Should we use a biophysical approach in the classification and management of air leakage after lung resection?
An alveolar-pleural fistula, or air leak, is the most common postoperative complication following elective lung resection. An air leak is a communication between the pulmonary parenchyma’s alveoli and the pleural space distal to a segmental bronchus. A prolonged air leak (PAL) is defined as an air leak for more than five days postoperatively. Patients with PAL have a significantly longer length of stay, which results in higher costs. Similar findings were observed in a cohort of patients undergoing lung cancer resection via video-assisted thoracoscopic surgery (VATS). PAL necessitates prolonged chest tube drainage, which increases postoperative pain, respiratory splinting, which increases the risk of pneumonia, venous thromboembolic complications due to decreased mobility, and the need for additional procedures such as chemical or mechanical pleurodesis (1).
Epidemiology of PALs
Upper lobectomy has been associated with a higher incidence of PAL (2). PAL has also been associated with advanced age, a low forced expiratory volume in the 1st second (FEV1%), a low body mass index (BMI), and the presence of pleural adhesions (3). Additionally, PAL may be surgeon dependent. While it is understandable that poor lung quality, such as that found in emphysema, increases the risk of postoperative air leak, it is unclear why different anatomical areas should experience varying degrees of air leak. One answer to this question is that those different areas of the lung experience varying degrees of pleural stress, which varies according to the lung’s shape and location. Since the shape of the lung is closely related to the shape of the chest wall, variations in the shape of the chest wall may be associated with variations in the extent of pleural stress. In a lobectomy, the remaining lung inflates into and conforms to the remaining space, assuming the shape of the resected lobe; this process occurs concurrently with other physiological changes such as contralateral lung hyperinflation mediastinal shifting, ipsilateral diaphragm elevation, and ipsilateral crowding of the intercostal spaces (4).
The incidence of an air leak lasting >5 days is 3.5% following wedge resection, 6.7% following segmentectomy, and 8.6% following lobectomy (2). The most often documented risk factors are decreased pulmonary function indicative of damaged and fragile lung parenchyma, upper lobe resections, a low BMI, and the development of pleural adhesions. Brunelli et al. recently developed an aggregate risk score for PAL based on four weighted variables:
where age >65 years =1; pleural adhesions =1; FEV1 80% =1.5; and BMI <18.5 =2. The score was used to create four risk classes with an incremental risk of PAL: 0 in class A (no risk factors present, score 0), 6.7 in-class B (score 1), 10.9 in-class C (score 1.5–3), and 25.7 in class D (>3.5) (5). In a cohort of patients who received VATS anatomical lung resections, Orsini et al. recently validated another PAL measure, the index for persistent air leak (IPAL). The IPAL is a risk model that incorporates seven variables associated with air leaks lasting more than seven days. The following formula is used to calculate the IPAL:
Where gender is F =0, M =4; pleural adhesion: no = 0, yes = 4; pulmonary resection: wedge = 0, segmentectomy or lobectomy = 7, bilobectomy = 11; bullectomy = 2, volume reduction = 14; location: middle or lower lobe = 0; upper = 4. The risk probability was calculated using the IPAL score formula:
A patient with an IPAL 5 has a <5% risk of developing PAL following pulmonary resection (low risk). With IPAL between 5 and 10, the risk of developing PAL is between 5% and 10% (moderate risk), while with IPAL >10, developing PAL is >10% (high risk) (6).
Terminology and nomenclature regarding pleural space management following lung resection
Four major European and North American Thoracic Surgery Organizations have published a joint to standardise terminology and nomenclature regarding pleural space management following lung resection position paper (7). Passive drainage occurs when the intrapleural pressure exceeds the atmospheric pressure; active drainage occurs when a sub-atmospheric pressure (negative) is applied to the pleural space via an external pump or by creating a column of liquid within the chest tube that extends below the pleural space’s level (siphoning effect). Pleural pressure levels assessed during the final hour before chest tube removal varied substantially in a series of straightforward pulmonary lobectomies conducted without external suction and with the chest tube kept on a water seal. Pressures in the same patients can range from positive to negative as minus 40 cmH2O in a brief period. To avoid ambiguity in the context of active drainage, the condition in which an external suction is applied to the drain has been designated as external suction applied. No external suction applied should be used in all other cases. A further critical distinction must be observed between regulated (variable) and unregulated (fixed) suction. Regulated suction is a type of active drainage achieved by applying an external source of suction that can adjust its level of negative pressure in response to pressure variations within the pleural space to maintain a pre-set pressure value. The suction that is not regulated or fixed is a type of active drainage that is provided by an external source of suction that is incapable of varying its level in response to the intrapleural pressure level (i.e., wall suction) (8).
