Chinese expert consensus on immunoprophylaxis of common respiratory pathogens in children (2021 edition)
Introduction
Respiratory tract infections (RTIs) are common respiratory disorders in children which cause uncontrolled systemic inflammation and may lead to symptoms incorporating septic shock, acute respiratory distress syndrome (ARDS), pneumonia, encephalitis, and multiple organ failure. RTIs are often associated with respiratory pathogens, primarily including measles virus (MeV), influenza virus, varicella-zoster virus (VZV), coronavirus (CoV), respiratory syncytial virus (RSV), adenovirus (AdV), Bordetella pertussis, Corynebacterium diphtheria, Haemophilus influenzae (Hi), Mycobacterium tuberculosis (MTB), Neisseria meningitidis (Nm), and Streptococcus pneumonia (Spn). Respiratory pathogen prevalence has exerted a huge disease burden on humans, such as the global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of December 14th, 2021, the pandemic had generated 270,031,622 confirmed cases worldwide, with 5,310,502 deaths (1). Vaccination is the most economical and effective way to prevent RTIs (as well as its associated complications) and to reduce the spread of pathogens. Common respiratory pathogen vaccines, such as influenza vaccine, measles vaccine, DTP (Diphtheria, Tetanus and Pertussis) vaccine, Hi vaccine, and Spn vaccine, can provide good immunoprotection for children. In recent years, the Centers for Disease Control and Prevention (CDC) at all levels in China have played an important role in publicizing vaccination knowledge, vaccination technology training, and managing vaccine-related adverse reactions, therefore immunoprophylaxis levels of common respiratory pathogens in children across China have been improved. However, when compared with vaccines of the National Immunization Program, the vaccination rate of non-immunization programs remains generally low. Therefore, it is necessary to increase public awareness of vaccinations, standardize vaccination services, and formulate corresponding vaccination guidelines.
Based on a comprehensive analysis of clinical evidence for the immunoprophylaxis of common respiratory pathogens in children at home and abroad (Table 1), in combination with the clinical situation in China and expert clinical experience, the Chinese expert consensus on immunoprophylaxis of common respiratory pathogens in children (2021 edition) was formulated. This consensus document applies to all CDC staff levels engaged in the prevention and control of related pathogens, vaccinators at vaccination sites, and medical staff in pediatric, respiratory, and infectious diseases departments at all levels in medical institutions. Based on research/study progress at home and abroad, this consensus document will be accordingly updated and regularly improved in the future. We present the following article in accordance with the RIGHT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1613/rc).
Table 1
| Serial number | Domestic/foreign policies and guidelines | Website |
|---|---|---|
| 1 | Technical Guidelines for Influenza Vaccination in China (2020-2021) | http://www.chinacdc.cn/jkzt/crb/bl/lxxgm/jszl_2251/202009/W020200911453959167308.pdf |
| 2 | Chinese CDC Vaccination Work Specifications | https://www.chinacdc.cn/jkzt/ymyjz/ymsysm_10961/201810/P020181010489030277647.pdf |
| 3 | Children's Immunization Procedures and Instructions under National Immunization Program Vaccine (2021) | http://www.nhc.gov.cn/jkj/s3581/202103/590a8c7915054aa682a8d2ae8199e222.shtml |
| 4 | Children’s Immunization Procedures and Instructions under National Immunization Program Vaccine (2016) | http://xxgk.zhoushan.gov.cn/art/2020/6/2/art_1229007389_44598579.html |
| 5 | White Paper on the Incidence of Pneumonia in Children and the Popularization of Vaccines in China | https://v.qq.com/x/page/y095247z3e3.html |
| 6 | Expert Consensus on Diagnosis, Treatment and Prevention of COVID-19 Infection in Children | http://rs.yiigle.com/CN101070202002/1185578.htm |
| 7 | Standards for Diagnosis and Treatment of Children with Adenovirus Pneumonia (2019) | http://www.nhc.gov.cn/yzygj/s7653/201902/bfa758ad6add48a599bc74b588a6e89a.shtml |
| 8 | 2019–2020 AAP Recommendations for Prevention and Control of Children with Influenza | https://pediatrics.aappublications.org/content/144/4/e20192478.long |
| 9 | Expert Advice on Diagnosis and Treatment of Children with Hemophilus influenzae Infection | http://rs.yiigle.com/CN112140201909/1161597.htm |
| 10 | Expert Consensus on Immunoprophylaxis of Pneumococcal Diseases (2020) | http://chinaepi.icdc.cn/zhlxbxen/ch/reader/view_abstract.aspx?file_no=085&flag=1 |
| 11 | Experts’ consensus on immunization with meningococcal vaccines in China | http://html.rhhz.net/zhlxbx/20190201.htm |
| 12 | Expert Consensus on Diagnosis, Treatment and Prevention of Streptococcus Pneumoniae Diseases in Chinese Children (2020) | http://rs.yiigle.com/cn101070202007/1197848.htm |
| 13 | WHO recommendations for routine immunization | https://www.who.int/immunization/policy/immunization_tables/en/ |
| 14 | Recommended Routine Immunizations for Children | https://www.who.int/immunization/policy/Immunization_routine_table2.pdf?ua=1 |
| 15 | Measles vaccines: WHO position paper | https://apps.who.int/iris/bitstream/handle/10665/255149/WER9217.pdf;jsessionid=5D3BAAA0540D20C49E5D4F75FFAFB6B7?sequence=1 |
| 16 | Diphtheria vaccine: WHO position paper | https://apps.who.int/iris/handle/10665/258683 |
| 17 | Meningococcal A conjugate vaccine: updated guidance | https://www.who.int/wer/2015/wer9008.pdf?ua=1 |
| 18 | WHO guidelines on tuberculosis infection prevention and control 2019 update | https://www.ncbi.nlm.nih.gov/books/NBK539297/ |
| 19 | Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices - United States, 2019 | https://www.cdc.gov/mmwr/volumes/69/wr/mm6903a5.htm |
| 20 | Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2020–21 Influenza Season | https://www.cdc.gov/mmwr/volumes/69/rr/rr6908a1.htm?s_cid=rr6908a1_w |
| 21 | Child and Adolescent Immunization Schedule Recommendations for Ages 18 Years or Younger, United States, 2022 | https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html |
| 22 | Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020 | https://pubmed.ncbi.nlm.nih.gov/33417592/ |
CDC, The Center for Disease Control and Prevention; WHO, The World Health Organization.
Immunoprophylaxis of virus-related diseases
Immunoprophylaxis of measles, mumps, and rubella
Measles, mumps and rubella are common acute infectious diseases in children, of which measles and rubella are eruptive infectious diseases. These diseases are not only widespread on a global level but they are also important infectious diseases that threaten children’s health in China. The causative pathogens of all three are RNA viruses, all with only one serotype. Infection sources for measles and rubella are patients with the virus, whereas mumps sources include patients with mumps and healthy carriers. These diseases are mainly spread via respiratory droplets and direct contact. Measles are generally susceptible to the population. The incidence peak typically occurs in winter and spring and is more common in children aged 6 months to 5 years. The incubation period is 6–18 days, with an approximate 10-day average. After this time, prodromal, eruption and recovery periods generally follow. Mumps occur all year round and frequently occur in winter and spring, with infections more common in children and adolescents aged 3–16 years. The incubation period is 14–25 days, with an average 18 days. Rubella occurs in all seasons, but mainly the winter and spring. It is more common in preschool or school-age children and the incubation period is generally 14–21 days.
Measles-Mumps-Rubella (MMR) viruses exhibit stable antigenicity, with vaccination being the most effective measure to prevent infections. The World Health Organization (WHO) recommends all children receive two doses of the measles-containing vaccine (MCV). In high-risk measles-death countries, it is recommended that the first dose of MCV is received at 9 months and the second dose of MCV at 15–18 months (the shortest interval between two doses is 4 weeks). In countries where the risk of infant-measles is low, the first dose of MCV may be administered at 12 months and the second at 15–18 months (2). However, recent data identified a rebound in countries and regions that had previously eliminated or were close to eliminating the infection (3). As a consequence, reported measles cases globally increased by 31% between 2016 and 2017 (4).
Children's rubella vaccinations reduce the spread of rubella virus and more importantly, prevent congenital rubella syndrome. The current strategy is to administer two doses at appropriate intervals. Experiences from several countries have shown that a history of rubella is not a reliable indicator of immunization, therefore all children without vaccine contraindications must be vaccinated. Maintaining a high vaccination rate with the children’s rubella vaccine reduces its prevalence and also the chance of infection among women of childbearing age. The rubella vaccine can be injected subcutaneously as a single component, or combined with vaccines such as the MMR triple vaccine. The American Academy of Pediatrics (AAP) recommends two vaccination doses to prevent rubella; the first dose is administered at 12–15 months and the second at 4–6 years old (5).
The mumps vaccine was originally produced as a monovalent vaccine, however, most countries currently use the MMR. China started producing the MMR vaccine in 2002, and because the vaccine prevents three diseases simultaneously, it was approved for domestic use the same year. In 2008, MMR vaccines were included in the National Immunization Program of China. According to recent reports, the effective rate of a single MMR vaccine dose is 85.4% [95% confidence interval (CI): 67.3–93.4%] which exerts limited effects on mumps prevention and control, however, the effective rate of two MMR vaccine doses is 88.5% (95% CI: 78.1–93.9%) (6) which is not as high as expected. In developed countries, mumps outbreaks occur periodically even among young people with high vaccine coverage. Therefore, some studies have suggested the third dose of MMR should be administered to at risk populations to control outbreaks (7).
