
Robotic versus thoracoscopic combined anatomic subsegmentectomy for early-stage lung cancer: early results of a cohort study
Introduction
For small pulmonary lesions, especially those with ground-glass opacity, as long as sufficient margins and staging of lymph node are fully acquired, a sublobar resection can be regarded as an option to lobectomy (1,2). Many recent studies have shown that sublobar resection results in equivalent oncologic outcomes in patients with stage I non-small cell lung cancer (NSCLC) (3-6). Recently, a phase III randomized trial (JCOG0802/WJOG4607L) (7)demonstrated that patients obtain more benefits from segmentectomy than lobectomy in terms of overall survival and post-surgery pulmonary function, which suggests that segmentectomy may be considered as the standard treatment for early-stage peripheral NSCLC.
As for intersegmental pulmonary nodules located between adjacent segments or at the edge of the diseased segments, single segmental resection is arduous to ensure a safe margin (8,9). Intersegmental pulmonary nodules resected by extended segmental resection is essentially a wedge resection with the added potential problem of insufficient margins (10). To solve this, combined anatomic subsegmentectomy (CAS) is performed to ensure a safe incisional margin by placing intersegmental nodules in the central area of the adjacent involved subsegments. The advantages of CAS are that it can offer a better margin for intersegmental nodules (10-12) and result in the retention of more lung parenchyma (9), since the removal of two subsegments is essentially equivalent to the removal of a lung segment.
However, hilar dissection in anatomical segmentectomies or CAS via video-assisted thoracic surgery (VATS) can be technically more complicated than lobectomy (13). With the increasing use of robotic surgery for sublobectomy, robotic systems are more likely to promote segmental lung resection because robotic-assisted thoracic surgery (RATS) has shown superiority over conventional VATS, with its use of three-dimensional vision, greater flexibility, and better assistance for surgeons (14). RATS also has disadvantages, such as unsatisfactory tactile feedback and higher costs (15,16).
To the best of our knowledge, there is no evidence to confirm if RATS CAS could provide the same perioperative and oncological results as VATS CAS, although there have been a few reports on the application of thoracoscopic and robotic surgery for subsegmental lung resection (9,12,17). Herein, we collected and presented the perioperative outcomes of 30 patients who underwent RATS CAS and 32 patients who underwent VATS CAS. The aim of our study was to retrospectively analyze and compare oncological safety, early results, and the costs of CAS using the RATS and VATS methods in early NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1895/rc).
Methods
Patients population
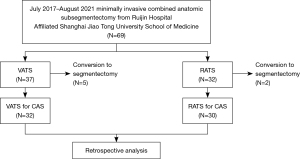
We conducted a retrospective study of 62 consecutive patients who underwent minimally invasive CAS using the RATS or VATS approach for early-stage NSCLC at Shanghai Ruijin Hospital between July 2017 and August 2021. Retrospective analysis of the data was performed using a system approved by the Investigation Review Board of Ruijin Hospital (KY201996), including appropriate patient identification of those enrolled for the purpose of privacy protection. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Investigation Review Board of Ruijin Hospital (No. KY201996). Because of its retrospective nature, informed patient consent was not required. CAS is defined as the removal of two or more subsegments during surgery, usually when the small pulmonary nodules involve two or more adjacent segments. In this study, we systematically reviewed the clinical data of 62 patients who underwent successful CAS. Some of these patients entered clinical experiments NCT03192904 or NCT03134534 at the department. As described in an earlier study (12), patients were required to meet the following eligibility criteria to receive CAS for early-stage lung cancer in the series: (I) nodules ≤2 cm in size with 50% or more ground-glass appearance on computed tomography (CT); (II) strongly suspected stage I NSCLC; (III) pulmonary lesions involving two or more adjacent segments; and (IV) patient performance status ≤1 or sufficient organ function. All patients made a choice for their surgical approach based on their own preference. Seven cases were excluded from this study due to the intraoperative change of surgical plan (Figure 1). Hook–wire localization assisted by CT angiography and bronchography (Xudong, China) was optionally applied based on patient’s condition.
