asthma Immunol 2015;114:178-186.e1. [Crossref] [PubMed]
- Rothe T, Spagnolo P, Bridevaux PO, et al. Diagnosis and Management of Asthma - The Swiss Guidelines. Respiration 2018;95:364-80. [Crossref] [PubMed]
- Beaurivage D, Boulet LP, Foster JM, et al. Validation of the patient-completed asthma knowledge questionnaire (PAKQ). J Asthma 2018;55:169-79. [Crossref] [PubMed]
- Beaurivage D, Boulay ME, Frenette E, et al. Development and validation of patient’s knowledge measurement tools: The model of the Questionnaire de Connaissances sur l’Asthme destine aux Patients Adultes (QCA-PA). Rev Mal Respir 2016;33:350-64. [Crossref] [PubMed]
- Anthoine E, Moret L, Regnault A, et al. Sample size used to validate a scale: a review of publications on newly-developed patient reported outcomes measures. Health Qual Life Outcomes 2014;12:176. [Crossref] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019. Available online: www.ginasthma.org
- Asthma group of Chinese Throacic S. Guidelines for bronchial asthma prevent and management (2020 edition) Asthma group of Chinese Throacic Society. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:1023-48. [PubMed]
- Beaton DE, Bombardier C, Guillemin F, et al. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25:3186-91. [Crossref] [PubMed]
- Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 1993;46:1417-32. [Crossref] [PubMed]
- Bland JM, Altman DG. Cronbach’s alpha. BMJ 1997;314:572. [Crossref] [PubMed]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951;16:297-334. [Crossref]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. [Crossref] [PubMed]
- Streiner DL. Building a better model: an introduction to structural equation modelling. Can J Psychiatry 2006;51:317-24. [Crossref] [PubMed]
- Giles SJ, Parveen S, Hernan AL. Validation of the Primary Care Patient Measure of Safety (PC PMOS) questionnaire. BMJ Qual Saf 2019;28:389-96. [Crossref] [PubMed]
- Carter JV, Pan J, Rai SN, et al. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery 2016;159:1638-45. [Crossref] [PubMed]
- Federman AD, Wolf MS, Sofianou A, et al. Self-management behaviors in older adults with asthma: associations with health literacy. J Am Geriatr Soc 2014;62:872-9. [Crossref] [PubMed]
- Gibson PG, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003;CD001117. [PubMed]
- Thoonen BP, Schermer TR, Jansen M, et al. Asthma education tailored to individual patient needs can optimise partnerships in asthma self-management. Patient Educ Couns 2002;47:355-60. [Crossref] [PubMed]
- Cabana MD, Le TT. Challenges in asthma patient education. J Allergy Clin Immunol 2005;115:1225-7. [Crossref] [PubMed]
- Smith S, Mitchell C, Bowler S. Patient-centered education: applying learner-centered concepts to asthma education. J Asthma 2007;44:799-804. [Crossref] [PubMed]
- Partridge MR, Hill SR. Enhancing care for people with asthma: the role of communication, education, training and self-management. 1998 World Asthma Meeting Education and Delivery of Care Working Group. Eur Respir J 2000;16:333-48. [Crossref] [PubMed]
- Su N, Lin J, Liu G, et al. An epidemiological survey on management and insights of asthma in China in 2009 to 2010. Zhonghua Nei Ke Za Zhi 2015;54:680-3. [PubMed]
- Wang WQ, Lin JT, Zhou X, et al. Evaluation of asthma disease perception from China national asthma control survey. Zhonghua Yi Xue Za Zhi 2018;98:467-71. [PubMed]
- Nong Y, Lin JT, Wang WQ, et al. A multi-center study for the association between the perception and control of disease among asthmatic patients in Chinese urban areas. Zhonghua Yi Xue Za Zhi 2017;97:1425-9. [PubMed]
- Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess 2003;80:99-103. [Crossref] [PubMed]
- Kritikos V, Krass I, Chan HS, et al. The validity and reliability of two asthma knowledge questionnaires. J Asthma 2005;42:795-801. [Crossref] [PubMed]