The accidental vaccination of pregnant women with live attenuated MMR vaccines is not an absolute indication for pregnancy termination. However, a 2017 WHO position paper on the measles vaccine suggested it was prudent to avoid measles vaccines and combined vaccines during pregnancy. For women of childbearing age with negative measles antibodies or low immunoglobulin G (IgG) antibody levels (<200 IU/mL), they were recommended to receive the measles vaccine but avoid pregnancy within 3 months after vaccination.
Similarly, in view of the risk of fetal malformations caused by the rubella virus, it was recommended that women of childbearing age who were not immunized shown by serum Rubella Virus-Immunoglobulin G (RV-IgG) testing should be intensively inoculated with one dose of MMR (8) and avoid pregnancy for at least 1 month after vaccination. “Immunization Procedures and Instructions for Children under the National Immunization Program (2020)” and the expert consensus on the vaccination of children with special conditions (9) can be referred to.
Recommendations
- If there are no contraindications, it is recommended that children aged 8 months receive the first dose of MMR and children aged 18 months receive the second dose of MMR.
- For children <18 years old who were born before expansion of the National Immunization Program, if they had not completed two vaccination doses containing measles components, the MMR could be used.
- MMR vaccines can be vaccinated at the same time and at different sites with other vaccines included in the National Immunization Programs, especially hepatitis A and DTP vaccines with crossed immunization times.
- If multiple vaccines must be administered but not at the same time, the MMR vaccine will be given priority. If it is not vaccinated with other injectable live attenuated vaccines at the same time, the interval must be ≥28 days.
- Individuals who inject immunoglobulin should be vaccinated with MMR at intervals of ≥3 months and avoid using immunoglobulin within 2 weeks after MMR inoculation.
- Allergies to known vaccine components or severe allergic reactions after a previous vaccination of the same vaccine: for primary immunodeficiency (except complement deficiency); children born to human immunodeficiency virus (HIV)-infected mothers or have other exposure risks and diagnosed with acquired immunodeficiency syndrome (AIDS)-related symptoms by a medical institution or have immunosuppressive symptoms are not vaccinated in principle.
- For children receiving chemotherapy, radiotherapy, and medium or high-dose systemic glucocorticoids (prednisone and other equivalent drugs ≥2 mg/kg/d or ≥20 mg/d), the MMR live attenuated vaccine should be temporarily delayed.
- For individuals with moderate or severe infectious diseases, the vaccination should be postponed until recovery.
Influenza immunoprophylaxis
Influenza virus belongs to the Orthomyxoviridae family and is a segmented single-negative-stranded RNA virus. According to the viral nucleoprotein and matrix protein, influenza virus is divided into A, B, C, D Type, according to the difference between surface hemagglutinin (HA) and neuraminidase (NA), influenza A viruses can be divided into multiple subtypes (10). Globally, the annual (or seasonal) influenza epidemic causes 3–5 million severe cases and 290,000–650,000 deaths which are related to respiratory tract disorders (11). There is no cross-immunity between different influenza virus types and subtypes, therefore populations can be repeatedly infected. The incubation period is 1–7 days. Patients with influenza and recessive carriers are the main infection sources. The virus is spread by droplets or by direct/indirect contact with mouth, nose, and eye discharge from patients. People are generally susceptible to the virus. Influenza viruses in China usually circulate once every 6 or 12 months, with epidemic peaks in winter and spring. Type A H3N2 is the most prevalent in winter and summer, whereas type A H1N1pdm09, type B Yamagata, and Victoria are often more prevalent in winter and spring (12,13). When young children have influenza, their clinical symptoms exhibit different characteristics depending on age, whereas older children’s symptoms are similar to adults. There are no significant differences in clinical symptoms and manifestations between influenza A and B in children, whereas influenza C is mostly manifested as mild upper RTIs.
Globally marketed influenza vaccines are mainly divided into inactivated influenza vaccines (IIV) and live attenuated influenza vaccines (LAIV) (11). The AAP recommends that all children >6 months, without medical contraindications, receive the influenza vaccine annually. Available vaccines include quadrivalent IIV (IIV4, e.g., Fluzone, FluLaval, Fluarix, and Afluri), trivalent IIV (IIV3), and tetravalent influenza spray vaccine (FluMist) (14). Annual influenza vaccination times for children are primarily based on age and previous vaccination history. Children >9 years only require one dose. For children between 6 months and 8 years who are vaccinated for the first time or received < two influenza vaccine doses in the past, they should receive two doses with an interval of ≥4 weeks. These guidelines are recommended for both IIV and LAIV (11).
The influenza vaccines approved for marketing in China are IIV3, IIV4, and live attenuated nasal spray influenza vaccines. IIV3 and IIV4 both protect against influenza A and B (15,16), but IIV4 displays better immunogenicity against influenza B than IIV3 (17). The domestic batches of influenza vaccines issued between 2020 and 2021 have come from 9 companies, including Hualan Biological Bacterin Co., Ltd., Shenzhen Sanofi Pasteur Biological Products Co., Ltd., and Changchun Institute of Biological Products Co., Ltd. The IIV4 vaccine currently used in China comes from Hualan Biological Bacterin Co., Ltd. and has been used in adults since the influenza season of 2018–2019, but has not yet been approved for children (18). In 2020, a newly-listed freeze-drying nasal trivalent LIAV (LAIV3) spray was recommended for children aged 3–17 years, produced by Changchun BCHT Biotechnology Co. China recommends that children between 6 months and 8 years who are vaccinated for the first time or with < two doses of influenza vaccine in the past should receive two doses (interval ≥4 weeks). Children aged 6 months to 8 years who have received two doses or more of the influenza vaccine in 2019–2020 or before, are recommended one dose. Children aged 9 years and older only need one dose, with recommendations to complete immunization before the end of October (11). For infants aged 6 months and below, no direct influenza vaccine is available. This population is protected via maternal vaccination during pregnancy and vaccinating family members and caregivers (11). IIVs can also be vaccinated simultaneously or sequentially with other inactivated vaccines and live attenuated vaccines, while LAIVs require a specific time interval to vaccinate with other live attenuated vaccines (11). The influenza vaccination is safe, but adverse reactions can occur; they are usually mild and disappear within a few days. Equally, serious abnormal reactions are rare. Contraindications for influenza vaccinations are for those who are allergic to any vaccine ingredients, e.g., excipients, formaldehyde, lysing agents, and antibiotics.
Recommendations
- If there are no contraindications to the influenza vaccination, children > 6 months should receive the vaccination annually, especially children in kindergartens and primary and secondary schools of key places.
- When children between 6 months and 8 years are vaccinated for the first time, or have received < two influenza vaccine doses in the past, they should receive two vaccine doses at an interval of ≥4 weeks. Children who have received ≥ two influenza vaccine doses in the past are recommended to receive one dose. Children aged ≥9 years only need one dose annually.
- Infants <6 months: as the influenza vaccine cannot be directly administered to this group, the population can prevent influenza by maternal vaccination during pregnancy and vaccinating family members and caregivers (11).
- Pregnant women: this cohort is at a higher risk of severe illness, death, and adverse pregnancy outcomes after influenza. Although a lack of data exists on the safety evaluation of influenza vaccination for pregnant women in China, foreign countries have generated sufficient evidence during pregnancy. Thus, vaccinations clearly prevent pregnant women from getting influenza and protect infants <6 months via maternal-fetal antibodies. Therefore, it is recommended pregnant women or women with pregnancy planning during the influenza season receive the influenza vaccine. Pregnant women can get the influenza vaccine at any stage of pregnancy (11).
- The peak time and duration of influenza activities vary across China. To ensure recipients acquire adequate immune protection before the high-incidence influenza season, it is best to complete vaccinations before the end of October (11).
- Generally, protective antibodies are produced 2–4 weeks after influenza vaccine administration. After 6–8 months, antibody titers begin to decay, therefore the influenza vaccine must be administered annually (11).
Varicella-zoster immunoprophylaxis
VZV belongs to the herpesvirus, α-herpesvirus subfamily. VZV infection is limited to humans and some advanced primates, and has only one serotype. Infection causes two different diseases, varicella and herpes zoster (HZ). Varicella is usually mild, but it may cause serious complications, including bacterial infections (e.g., cellulitis and pneumonia) and neurological complications (meningitis), or it may be fatal (19). HZ is usually accompanied by neuropathic pain; post-herpetic neuralgia is the most common complication. The pain may last for several months while seriously affecting the quality of life of patients. The incubation period for VZV is 10–21 days. Varicella and HZ patients are typically infection sources, which are mainly spread by droplets and contact. However, it is generally accepted HZ is mainly caused by reactivation of a latent viral infection after varicella in infants. The population is generally susceptible, with the disease occurring throughout the year, but peaking in winter and spring. Typically, varicella infections may be divided into prodromal and eruption periods. The former is usually asymptomatic or mild and may include low-grade fever and irritability. During the latter period, a rash is first observed on the trunk and later extends to the face and limbs. Varicella is mostly a self-limiting disease and is self-healing in approximately 10 days.