Operative preparations and postoperative treatment protocols were similar for RATS CAS and VATS CAS. The clinical data of baseline characteristics, perioperative results, and pathological outcomes were collected from the electronic medical records of each patient. The perioperative period corresponds to the entire process surrounding the operation, including the periods before, during, and after the surgery. Specifically, this begins from the time that the surgical treatment was confirmed (approximately 5–7 days before surgery) until the treatment related to the surgery was completed (7–12 days after surgery). The follow-up period to determine mortality outcomes was approximately 30 days via telephone. The percent forced expiratory volume in 1 second (%FEV1) was divided into three categories: ≥80%, 50–80%, and<50%, based on the Global Initiative for Chronic Obstructive Disease classification of airflow limitation severity in chronic obstructive pulmonary disease. Clavien–Dindo classifications were used to describe perioperative complications. The 8th edition of the Tumor Node Metastasis (TNM) classification was applied to evaluate the pathological stages of the patients. Since the postoperative pathological N outcomes of all patients in this study were pN0, N upstaging was no longer mentioned. Regarding the cost analysis, the finance department was responsible for calculating of total cost for every patient, representing both the total direct and indirect costs. Direct cost refers to any item used for patient care, including all disposable items during operation, staplers, laboratory tests, imaging tests, and medications. Indirect cost refers to extra cost during in-hospital stay, comprised of overhead costs and amortization of capital equipment, which includes the cost of purchasing and maintaining these surgical platforms, specifically the videoscope or the da Vinci robot.
Operative techniques
RATS CAS was performed by using the da Vinci robot (Model S/Si; Intuitive Surgical, California, USA) equipped with 4 arms (Video 1) as we mentioned in the earlier studies (12,13). Meanwhile, videoscope guidance (Karl Storz, Tuttlingen, Germany) was applied during the VATS CAS procedure via 1 incision (Video 2). The main steps for both types of CAS were performed in a similar manner. The first step is dissection of the anatomical target structure at the segmental hilum with silk thread or staplers. Subsequently, the surgeons need to precisely identify inter-subsegmental planes. The surgeons should then accurately separate the intersegmental plane. Arteries and veins were clipped with Hem-o-Lok (Teleflex, Morrisville, NC) or stapled with a vascular stapler. Bronchus was subsequently isolated and stapled. The imaginary intersegmental plane was stapled after ventilating and deflating the remnant lung. All operations were performed by one general thoracic surgeon (H. L.), and clinical assessments were conducted according to National Comprehensive Cancer Network Guidelines. it was imperative to first confirm N0 status. N1 and N2 node resection and mapping is a routine of lung cancer surgery in this study, as well as No.12 and No.13 lymph nodes. and a minimum of three N2 stations should be sampled or a standardized lymph node dissection. If enlarged nodes (>1 cm) or positive margins were identified, a frozen section analysis was performed.
Statistical analysis
Statistical analysis was performed by the SPSS software (version 22.0; International Business Machines Corporation, Armonk, NY, USA). Given that the measured data had a normal distribution, homogeneity of variance was analyzed using T-tests or Wilcoxon rank sum tests, and reported as mean ± standard deviation. Categorical data were compared using the χ2 tests or Fisher’s exact tests and reported as n (%).
Results
Patient characteristics
A total of 62 patients who underwent CAS via RATS (n=30) or VATS (n=32) met the selection criteria and were enrolled in this study. The patients’ demographic and clinical characteristics are summarized in Table 1. The RATS and VATS cohorts were comparable in terms of age, gender, body mass index, %FEV1, American Society of Anesthesiologists (ASA) score, smoking history, and approximate tumor size. No significant differences were observed in the baseline characteristics of the patients.