The physiology of air leak for dummies
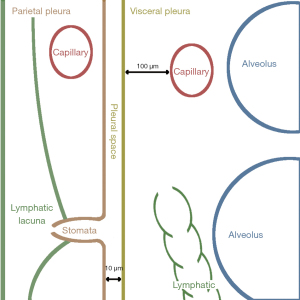
The pleural gap is a marvel of bioengineering because it performs two functions: it maintains the lung optimally enlarged in the chest. It permits flawless reciprocal sliding of the visceral and parietal pleura with an incredibly low coefficient of friction. This is achieved by keeping pleural fluid pressure at the sub-atmospheric region, which is governed by the balance of pleural fluid filtration and drainage. Behind the apparent simplicity of these concepts, there is a well-designed and complicated system responsible for maintaining this balanced state (9). The anatomical arrangement of the pleural space is depicted schematically in Figure 1. It is defined by the visceral pleura, which surrounds the lung, and the parietal pleura, which covers the rib cage and diaphragm. Pleural membranes are made of a layer of 4 µm thick mesothelial cells joined on the luminal side by tight junctions and the subpleural basal side by desmosomes. Microvilli 1–3 µm in length and varying density from 2 to 30/m2 cover the mesothelial cells irregularly. Microvilli trap glycoproteins and hyaluronic acid in high concentrations. In humans, the visceral pleura can reach a thickness of 100 µm, whereas the parietal pleura is only five times thick. The parietal pleura is densely packed with lymphatics that open directly onto the mesothelial surface via the so-called lymphatic stomata. Stomata range in diameter from 1 to 40 meters and have a density of 0.01/m2 on the intercostal surface to 0.8/m2 on the diaphragm. The parietal mesothelium of the mediastinum is so densely packed with stomata that it is classified as a cribriform membrane (Kampmeier foci). Stomata are frequently clustered and form/constitute a network of submesothelial lacunae. On the parietal pleura, lymphatic stomata are scattered fractally, indicating that their greater density in some regions represents a more considerable draining capacity demand. Because the lymphatics of the visceral pleura do not link directly to the pleural space, unlike the lymphatics of the parietal pleura, they are not engaged in the drainage of pleural fluid. The parietal pleura receives blood from the systemic circulation, whereas venous drainage occurs via the intercostal veins. The systemic circulation provides the blood supply to the visceral pleura via the bronchial arteries and empties mostly into the pulmonary veins (8).
Let us assume that the pleural space is represented by a simple column of water surrounding the lung. In a gravitational field, fluids in static conditions (that is/namely without flow) exhibit an attractive property regarding the gravity-dependent distribution of pressures: as illustrated in Figure 2A, pressure increases by 7.4 mmHg for every 10 cm down the water column; this is referred to as be the hydrostatic gradient. Naturally, one could also consider a pressure decrease of 7.4 mmHg for every 10 cm increase. Consider the following case simulating the pleural cavity’s condition and imagining a system draining pleural fluid from the cavity’s bottom (lymphatics of the diaphragmatic region when standing). Consider also that the draining system can produce a pressure of 0 mmHg (atmospheric): it is self-evident that the pleural liquid pressure should gradually increase as one ascends the cavity. Pleural liquid pressure should reach 0.35 m at the top of the cavity if the pleural cavity is 0.35 m in height. Although direct measurements of pleural liquid pressure in humans are not available, reasonable extrapolations from data from large mammals indicate that pleural liquid pressure in supine humans is in the range of 7.4 mmHg at the mid-heart level (Figure 2B).