Market based varicella vaccines are all live attenuated varicella vaccines and measles, mumps, rubella and varicella (MMRV) combined vaccine. Study vaccines include new live attenuated vaccines, inactivated vaccines, nucleic acid vaccines, and combined vaccines. The AAP recommends regular varicella vaccination, with the first dose at 12–15 months and the second at 4–6 years (5). The WHO recommends the first dose at 12–18 months. In total, one or two doses are given. The interval between doses is 4–12 weeks (subject to specific vaccination instructions) (20). Adverse reactions in healthy children after varicella vaccination are mostly mild; they include pain and redness at the injection site, fever, and a mild rash (21). In most cases, reactions are transient and disappear after 2–3 days. Currently, the listed HZ vaccines are mainly live attenuated vaccines and adjuvant HZ subunit vaccines. This latter category was recently listed in China, however, the live attenuated vaccines and adjuvant HZ subunit vaccines are used for the elderly. Currently, no HZ vaccines are available for children.
In 1997, China introduced varicella vaccines, with domestically produced vaccines launched in 2000. Thus far, China has not included this vaccine in its National Immunization Program. It is recommended that the two-dose varicella vaccination be included in the National Immunization Program. For pregnant women, the WHO recommends no varicella vaccination during pregnancy. If women of childbearing age are vaccinated, pregnancy should be postponed by 4 weeks (22). There may be a risk after vaccination with live vaccines or attenuated vaccines (23). The CDC also recommends that varicella vaccines are not administered to pregnant women (24). Importantly, there are no requirements to terminate pregnancy after unintentional varicella vaccination in the first trimester (25). A 10-year data survey reported no evidence to suggest the risk of congenital varicella syndrome and congenital birth defects in live births were related to varicella vaccinations of pregnant women, however, a larger number of study samples are still required to confirm this (26).
Recommendations
- If there are no contraindications, healthy children aged ≥1 year may be vaccinated with two doses of live attenuated varicella vaccines. It is recommended that the first dose is received at 12–24 months and the second at 4–6 years.
- If two doses are not completed, supplement two doses (≤14 years at least 3 months apart, and for ≥15 years old at least 4 weeks apart).
- For pregnant women, the varicella vaccine is forbidden. If women of childbearing age are vaccinated, pregnancy should be postponed by 4 weeks.
Novel coronavirus (CoV) immunoprophylaxis
CoV belongs to the coronaviridae family of nidovirales and may be divided into α-, β-, γ-, and δ-CoVs. Severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2 are all highly pathogenic human coronaviruses (HCoVs) which may cause severe respiratory distress syndrome, with high mortality rates. The WHO defined the disease caused by SARS-CoV-2 infection as corona virus disease 2019 (COVID-19) and recognized COVID-19 as a pandemic on March 11th, 2020. As of December 14th, 2021, global COVID-19 infections have exceeded 270.03 million, with >5 million deaths. Infections in the United States have exceeded 49.63 million and deaths exceeded have 0.79 million (1,27). The reported cases of SARS-CoV-2 infections in children in China are mostly asymptomatic infections and mild cases, with rare severe cases (28).
SARS-CoV-2 is highly infectious. Symptomatic patients are the main infection sources, with asymptomatic carriers also infection sources. The main routes of transmission are droplets and close contact. The infection may be spread by aerosols in confined spaces and poorly ventilated areas. Fecal-oral transmission routes require confirmation. The population is generally susceptible. When compared with infected adults, children display a lower prevalence, milder symptoms, and a relatively lower mortality rate (28-30). The majority of SARS-CoV-2 infections in Chinese children have occurred in boys, with high-risk populations in the 1–12 year(s) old category. Most children have a close contact history with confirmed or suspected patients with COVID-19, and most of them have had family clusters (31). The incubation period for the infection is 1–14 days, but generally this is 3–7 days. In most cases, clinical manifestations may be asymptomatic or manifested as fever, fatigue, dry cough, however, a small number of patients will be accompanied by nasal congestion, rhinorrhoea, sore throat, other upper respiratory symptoms, abdominal discomfort/pain, nausea, vomiting, diarrhea, and other gastrointestinal symptoms. Some experience olfactory and taste disorders. Severe cases often experience dyspnoea and/or hypoxemia 1 week after infection onset. In other severe cases, the infections rapidly progress to ARDS, septic shock, irreversible metabolic acidosis, coagulation disorder, and multiple organ failure.
SARS-CoV-2 vaccine research and development (R&D) has progressed rapidly and has encompassed the vast majority of existing vaccine R&D technologies, including inactivated vaccines, RNA vaccines, DNA vaccines, viral vector vaccines (non-replicating or replicating vector vaccines), live attenuated vaccines, protein subunit vaccines, and virus-like particle vaccines. According to official WHO data, as of July 6th, 2021, 105 global vaccines had entered clinical studies, with 184 undergoing preclinical studies (32). Phase III data for BNT162b2 by Pfizer/BioNTech reported that two BNT162b2 doses could reach 95% effectiveness in preventing infections in individuals ≥16 years (33), whereas for 12–15 years old, the effective prevention rate could reach 100% and was well tolerated (34). The phase III clinical mRNA-1273 vaccine developed by Moderna showed an effective rate of 94.1% (35) and was 100% efficacious in preventing severe COVID-19 without increasing the risk of thrombosis (36).
The Chinese SARS-CoV-2 vaccine has also made rapid progress. Currently, the National Medical Products Administration has approved five COVID-19 vaccines for conditional listing. They include inactivated vaccines developed by the Wuhan Institute of Biological Products Co., Ltd., a subsidiary of Sinopharm, inactivated vaccines from the Beijing Institute of Biological Products Co., Ltd., a subsidiary of Sinopharm, an adenovirus vector vaccine developed by the Academy of Military Sciences of PLA (People’s Liberation Army of China) in conjunction with CanSino Biologics Inc., a recombinant protein vaccine from Anhui Zhifei Longcom Biopharmaceutical Co., Ltd., and an inactivated vaccine from Beijing Kexing Zhongwei Biotech Co., Ltd. Phase III clinical studies performed overseas showed that the Kexing Zhongwei SARS-CoV-2 inactivated vaccine (CoronaVac) exerted 91.25% protective effects against COVID-19 after 14 days inoculation with two vaccine doses according to the 0, 14-day vaccination schedule.
Interim phase III clinical trial analyses of the adenovirus vector vaccine, Ad5-nCoV (Academy of Military Sciences of PLA with CanSino Biologics Inc., Pakistan) showed that a single dose exerted 100% protective affects against severe COVID-19. The overall protection effect was 74.8%.
Currently, SARS-CoV-2 continues to mutate across global populations. According to the Global Initiative on Sharing All Influenza Data, the prevalent mutant strains are primarily, α, β, γ, and Δ (37). Study data have shown that the neutralization titer of the α-mutant strain in sera of the COVID-19 vaccine, BBIBP-CorV (Beijing Institute of Biological Products Co., Ltd., China) vaccinators is higher than that of wild type (1.4 times), while the neutralization titer of the β-mutant strain is 0.4 times higher than that of wild type. The neutralization titer of the α-mutant strain in the sera of the CoronaVac vaccine (Kexing Zhongwei COVID-19 vaccine) inoculators is significantly lower than that of wild type (0.5 times, P=0.004), and the neutralization titer for the β-mutant is 0.3 times that of wild type (P<0.001) (38).
Recommendations
- Vaccinations effectively prevent viral infections. Sinopharm China’s bioCOVID-19 inactivated vaccine is approved for emergency use in populations aged 3–17. The recommended interval between the 2 doses is ≥3 weeks, and the second dose should be completed as soon as possible within 8 weeks.
- To reduce the pressure from medical institutions on the superposition of respiratory infectious diseases and COVID-19 infections, children should be immunized against other common respiratory pathogens, including routine influenza vaccines, Hi vaccines, Spn vaccines, pertussis vaccines, MMR vaccine, etc. Multisystem inflammatory syndrome in children (MIS-C) may be related to immune disorders caused by COVID-19 infection and immune-related side effects should be closely monitored after vaccination of children.
RSV immunoprophylaxis
RSV is an enveloped single negative-stranded RNA virus, originally belonging to the pneumoviridae of paramyxoviridae. In 2015, the International Committee on Taxonomy of Viruses changed RSV origins to orthopneumovirus of pneumoviridae (39), which is mainly divided into two types: A and B subtypes (40). Globally, RSV is a key pathogen causing acute RTIs in children <5 years. It is the primary factor implicated in the hospitalization of infants and young children with viral RTIs, which seriously endanger health, especially in premature infants and those with congenital heart disease or primary immunodeficiency (41). The RSV incubation period is 2–8 days (more often 4–6 days) (42). The infection sources are symptomatic RSV patients and asymptomatic recessive carriers. The highly infectious RSV is spread by droplets or contact. Infants, the elderly, and those with weakened immune systems are all susceptible to infection.
The RSV epidemic season generally shows a peak trend every other year and appears related to certain climatic factors. RSV subtypes A and B are alternately prevalent, with the dominant subtype changing every 2 years. Compared with the year when subtype B is dominant, the start of RSV season in years with high prevalence of subtype A is 3 to 5 weeks earlier, and the season duration is also accordingly extended by approximately 6 weeks (43). RSV infections cause bronchitis, bronchiolitis, and interstitial pneumonia, or mild “cold” symptoms. Children exhibit obvious symptoms, such as nasal obstruction, cough (mainly dry), and fever accompanied by mild conjunctivitis and pharyngitis. Moderately and severely infected individuals experience dyspnoea, wheezing, lip bruising, flaring nares, and three concave sign (the suprasternal fossa, supraclavicular fossa, and intercostal space appear concave when inhaling).
Currently, no licensed RSV vaccines are available for vaccinations. RSV fusion protein humanized mouse monoclonal antibody Palivizumab is currently the only monoclonal antibody approved by the U.S. Food and Drug Administration (FDA), but it is not marketed in China. The AAP recommends palivizumab for premature and high-risk infants (those with congenital heart disease, chronic lung disease, immunodeficiency, etc.) with a gestational age of <29 weeks, or during the RSV epidemic season, where up to five palivizumab doses (15 mg/kg per dose; one dose/month via intramuscular injection) are given to infants in the first year after birth who meet precautionary conditions. Palivizumab is not recommended for the second year after birth, unless oxygen inhalation for at least 28 days after birth or medical support (e.g., oxygen inhalation, glucocorticoid therapy or long-term diuretic therapy) are needed (44).
Nirsevimab is a recombinant humanized immunoglobulin G, subclass 1, κ light chain (IgG1κ) monoclonal antibody under development. It neutralizes RSV by targeting the prefusion-conformation of the RSV fusion protein (F). Nirsevimab has a higher potency and a longer half-life than palivizumab (average 59.3 days). In an interventional, randomized, double-blind phase IIb trial, 1,453 healthy premature infants with a gestational age of 29–35 weeks received a single intramuscular injection of Nirsevimab of 50mg (n=969) or placebo at the beginning of the RSV epidemic season (n=484). Within 150 days after administration, the incidence of RSV-related lower RTI in the Nirsevimab group (2.6% vs. 9.5%; P<0.001) was 70.1% (95% CI: 52.3–81.2%) lower than the placebo group. The RSV-related hospitalization rate for lower RTIs was also reduced by 78.4% (95% CI: 51.9–90.3%). The preventive effects were unaffected by the region and the epidemic RSV subtype, safety outcomes were similar to the placebo group, and no significant hypersensitivity reactions were observed (45). Nirsevimab effectively neutralizes RSV A and RSV B subtypes via a single administration before the RSV epidemic season and provides protective effects against different RSV subtypes, thereby reducing RSV disease burden and improving the cost-effectiveness and compliance of RSV prevention. Currently, Nirsevimab has been granted fast tracking and breakthrough drug status by the FDA.
Recommendations
- Currently, no RSV vaccines exist in China. “Social vaccines” are effective measures for RSV prevention, including healthy lifestyles, good hygiene habits, and personal daily protection. Transmission routes are limited by wearing masks, washing hands, and maintaining a safe social distance.
- Immunoprophylaxis of other common respiratory pathogens should be performed, including routine vaccination with the influenza vaccine, Hi vaccine, Spn vaccine, etc., to mitigate or reduce multiple mixed infections caused by RSV and other respiratory pathogens.
Adenovirus immunoprophylaxis
Currently, >90 human adenovirus serotypes (HAdV) have been defined through whole genome sequencing and are divided into 7 species: HAdV-A–G (46). HAdV show different tissue orientations and clinical manifestations. RTIs are mainly caused by types B (including types 3, 7, 11, 14, 21, and 55), C (types 1, 2, 5, and 6), and E (type 4) (47). Adult serotypes 1, 7, 14, and 21 are the most common, while in children the most common are types 1–7 and 55 (46). Most HAdV infections occur in patients from 6 months to 5 years (48), with the peak occurring before 2 years, accounting for 2–7% of the entire respiratory tract disorder and 4%-20% of pneumonia (49). HAdV infection is mostly a self-limiting disease, but in low numbers of children, severe RTI, acute respiratory failure, and even death are often recorded. In addition, 14%–60% of patients with HAdV pneumonia may have pulmonary sequelae (47) which seriously affect the quality of life of children.
HAdV infections may be caused by infected individuals (inhalation of atomized droplets, conjunctival inoculation, and fecal-oral infection transmission), exogenous modalities (pillows, bed sheets, etc.) or viral reactivation in the body. Asymptomatic HAdV carriers may carry the infection for several weeks or months. HAdV infections can occur throughout the year, mostly in winter and spring; in southern China, the detection rate is highest in the summer months, May to August (50). HAdV invades most tissues and cells in the body, among which, RTIs are the most common and mainly affecting infants and young children aged 6–24 months. Children with adenovirus pneumonia have a rapid onset and apparent poisoning symptoms, mainly manifested as high fever, severe cough, and dyspnoea. Approximately one-third of adenovirus pneumonia cases develop into severe pneumonia (51). The type 3 and 7 HAdV infections are the most common (52), which manifest as respiratory symptoms, with respiratory failure, atelectasis, ARDS, and other pulmonary complications.
The United States uses oral HAdV-4 combined with HAdV-7 bivalent vaccines in the military which effectively reduce adenovirus infection incidences (53). In recent years, Chinese scientists have also commenced adenovirus vaccine development, especially for the severe RTIs caused by HAdV-3, 7, and 55 types. Recently, adenoviral RTIs in children have become prevalent across China, some of which have caused serious or life-threatening complications. Thus, the development of an adenovirus vaccine for children is imminent. However, due to the many adenovirus types, certain difficulties in vaccine development must be overcome.
Recommendations
- Currently, no HAdV vaccines have been approved for the prevention of HAdV infection in children, however, the “social vaccine” approach is an effective preventative mechanism.
Immunoprophylaxis of bacterial-related diseases
Immunoprophylaxis of B. pertussis
The B. pertussis pathogen causes pertussis. In 2018, the WHO reported 151,074 global pertussis cases, with disease incidence rebounding in many countries suggesting a clear upward trend (54). Individuals with a pertussis infection experience several complications, including pneumonia (most common), subconjunctival hemorrhage, pulmonary arterial hypertension, and encephalopathy. Study data indicated that the diagnostic rate of pneumonia in hospitalized children with pertussis was as high as 62.0% (55), and the mortality rate of 5-month-old infants with pertussis-mediated pneumonia was as high as 12.5% (56). The incubation period is 6–20 days, with patients, recessive carriers, and bacterial disease carriers all good infection sources. The infection is mainly spread by droplets and aerosols, with intra-family transmission an important interpersonal transmission modality. The pertussis prevalence rate in China is sporadic throughout the year, with peak infections in summer in most areas. More men are infected and most cases are children, however, the incidence rate has been rising in recent years (57). Pertussis infection is generally divided into a catarrhal period (1–2 weeks), spasmodic cough period (2–6 weeks), and recovery period (2–3 weeks). Clinical manifestations in infants and young children are more typical, but clinical symptoms in adolescents and adults are atypical, or only showing persistence or with chronic cough.
Pertussis vaccines are divided into whole-cell pertussis (wP) and acellular pertussis vaccines (aP). The both are combined with diphtheria and tetanus toxoids to form a diphtheria, tetanus, and wP combined vaccine (DTwP), and a diphtheria, tetanus and aP combined vaccine (DTaP). After China implemented the National Immunization Program in 1978, DTwP was popularized at a national level. In 2011, the DTwP replacement by DTaP was effectively completed. Current commonly used combination vaccines containing pertussis components mainly include DTaP and DTaP-Hi type b (conjugate) combined vaccine (DTaP-Hib) prepared on the basis of DTaP, and DTaP-inactivated poliomyelitis vaccine Hi type b (conjugate) combined vaccine (DTaP-IPV-Hib). In term of equivalent immunogenicity and safety, multivalent vaccines can often simplify the immunization procedure and improve vaccination compliance and coverage. DTaP exhibits good safety levels and local and systemic adverse reactions are generally mild and transient. The WHO recommended that all children be vaccinated against pertussis. The initial vaccination age is ≥6 weeks and no later than 8 weeks. Three basic immunization doses should be completed within 6 months of birth (58).
Currently in the Chinese National Immunization Program, the vaccination procedure of the vaccines containing pertussis components is 4 doses, the basic immunization stage is 3, 4, and 5 months of age, 1 dose each, and the booster immunization stage is 1 dose at 18 months of age. China's immunization program is not only the first dose of vaccination is significantly delayed, but the fifth dose (4–6 years old) and the sixth dose (11–18 years old) for booster immunization are less than that in other countries. As a result, while domestically DTaP vaccination rates are maintained at relatively high levels, the pertussis incidence is increasing (57). The American Advisory Committee on Immunization Practices (ACIP) recommends that children between 2 months and 6 years be routinely vaccinated with five DTaP doses at 2, 4, 6, 15–18 months, and 4–6 years. Children or adolescents between 11 and 18 years should be vaccinated once, preferably at 11–12 years, with one dosing boost every 10 years (59). A meta-analysis showed that after the last DTaP vaccination, the pertussis risk increased by 1.33 times per annum (95% CI: 1.23–1.43) over time. Assuming a vaccine efficacy of 85%, it was estimated that only 10% of children's immune protection can be obtained 8.5 years after the last DTaP vaccination (60). Therefore, after the routine four DTaP vaccination doses, one booster immunization dose for 6-years-old should be considered, and the type of vaccine is selected according to the age at the time of replanting. DTaP is used for 3 months to 5 years old. Diphtheria-tetanus (DT) combined vaccine adsorbed (for children) is used for 6–11 years old, and adults and adolescents’ type is used for ≥12 years old. Individuals ≥12 years must use DTaP.