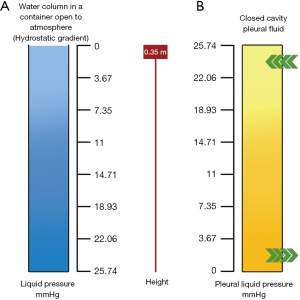
Most fluid drainage occurs at the cavity’s bottom via lymphatic drainage. In contrast, the low pressure at the cavity’s top facilitates microvascular filtration from the parietal pleura’s capillaries into the pleural space via the parietal pleura’s capillaries. Indeed, the vertical distribution of pleural liquid pressure does not accurately reflect the hydrostatic state. The pleural fluid should flow from the top to the bottom, but it is so tiny that it has a negligible effect on the hydrostatic gradient, despite the high flow resistance within the pleural space. Additionally, a flow of pleural fluid from the costal side to the lymphatic-rich mediastinal regions was demonstrated. The polarisation of filtration/drainage for pleural fluid in a more realistic fluid dynamics model in the pleural space should be controlled. Most fluid production occurs in the parietal pleura’s fewer dependent regions. Due to the greater thickness of the visceral pleura, it is excluded from pleural fluid turnover under physiological conditions; in fact, its permeability is tenfold the one of the parietal pleura. Finally, pleural fluid is drained from the diaphragmatic, costal, and mediastinal regions via the parietal pleura’s lymphatic stomata. Initial lymphatics have a flow velocity of about 2 millimetres per minute. Specific data indicate that mesothelial cells’ active water transport may contribute to pleural fluid turnover. The negative pleural liquid pressure maintains the visceral and parietal pleura in proximity, thereby maintaining a low volume of pleural fluid (0.3 mL/kg).
The visceral and parietal pleura are pushing against one another physically. These molecules function as an efficient lubricant, allowing for easy reciprocal sliding of the pleural membranes while maintaining a low coefficient of friction (0.02, as with ice sliding on ice). No friction is encountered between reciprocally sliding pleural membranes despite this mechanical arrangement. This is because phospholipids are adsorbed and stratified in multiple layers on the surfaces of pleural membranes. Due to the common repulsive effects between charges having the same sign (negative), phospholipids have hydrophobic tails carrying negative charges. As a result, the parietal and visceral pleura remain separate. In practice, despite their opposition, the opposing pleurae will never come into touch.
Acknowledgments
Funding: This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5×1000 funds.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease, for the series “Prolonged Air Leak after Lung Surgery: Prediction, Prevention and Management”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1870/coif). The special series “Prolonged Air Leak after Lung Surgery: Prediction, Prevention and Management” was sponsored by Bard Limited. Bard Limited has no interference on the contents of the special series. FZ served as the unpaid Guest Editor of the series. LB serves as an unpaid board member of the Journal of Thoracic Disease. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clark JM, Cooke DT, Brown LM. Management of Complications After Lung Resection: Prolonged Air Leak and Bronchopleural Fistula. Thorac Surg Clin 2020;30:347-58. [Crossref] [PubMed]
- Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: is it a patient or surgeon related problem? Ann R Coll Surg Engl 2012;94:422-7. [Crossref] [PubMed]
- Murakami K, Hamaji M, Morita S, et al. Prolonged air leak after reoperative pulmonary resection (with prior ipsilateral chest surgery). Interact Cardiovasc Thorac Surg 2020;31:544-6. [Crossref] [PubMed]
- Casha AR, Caruana-Gauci R, Manche A, et al. Pleural pressure theory revisited: A role for capillary equilibrium. J Thorac Dis 2017;9:979-89. [Crossref] [PubMed]
- Pompili C, Falcoz PE, Salati M, et al. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: An analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957-65. [Crossref] [PubMed]
- Orsini B, Baste JM, Gossot D, et al. Index of prolonged air leak score validation in case of video-assisted thoracoscopic surgery anatomical lung resection: results of a nationwide study based on the French national thoracic database, EPITHOR. Eur J Cardiothorac Surg 2015;48:608-11. [Crossref] [PubMed]
- Brunelli A, Beretta E, Cassivi SD, et al. Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC. Eur J Cardiothorac Surg 2011;40:291-7. [Crossref] [PubMed]
- Pompili C, Miserocchi G. Air leak after lung resection: pathophysiology and patients' implications. J Thorac Dis 2016;8:S46-54. [Crossref] [PubMed]
- Beatchey W. Respiratory Care Anatomy and Physiology. 4th Edition. St Louis: Elsevier, 2022.