A retrospective survey of the vaccine history of pertussis cases in Shenzhen, China reported that children with pertussis were mainly under 1 year old, and that 73.05% of the children had not received vaccines containing anti-pertussis components. Children at this age experience close relationships with family members and their activity capacity and scope are limited. Thus, clinicians must be alert to the “adult/adolescent-infant” transmission mode. Vaccinate all close contacts infants <12 months old, to reduce the risk of pertussis transmission (61), the so-called “cocoon strategy”. At present, the United States, France, Switzerland, and Germany, and other countries have implemented the “cocoon strategy” (62). However, due to the difficulty of vaccination operation, it is difficult to achieve the expected goal.
“Maternal immunity” is another strategy that reduces pertussis infections in infants and young children. Approximately 40 countries have recommended that pregnant women receive the DTaP vaccine to prevent pertussis and protect the baby after birth (63). Pregnant women vaccinated with the DTaP vaccine prevent approximately 70–90% of pertussis infections and reduce 90.5% of pertussis-related hospitalizations in infants <3 months old (63). However, China lacks study data on pregnant women vaccinated with the DTaP vaccine.
Recommendations
- It is recommended that children are vaccinated with four doses of DTaP or DTaP-Hib or DTaP-IPV-Hib in time, including three basic immunization doses at 3, 4, and, 5 months, and one immunization booster dose at 18–24 months. The vaccine type should be selected according to the age at the time of vaccination. DTaP is used for 3 months old to 5 years old, and two additional booster immunizations are recommended after the completion of the whole vaccination. DT is used for 6–11 years old, and DTaP used for ≥12 years old.
Diphtheria immunoprophylaxis
Diphtheria is an acute respiratory infectious disease caused by C. diphtheria which is a Gram-positive toxin-producing aerobe. Based on morphology, colony, biochemical and pathogenic characteristics, it is divided into gravis, intermedius, mitis and belfanti types. The four biological types can cause diphtheria epidemics. Since 1962, and notably after implementation of the National Immunization Program in 1978, the diphtheria incidence has dropped significantly in China. Since 2006, no diphtheria reports have been recorded (64), thereby controlling disease incidence and eliminating diphtheria. However, in recent years, diphtheria has become endemic in countries where the vaccination coverage rate is not up to standard (65). Infections are mainly concentrated in India, Nepal, Indonesia, Thailand, and other countries surrounding China (65,66). Humans are the only known hosts of C. diphtheria which is spread via droplets and secretions from contacts with damaged skin. Infection sources include diphtheria patients or carriers, and the incubation period is 2–5 days. Critically, diphtheria patients are infectious at the end of the incubation period. The population is generally susceptible, with children being the most susceptible. Newborns acquire immunity through the placenta and breast milk, but this practically disappears in 12 months with susceptibility gradually increasing with age. Diphtheria transmission may involve any mucosa, including the nose, pharynx, tonsils, throat, skin, eyes, and genitals. The exotoxin is known as diphtheria toxin and is the main cause of disease. It causes inflammatory tissue necrosis, elevated inflammatory cell infiltration, fibrin exudation, and the local formation of characteristic diphtheria pseudomembranes. According to incidence rates, infections are divided into faucial diphtheria, laryngeal diphtheria, nasal diphtheria, and diphtheria in other sites. Faucial diphtheria is the most common in adults and older children, whereas other diphtheria types are more common in young children.
Currently, there is no single diphtheria toxin antigen vaccine. Diphtheria toxin vaccine for children is mostly combined with tetanus toxin to form DT vaccines, or combined with tetanus and the pertussis antigen to form DTwP or DTaP. The WHO recommends three DTP vaccine doses as a primary immunization to establish lifelong immunity against infection; specifically, the first dose at 6 weeks with the interval between the next two doses being at least 4 weeks. If possible, the third primary immunization dose should be completed before 6 months. If the start or end of the primary immunization is delayed, the missing doses should be administered as soon as possible and the interval between the two doses should be at least 4 weeks. After this, combination vaccine containing diphtheria toxoid antigen booster doses must be administered in childhood and adolescence (i.e., vaccination at 12–23 months, 4–7 years, and 9–15 years) to provide immune protection in adolescence and adulthood. Ideally the interval between the two booster doses is at least 4 years (20).
China currently implements five diphtheria toxin-containing vaccine doses as routine immunization procedures in children of target age; one DTaP vaccine dose at 3, 4, 5, and 18–24 months and one DT vaccine dose at 6 years. The diphtheria toxin vaccine is one of the safest vaccines available, with rare serious adverse reactions. Similarly, no serious adverse reactions to vaccinations have been identified in pregnant women and newborns (67). The high levels of maternal antitoxin antibodies may affect the baby’s immune responses, resulting in a decrease in the baby’s immune response after the first two doses of diphtheria toxin-containing vaccine. The 2017 WHO position paper on the diphtheria vaccine pointed out that the prevention of neonatal diphtheria does not need to be vaccinated to pregnant women (68).
Recommendations
- One dose of DTaP vaccine is recommended for postnatal infants at 3, 4, 5, and 18 months and one dose of DT vaccine is recommended at 6 years old.
- It is not recommended to prevent neonatal diphtheria by giving pregnant women the DTaP vaccine.
MTB immunoprophylaxis
Tuberculosis is caused by MTB and is one of the most important infectious diseases seriously endangering human health. MTB is a facultative intracellular parasite that belongs to the mycobacterium family. In 2020, a WHO global tuberculosis report indicated that in 2019, 9.96 million new cases were reported worldwide, of which 1.19 million were children, accounting for 12% of infected cases. Approximately 1.4 million people died of tuberculosis, including 194,000 children (69). Thus, tuberculosis has become one of the infectious diseases with a high mortality rate caused by a single pathogen in the world. China is one of 30 countries with a high tuberculosis burden and a high tuberculosis, multidrug-resistant tuberculosis (MDR-TB), and tuberculosis/HIV co-infection incidences. Pulmonary tuberculosis patients are the main infection sources who transmit the bacteria via the respiratory tract, air droplets, and the digestive tract. Children with a family member history of exposure to tuberculosis are 8–9 times more likely to develop tuberculosis when compared with children with no tuberculosis exposure history. The risk of developing active tuberculosis within 12 months after an initial infection is higher, especially in the first 6 months People are generally susceptible to MTB. The younger the age, the higher the risk of MTB infection. Typical symptoms of pulmonary tuberculosis in children include, chronic cough, unexplained weight gain or loss, intermittent fever, night sweats, and hemoptysis.
Bacilli Calmette-Guerin (BCG) is the only effectively prevents tuberculosis. Since its advent, approximately 4 billion children have benefited from the BCG vaccine. Studies have confirmed the BCG effectively prevents tuberculous meningitis and miliary pulmonary tuberculosis which are highly fatal to infants and young children. The WHO recommends (20) that countries should choose a reasonable BCG vaccination plan based on the epidemiological characteristics of its tuberculosis type. In countries with a high burden of tuberculosis (including China), all newborns should be BCG vaccinated after birth. In countries with a low tuberculosis incidence, the BCG vaccination may be limited to high-risk newborns and infants, or older children who have negative tuberculin skin tests (TST). Because the BCG is a live attenuated vaccine, it may cause malformations during fetal development in pregnancy, therefore, WHO does not recommend the vaccination for pregnant women (20).
Since 1978, BCG vaccinations have been included in planned immunizations across China. The “Immunization Procedures and Instructions for Children under the National Immunization Program (2020)” recommended that newborns should be given one dose at birth, and premature infants of gestational age >31 weeks with stable clinical conditions could be BCG vaccinated. Premature infants of gestational age ≤31 weeks could be vaccinated before discharge when clinical conditions were stable (70). Because delayed BCG vaccinations of premature infants and low-birth-weight infants are widespread in China, BCG vaccination is usually started when the newborn's weight reaches 2,500 g. A recent systematic review and meta-analysis reported that safety, reactogenicity, and TST positive rates of early BCG vaccinated and delayed BCG vaccinated clinical condition stable premature infants and low-birth-weight infants were similar (71).
Recommendations
- Newborns should be vaccinated with an intradermal BCG within 24 h after birth (preferably at the same time as the hepatitis B vaccine). If they are not vaccinated at this time, they should be vaccinated within 3 months after birth.
- Premature infants in middle and late stages (gestational age >31 weeks) and low-birth-weight infants (<2,500 g) may be vaccinated intradermally with BCG at birth or at discharge at the latest if they are healthy and clinically stable. Premature infants at gestational age ≤31 weeks may be vaccinated before discharge when clinically stable.
- If there is a family member with immune deficiency disease, it must be excluded neonatal immune deficiency disease before the BCG vaccination.
- For children born to HIV-infected mothers, HIV-uninfected children will be vaccinated with BCG, but for children with an unknown HIV-infection status, vaccinations will be suspended, and HIV-infected children will not be vaccinated.
- The BCG vaccination of patients receiving immunosuppressants should be avoided.
- For TST-negative children and adolescents, a catch-up intradermal BCG may be appropriate.
- BCG vaccinations are not recommended for pregnant women.
Immunoprophylaxis of epidemic meningitis
N. meningitidis (Nm) is usually referred to as meningococcus Nm. Based on its capsular polysaccharide, the bacteria are divided into at least 13 serogroups, such as A, B, C, W, Y, and X, etc. Diseases caused by Nm are global. Infected individuals may be asymptomatic or may cause severe invasive disease. The clinical manifestations are mainly sepsis and meningitis—also called epidemic meningitis. In severe cases, septic shock and brain parenchymal damage may occur, with some patients rapidly succumbing to death. Epidemic meningitis infection sources are typically patients or asymptomatic carriers, with disease spreading via respiratory droplets and secretions. The population is generally susceptible to Nm.
The infection is mainly in winter and spring and due to different geographical locations, the onset season may be slightly different. In northern Chinese regions, infections start at the end of winter every year and reach the highest peak levels from February to April. Then, they gradually decrease from May. In southern regions, infections occur 1–2 months later. Different from the predominant epidemics of group B and C in foreign countries, the current epidemic of epidemic meningitis in China is still dominated by group A and C, and there are differences in the epidemic serogroups and clonal groups in different regions (72). The most common Nm infection is acute bacterial meningitis. Infected children usually display fever, chills, vomiting, photophobia, and severe headaches. In addition to the central nervous system, Nm also colonizes and multiplies in various tissues or organs, resulting in disease. Common infection sites include the pericardium and joints, leading to pericarditis and pyogenic arthritis.
Meningococcal vaccines include capsular polysaccharide vaccines, polysaccharide-protein conjugate vaccines, and group B protein vaccines. Of these, polysaccharide vaccines are used to control outbreaks and include group A, bivalent (AC), trivalent (ACW), and tetravalent (ACWY) vaccines. Polysaccharide vaccines are ineffective for <2 years old, however, above this age, effective protection period is about 3 years with no group immune effects. Combined vaccine (used for routine immunizations and outbreaks) can produce immunity for >5 years, and produce group immunity. Vaccines can be administered to infants and children <1 year old, including C group, A group, AC group, and ACWY group.
Group B protein vaccines include outer membrane and recombinant proteins (group B, British routine immunization) which are mainly used to control outbreaks. The use of meningococcal vaccines for target-age children should follow the requirements of the Immunization Procedures and Instructions for Children under the National Immunization Program (2020): Group A meningococcal polysaccharide vaccine (Group A epidemic meningitis polysaccharide vaccine, MPSV-A) is administered two times, one dose at 6 and 9 months. Group A and C meningococcal polysaccharide vaccine (group A and C epidemic meningitis polysaccharide vaccine, MPSV-AC) is administered two times, one dose each at 3 and 6 years old. When emergency vaccinations are performed for epidemic meningitis, the corresponding vaccine should be selected according to the bacterial flora and epidemiological characteristics underpinning the epidemic. For children <24 months, if the prescribed doses have been administered according to epidemic meningitis conjugate vaccine instructions, it may be regarded as completion of the immunization dose of the group A epidemic meningitis vaccine. At 3 and 6 years old, one epidemic meningitis polysaccharide vaccine dose is given according to the schedule. Administration of the meningococcal vaccine to pregnant women is effective in preventing meningitis in infants and young children. Currently, these study data are mainly derived from small sample clinical studies (73).
Recommendations
- MPSV-A must be administered twice, one each at 6 and 9 months. MPSV-AC must be administered twice, one each at 3 and 6 years old.
- The interval between two MPSV-A doses is ≥3 months. The interval between the first MPSV-AC dose and the second MPSV-A dose is ≥12 months. The interval between two MPSV-AC doses is ≥3 years. Repeated vaccinations should be avoided within 3 years.
- When performing an emergency vaccination against epidemic meningitis, the corresponding epidemic meningitis vaccine should be selected according to bacterial flora and epidemiological characteristics behind the epidemic.
- For children <24 months, if the epidemic meningitis conjugate vaccine prescribed doses have been vaccinated, it may be regarded as completion of the group A epidemic meningitis vaccine immunization. At 3 and 6 years old, one epidemic meningitis polysaccharide vaccine dose is performed according to procedures.
- Women who are at risk of meningitis infection during pregnancy or who travel to areas endemic for the disease may be vaccinated against meningococcal meningitis.
Immunoprophylaxis of Hi
Hi is a pathogen often isolated from children’s respiratory and reproductive tract specimens. It belongs to the hemophilus family and is generally divided into typable Hi and non-typable Hi (NTHi). Based on differences in capsular polysaccharide antigens, Hi is divided into six types: a, b, c, d, e, f, namely Hia, Hib, Hic, Hid, Hie, Hif, of which Hib is highly pathogenic and often leads to invasive meningitis, pneumonia, sinusitis, and epiglottitis.
The source of Hi infections are Hi patients and nasopharyngeal carriers, with disease spread via droplets and direct contact. The population is generally susceptible, especially newborns (several weeks old) to 2–3 years old. The Hi epidemic is seasonal; spring and winter are the high incidence infection seasons, however due to differences in climatic conditions, the peak seasons of Hi infection may vary from region to region (74). In recent years, with the widespread administration of the Hib vaccine, Hib infections have become rare, and NTHi has become the most common Hi disease pathogen in the world. Between 2007 and 2014, NTHi accounted for 78% of invasive Hi infection patients in 12 European countries (75). The common clinical manifestations of Hi infection include meningitis, epiglottitis, pneumonia, cellulitis, and blood infection.
Vaccines preventing Hi infection in children include the Hib single vaccine and Hib combined vaccines which include the epidemic meningitis-Hib vaccine, DTaP-Hib vaccine, DTaP-IPV-Hib vaccine, DTaP-IPV-Hib-HepB vaccine, etc., among which DTaP-IPV-Hib- HepB vaccine has not yet been marketed in China. Both Hib single and Hib combined vaccines exert good protective effect toward Hib-related diseases, with rare local and systemic reactions at injection sites. The ACIP has recommended four Hib vaccine doses, routinely administered at 2, 4, 6, and 12–15 months (5).
The Hib-vaccine is a non-immunization program vaccine in China, with vaccination rates lower than National Immunization Program vaccines. A meta-analysis reported that as of December 31st, 2016, the total coverage rate of the Hib conjugate vaccine in 12 mainland Chinese provinces was 54.9% (95% CI: 52.9–57.0%), the coverage rates of 1, 2, 3, and 4 doses of the vaccine were 26.7%, 14.8%, 13.5%, 14.3%, respectively, with the coverage rate in eastern regions higher than central and western regions (76). According to WHO, 189 member states included the Hib vaccine in their immunization programs in 2019, and the global coverage rate of the three-dose Hib vaccine was 85% (4). The results of a multi-level sampling survey in four Hangzhou districts, China showed that children whose household registration is a permanent resident and received notification of Hib vaccination are the motivating factors for Hib vaccination. However, the high price of Hib vaccine is not only an obstacle to the vaccination of Hib vaccine (77).
Recommendations
- If no contraindications are evident, it is recommended that children <5 years be vaccinated with the Hib-vaccine as soon as possible.
- The Hib vaccine immunization procedure is as follows:
- Infants <6 months are given one dose every 1 or 2 months for a total of three doses, then the fourth booster immunization dose at 18 months;
- Infants at 6–12 months old are given one dose every 1 or 2 months for a total of two doses, then the third booster immunization dose at 18 months;
- Children aged 1.5–5 years old need require only one dose.
- Epidemic meningitis-Hib vaccine, DTaP-Hib vaccine, DTaP-IPV-Hib vaccine procedures can be referred to the manufacturer's vaccine instructions.
Immunoprophylaxis of Spn
Spn is a genus of α-hemolytic streptococcus. Capsular polysaccharides constitute its main virulence factors and are also the basis for serotyping. Serotypes without a capsule are non-typable. Currently, >90 serotypes have been identified of which >20 cause clinical disease, especially in children and the elderly. Globally, Spn-related diseases lead to high morbidity and mortality rates in children and are serious public health issues. The 2016 Global Burden of Disease, Injury, and Risk Factors Study showed that Spn was the leading cause of morbidity and death from lower RTIs (78). In 2015, among HIV-uninfected children aged 1–59 months, 294,000 global deaths were caused by Spn, of which 81% had pneumonia (79).
Spn is mainly spread by respiratory droplets. The population is generally susceptible, especially children <5 years old. Spn infections are more prevalent in winter and early spring and different Spn serotypes are prevalent in different countries/regions. Currently, the prevalent serotypes in Chinese children mainly include, 19A, 19F, 14, 23F, and 6B (80). Clinical infections may be divided into non-invasive pneumococcal disease (NIPD) and invasive pneumococcal disease (IPD) and are based on infection sites. NIPD mainly includes acute otitis media, sinusitis, and non-bacteremic pneumonia, while IPD mainly includes bacteremia, meningitis, and bacteremic pneumonia. Spn mediated pneumonia usually manifests as typical lobar pneumonia in older children, and bronchial pneumonia in younger children. Spn meningitis is the most serious and common IPD, often occurring with acute onset, mostly with fever as the initial symptom, followed by coma and convulsions, shock, meningitis, and pneumonia.
The pneumococcal conjugate vaccine (PCV) is mainly administered to children. The 7-valent (PCV7) vaccine has been withdrawn and replaced by 10-valent and 13-valent (PCV10, PCV13) vaccines. A pneumococcal polysaccharide vaccine (PPV) which is only 23-valent (PPV23) is also available on the market. PCV, PPV vaccines are administered separately, and the safety of vaccinate with other vaccines at the same time and revaccination are good (81), and it can reduce the colonization rate of Spn in the nasopharynx and Spn drug resistance. Common adverse reactions such as redness, swelling, pain, fever, chills, headache, and other systemic reactions at injection sites are transient; in most cases, they disappear within 1–2 days.
PCV13 and PPV23 are currently the most widely used Spn vaccines in clinical practice. PCV13 is suitable for infants and young children aged 6 weeks to 18 months. The serotypes covered include 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19F, and 23F. The immunogenicity of PPV vaccines is not as good as PCV, therefore PPV23 is unsuitable for young infants, but is suitable for children at high risk of Spn infection >2 years old. The serotypes include, 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F (81).
The development of new Spn vaccines is still ongoing, and includes expanding the coverage of bacterial types from 15 to 20 of more valence PCV, DNA vaccines, and protein and other antigen vaccines. Currently, PCV13 is a non-immunization program vaccine in China requiring administration in accordance with the principles of informed consent and voluntary vaccination at their own expense. It is recommended that children be immunized at 2, 4, and, 6 months, or 3, 4, and, 5 months for basic immunizations, and 12–15 months for booster immunizations. The first basic immunization dose may be given as early as 6 weeks, with the interval between doses being 4–8 weeks. PPV23 only requires a once-off administration in children as young as 2 years old (5).
Recommendations
- will be vaccinated with a PCV13 dose and then a PPV23 dose 8 weeks after inoculation to expand the range of immunoprotective Spn serotypes. For those requiring revaccination, the interval between vaccinations should be at least 5 years.
- PCV13: the recommended routine immunization procedures are 1 dose each at 2, 4, and 6 months, and 1 dose of booster vaccination at 12–15 months. The first dose may be given as early as 6 weeks and the interval between each dose is 4–8 weeks. Older infants and children who have not been vaccinated according to immunization procedures: 7–11 months old infants should be given two doses at least 1 month apart each time. After 12 months, the 3rd dose should be given with an interval of at least 2 months from the 2nd vaccination. Children aged 12–23 months should receive two doses at least 2 months apart. Children aged 24 months to 5 years should receive one dose.
- PPV23: children aged ≥2 years who are at risk of Spn disease will be vaccinated with a PCV13 dose and then a PPV23 dose 8 weeks after inoculation to expand the range of immunoprotective Spn serotypes. For those requiring revaccination, the interval between vaccinations should be at least 5 years.
Conclusions
With a continuously developing society and improving economic conditions, the effective implementation of management and nursing measures in rolling out continuous vaccine strategies has meant that childhood incidence and mortality rates from RTIs have dropped significantly in the past ten years. Vaccines are one of the most successful discoveries in the modern medicine era, with current immunization programs highly effective in preventing, controlling, and eliminating common respiratory pathogen infections in children. Globally, more than 1 billion children have been vaccinated, and it is estimated that 2–3 million deaths potentially avoided every year (4). Bases on different immunization methods, vaccines may be divided into active immunization and passive immunization procedures. Similarly, based on the type and quantity of vaccine antigens, vaccines may be divided into monovalent, multivalent, and combined vaccines. Since 2007, China has expanded the scope of its National Immunization Program; vaccine types have been expanded from the original 6 to 14, and preventable infectious diseases have been expanded to 15 vaccine types. If a child needs to complete all immunizations, more than 20 vaccine doses are required before the age of 2. Too many vaccinations will not only increase social costs but also increase the risk of adverse events following immunization.
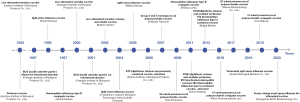
The development of a combined vaccine is the study direction; this will reduce the number of injections, the rate of missed and delayed immunizations, generate higher compliance and immunization coverage, and reduce management, storage, and transportation costs. Strictly speaking, PCV and PPV are combined vaccines. In foreign markets, six combination vaccines are currently available, e.g., the DTaP-IPV-Hib-HepB vaccine concurrently prevent pertussis, diphtheria, tetanus, poliomyelitis, hepatitis B, and Hi type b infections. Five combination vaccines are currently available in China. The only vaccines included in the immunization program are DTP and MMR vaccines, which means China still lags far behind international levels (Figure 1).
Influenza, Hib, and Spn vaccines are non-immunization program vaccines used in most areas of China, and individuals need to be vaccinated at their own expense voluntarily. It is our goal to include these vaccines in the National Immunization Program as soon as possible. To promote the coverage of children’s vaccinations in China and reduce the harm and economic burden of common respiratory pathogens to public health, the CDC has played an active role at all levels: on one hand, scientific popularization, health education, risk communication, and vaccine policy promotion activities have been successfully organized and conducted, whereas on the other hand, a vaccination plan has been formulated, implemented, and organized. This includes controlling technical aspects such as dosage form selection, vaccination procedures, and vaccination timing. However, concomitant with ongoing social and economic growth, improvements in health care levels, and increased immunoprophylaxis awareness, several immunoprophylaxis strategies require urgent attention. To realize the full potential of existing vaccines as barriers to respiratory pathogen infections, international and national communities must work together to broadly increase vaccine coverage.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the RIGHT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1613/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1613/coif). NZ serves as the Editor-in-Chief of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Coronavirus disease (COVID-19) pandemic. 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- World Health Organization. Measles vaccines: WHO position paper, April 2017 - Recommendations. Vaccine 2019;37:219-22. [Crossref] [PubMed]
- World Health Organization. Measles. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/measles
- World Health Organization. Immunization coverage. 2020. Available online: https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage
- American Academy of Pediatrics. Recommended Child and Adolescent Immunization Schedule for ages 18 years or younger, United States, 2020. Available online: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html#note-mmr
- Domínguez A, Torner N, Castilla J, et al. Mumps vaccine effectiveness in highly immunized populations. Vaccine 2010;28:3567-70. [Crossref] [PubMed]
- Marlow MA, Marin M, Moore K, et al. CDC Guidance for Use of a Third Dose of MMR Vaccine During Mumps Outbreaks. J Public Health Manag Pract 2020;26:109-15. [Crossref] [PubMed]
- Haralambieva IH, Ovsyannikova IG, Kennedy RB, et al. Rubella virus-specific humoral immune responses and their interrelationships before and after a third dose of measles-mumps-rubella vaccine in women of childbearing age. Vaccine 2020;38:1249-57. [Crossref] [PubMed]
- Pediatrician branch of Guangdong Medical Association. Expert consensus on vaccination for children with special status (Guangdong). Chinese Journal of Applied Clinical Pediatrics 2020;35:401-10.
- Sautto GA, Kirchenbaum GA, Ross TM. Towards a universal influenza vaccine: different approaches for one goal. Virol J 2018;15:17. [Crossref] [PubMed]
- National Immunization Advisory Committee Technical Working Group. Technical guidelines for seasonal influenza vaccination in China (2020-2021). Chinese Journal of Preventive Medicine 2020;54:1035-59. [PubMed]
- Ye C, Zhu W, Yu J, et al. Understanding the complex seasonality of seasonal influenza A and B virus transmission: Evidence from six years of surveillance data in Shanghai, China. Int J Infect Dis 2019;81:57-65. [Crossref] [PubMed]
- Zhou L, Yang H, Kuang Y, et al. Temporal patterns of influenza A subtypes and B lineages across age in a subtropical city, during pre-pandemic, pandemic, and post-pandemic seasons. BMC Infect Dis 2019;19:89. [Crossref] [PubMed]
- Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2020-21 Influenza Season. MMWR Recomm Rep 2020;69:1-24. [Crossref] [PubMed]
- Pepin S, Szymanski H, Rochín Kobashi IA, et al. Safety and immunogenicity of an intramuscular quadrivalent influenza vaccine in children 3 to 8 y of age: A phase III randomized controlled study. Hum Vaccin Immunother 2016;12:3072-8. [Crossref] [PubMed]
- Montomoli E, Torelli A, Manini I, et al. Immunogenicity and Safety of the New Inactivated Quadrivalent Influenza Vaccine Vaxigrip Tetra: Preliminary Results in Children ≥6 Months and Older Adults. Vaccines (Basel) 2018;6:14. [Crossref] [PubMed]
- Chu K, Xu K, Tang R, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine: A randomized, double-blind, controlled phase III study in healthy population aged ≥3 years. Vaccine 2020;38:5940-6. [Crossref] [PubMed]
- Feng LZ, Peng ZB, Wang DY, et al. Technical guidelines for influenza vaccination in China (2018-2019). Chinese Journal of Epidemiology 2018;39:1413-25. [PubMed]
- World Health Organization. Varicella. 2020. Available online: https://www.who.int/immunization/policy/Immunization_routine_table2.pdf?ua=1
- World Health Organization. Summary of WHO Position Papers - Recommended Routine Immunizations for Children. 2020. Available online: https://www.who.int/immunization/diseases/varicella/en/
- Hao B, Chen Z, Zeng G, et al. Efficacy, safety and immunogenicity of live attenuated varicella vaccine in healthy children in China: double-blind, randomized, placebo-controlled clinical trial. Clin Microbiol Infect 2019;25:1026-31. [Crossref] [PubMed]
- Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec 2014;89:265-87. [PubMed]
- Practice bulletin no. 151: Cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol 2015;125:1510-25. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule for ages 19 years or older, United States, 2021. Available online: https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html
- Wang X. Interpretation of the clinical practice guidelines to varicella zoster virus infection during pregnancy by ACOG. Chinese Journal of Practical Gynecology and Obstetrics 2016;32:508-10.
- Wilson E, Goss MA, Marin M, et al. Varicella vaccine exposure during pregnancy: data from 10 Years of the pregnancy registry. J Infect Dis 2008;197:S178-84. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. United States COVID-19 Cases and Deaths by State. 2020. Available online: https://covid.cdc.gov/covid-data-tracker/#cases
- Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med 2020;382:1663-5. [Crossref] [PubMed]
- Long XR, Zhu J, Zhao RQ, et al. Epidemiology and clinical features of highly pathogenic human coronavirus infection in children. Chinese Journal of Pediatrics 2020;58:351-4. [PubMed]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-42. [Crossref] [PubMed]
- Liu J, Luo WJ, Deng ZH, et al. Clinical and epidemiological characteristics of 91 children conformed with COVID-19. Chinese Journal of Nosocomiology 2020;30:1625-29.
- World Health Organization. COVID-19 vaccine tracker and landscape. 2021. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-15. [Crossref] [PubMed]
- Mahase E. Covid-19: Pfizer reports 100% vaccine efficacy in children aged 12 to 15. BMJ 2021;373: [Crossref] [PubMed]
- Vaccines and Related Biological Products Advisory Committee Meeting. FDA Briefing Document, Moderna COVID-19 Vaccine. 2021. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- Griffin S. Covid-19: AstraZeneca vaccine prevents 79% of symptomatic disease and 100% of severe disease, US study finds. BMJ 2021;372: [Crossref] [PubMed]
- GISAID. Map of tracked variant occurrence. 2021. Available online: https://www.gisaid.org/hcov19-variants/
- Wang GL, Wang ZY, Duan LJ, et al. Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization. N Engl J Med 2021;384:2354-6. [Crossref] [PubMed]
- China National Clinical Research Center for Respiratory Diseases. Chinese Medical Doctor Association Committee on Respirology Pediatrics, et al. Chinese experts’ consensus statement on diagnosis, treatment and prevention of respiratory syncytial virus infection in children. Chinese Journal of Applied Clinical Pediatrics 2020;35:241-50.
- Chen X, Xu B, Guo J, et al. Genetic variations in the fusion protein of respiratory syncytial virus isolated from children hospitalized with community-acquired pneumonia in China. Sci Rep 2018;8:4491. [Crossref] [PubMed]
- Global Burden of Disease Pediatrics Collaboration. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr 2016;170:267-87. [Crossref] [PubMed]
- Manti S, Cuppari C, Lanzafame A, et al. Detection of respiratory syncytial virus (RSV) at birth in a newborn with respiratory distress. Pediatr Pulmonol 2017;52:E81-4. [Crossref] [PubMed]
- Yu J, Liu C, Xiao Y, et al. Respiratory Syncytial Virus Seasonality, Beijing, China, 2007-2015. Emerg Infect Dis 2019;25:1127-35. [Crossref] [PubMed]
- American Academy of Pediatrics Committee on Infectious Diseases. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134:415-20. [Crossref] [PubMed]
- Griffin MP, Yuan Y, Takas T, et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N Engl J Med 2020;383:415-25. [Crossref] [PubMed]
- Lynch JP 3rd, Kajon AE. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin Respir Crit Care Med 2016;37:586-602. [Crossref] [PubMed]
- Li L, Woo YY, de Bruyne JA, et al. Epidemiology, clinical presentation and respiratory sequelae of adenovirus pneumonia in children in Kuala Lumpur, Malaysia. PLoS One 2018;13:e0205795. [Crossref] [PubMed]
- Xie L, Zhang B, Xiao N, et al. Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China. J Med Virol 2019;91:392-400. [Crossref] [PubMed]
- Shachor-Meyouhas Y, Hadash A, Kra-Oz Z, et al. Adenovirus Respiratory Infection among Immunocompetent Patients in a Pediatric Intensive Care Unit During 10-year period: Co-morbidity is common. Isr Med Assoc J 2019;21:595-8. [PubMed]
- Yao LH, Wang C, Wei TL, et al. Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017-2018. Virol J 2019;16:78. [Crossref] [PubMed]
- Lu MP, Ma LY, Zheng Q, et al. Clinical characteristics of adenovirus associated lower respiratory tract infection in children. World J Pediatr 2013;9:346-9. [Crossref] [PubMed]
- Li Y, Zhou W, Zhao Y, et al. Molecular typing and epidemiology profiles of human adenovirus infection among paediatric patients with severe acute respiratory infection in China. PLoS One 2015;10:e0123234. [Crossref] [PubMed]
- Hoke CH Jr, Snyder CE Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine 2013;31:1623-32. [Crossref] [PubMed]
-
World Health Organization 2020 . Available online: https://www.who.int/health-topics/pertussis#tab=tab_1 - Xu M, Lei YL, Tan K, et al. Clinical analysis of 309 hospitalized children with pertussis associated pneumonia. Chinese Journal of Pediatrics 2018;56:686-90. [PubMed]
- Barger-Kamate B, Deloria Knoll M, Kagucia EW, et al. Pertussis-Associated Pneumonia in Infants and Children From Low- and Middle-Income Countries Participating in the PERCH Study. Clin Infect Dis 2016;63:S187-96. [Crossref] [PubMed]
- Ning GJ, Gao Y, Wu D, et al. Epidemiology of pertussis in China, 2011-2017. Chinese Journal of Vaccines and Immunization 2018;24:264-67, 73.
- WHO. Pertussis vaccines: WHO position paper, August 2015--Recommendations. Vaccine 2016;34:1423-5. [Crossref] [PubMed]
- Liang JL, Tiwari T, Moro P, et al. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018;67:1-44. [Crossref] [PubMed]
- McGirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics 2015;135:331-43. [Crossref] [PubMed]
- Duan LN, Fang Q, Liu G, et al. Retrospective investigation on vaccine immunization history of 334 cases of pertussis in Shenzhen. Chinese Journal of Infectious Diseases 2020;38:294-96.
- Jia JH, Guo Q, Wan CM. Resurgence and vaccine strategies of pertussis. Chinese Journal of Pediatrics 2020;58:686-89. [PubMed]
- Kandeil W, van den Ende C, Bunge EM, et al. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev Vaccines 2020;19:621-38. [Crossref] [PubMed]
- Chinese Centers for Disease Control and Prevention. Experts from Chinese Centers for Disease Control and Prevention answer questions about vaccination. 2020. Available online: http://www.chinacdc.cn/jkzt/ymyjz/ymlbgd/201807/t20180725_189214.html
- Clarke KEN, MacNeil A, Hadler S, et al. Global Epidemiology of Diphtheria, 2000-20171. Emerg Infect Dis 2019;25:1834-42. [Crossref] [PubMed]
- World Health Organization. Diphtheria reported cases. 2020. Available online: https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencediphtheria.html
- Demicheli V, Barale A, Rivetti A. Vaccines for women for preventing neonatal tetanus. Cochrane Database Syst Rev 2015;CD002959. [Crossref] [PubMed]
- Diphtheria vaccine: WHO position paper – August 2017. Wkly Epidemiol Rec 2017;92:417-35. [PubMed]
- World Health Organization. Global Tuberculosis Report 2018. Geneva: WHO, 2019. 2020. Available online: https://www.who.int/tb/publications/global_report/en/
- Immunization procedures and instructions for children under the national immunization plan (2020 Edition). Available online: http://www.pengze.gov.cn/zw/02/04/003/0007/202012/P020201201617772985589.pdf
- Badurdeen S, Marshall A, Daish H, et al. Safety and Immunogenicity of Early Bacillus Calmette-Guérin Vaccination in Infants Who Are Preterm and/or Have Low Birth Weights: A Systematic Review and Meta-analysis. JAMA Pediatr 2019;173:75-85. [Crossref] [PubMed]
- Li JH, Wu D, Yin ZD, et al. Analysis of epidemic characteristics for meningococcal meningitis in China during 2015-2017. Chinese Journal of Preventive Medicine 2019;53:159-63. [PubMed]
- Zhang DW, Li JX, Hu JL, et al. Research progress of maternal immunization. Chinese Journal of Preventive Medicine 2019;53:534-9. [PubMed]
- The Subspeciality Group of Infectious Diseases. the Society of Pediatrics, Chinese Medical Association; the Group of Infectious Disease Surveillance of Pediatrics; the Editorial Board, Chinese Journal of Pediatrics, et al. Expert consensus on diagnosis and treatment of Haemophilus influenzae infection in children. Chinese Journal of Pediatrics 2019;57:663-8.
- Whittaker R, Economopoulou A, Dias JG, et al. Epidemiology of Invasive Haemophilus influenzae Disease, Europe, 2007-2014. Emerg Infect Dis 2017;23:396-404. [Crossref] [PubMed]
- Yang Y, Yang Y, Scherpbier RW, et al. Coverage of Haemophilus influenzae Type b Conjugate Vaccine for Children in Mainland China: Systematic Review and Meta-analysis. Pediatr Infect Dis J 2019;38:248-52. [Crossref] [PubMed]
- Liu Y, Xu EP, Wang J, et al. Influencing Factors of Haemophilus Influenzae Type b Polysaccharide Conjugate Vaccine Vaccination in Children in Hangzhou City. Chinese Journal of Vaccines and Immunization 2015;21:84-7.
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018;18:1191-210. [Crossref] [PubMed]
- Wahl B, O'Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health 2018;6:e744-57. [Crossref] [PubMed]
- Men W, Dong Q, Shi W, et al. Serotype distribution and antimicrobial resistance patterns of invasive pneumococcal disease isolates from children in mainland China-a systematic review. Braz J Microbiol 2020;51:665-72. [Crossref] [PubMed]
- Chinese Preventive Medicine Association, Vaccine and Immunology Branch of Chinese Preventive Medicine Association. Expert consensus on immunization for prevention of pneumococcal disease in China (2017). Chinese Journal of Epidemiology 2018;39:111-38. [PubMed]