Table 1
| Characteristic | RATS (n=30) | VATS (n=32) | P value |
|---|---|---|---|
| Age, years | 46.97±10.81 | 50.47±12.60 | 0.25 |
| Gender | 0.65 | ||
| Male | 12 (40.0) | 11 (34.38) | |
| Female | 18 (60.0) | 21 (65.62) | |
| BMI | 23.44±2.48 | 22.70±2.38 | 0.24 |
| %FEV1 | 0.55 | ||
| ≥0.8 | 20 (66.67) | 19 (59.28) | |
| 0.5–0.8 | 10 (33.32) | 13 (40.62) | |
| <0.5 | 0 | 0 | |
| ASA score | 0.42 | ||
| 1 | 8 (26.67) | 6 (18.75) | |
| 2 | 21 (70.0) | 26 (81.25) | |
| 3 | 1 (3.33) | 0 | |
| Tobacco use | 0.46 | ||
| Current smokers | 3 (10.0) | 1 (3.12) | |
| Abstained for at least 1 year | 5 (16.67) | 4 (12.5) | |
| Never | 22 (73.33) | 27 (84.38) | |
| Hook-wire localization | 13 (43.33) | 11 (32.35) | 0.47 |
| Tumor size (cm) | 0.83±0.27 | 0.8±0.22 | 0.67 |
Mean ± standard deviation (SD) is used to describe continuous data, and n (%) is used to describe categoric data. RATS, robot-assisted thoracic surgery; VATS, video-assisted thoracic surgery; BMI, body mass index; %FEV1, percent forced expiratory volume in 1 second; ASA, American Society of Anesthesiologists.
Types of CAS
The types of CAS performed by RATS or VATS are shown in Table 2. The nodules are more commonly located on the right lung; specifically, 19 patients (63.33%) in the RATS group, and 22 patients (68.75%) in VATS group underwent right-sided CAS. Of all the cases, S2b + S3a was the most common CAS performed in both lung lobes, followed by S1a + S2. There was no significant difference in nodule location (P=0.65) or the type of CAS performed (P=0.19).
Table 2
| Surgery category | RATS (n=30) | VATS (n=32) | P value |
|---|---|---|---|
| Location of nodules | 0.65 | ||
| Right | 19 (63.33) | 22 (68.75) | |
| Left | 11 (36.67) | 10 (31.25) | |
| Type of CAS | 0.19 | ||
| Right lung | |||
| S1 + S2a | 0 | 2 | |
| S1 + S3b | 2 | 1 | |
| S1a + S2 | 4 | 4 | |
| S1a + S2a | 1 | 4 | |
| S1b + S3 | 0 | 3 | |
| S1b + S3b | 0 | 1 | |
| S2 + S3a | 0 | 1 | |
| S2b + S3a | 6 | 5 | |
| S6 + S8a | 2 | 0 | |
| S6b + S* | 1 | 0 | |
| S6b + S8a | 1 | 1 | |
| S8a + S9 | 2 | 0 | |
| Left lung | |||
| S1+2 + S3c | 3 | 0 | |
| S1+2a + b + S3c | 0 | 2 | |
| S1+2a + S3 | 2 | 1 | |
| S1+2c + S3a | 0 | 2 | |
| S1+2c + S4 | 1 | 3 | |
| S6 + S10a + S* | 1 | 0 | |
| S6 + S8a | 1 | 0 | |
| S6b + S7+8 | 1 | 0 | |
| S6b + S8a | 1 | 1 | |
| S9b + S10 | 1 | 0 | |
| S9b + S10c | 0 | 1 | |
N (%) is used to describe categoric data. RATS, robot-assisted thoracic surgery; VATS, video-assisted thoracic surgery; CAS, combined anatomic subsegmentectomy; S*, subsuperior segment.
Perioperative results
The perioperative results are summarized in Table 3. There were no in-hospital and 30-day mortalities. There was no significant difference between RATS and VATS in the operative duration (127.23±22.99 vs. 128.13±35.42 min; P=0.91), intraoperative blood loss {50 mL [interquartile range (IQR), 30–100 mL] vs. 100 mL (IQR, 50–100 mL); P=0.70}, and the rate of overall complications (23.33% vs. 29.41%; P=0.58). Furthermore, no significant difference was found in length of hospital stay [4 days (IQR, 3.5–5 days) vs. 3.75 days (IQR, 3–4.75 days); P=0.71] and mean duration of drainage [2.5 days (IQR, 2–3 days) vs. 2 days (IQR, 2–3 days); P=0.12]. One readmission occurred in the RATS group because of multiple small discrete lung nodules distributed throughout both lungs, resulting in a second surgery.
Table 3
| Characteristic | RATS (n=30) | VATS (n=32) | P value |
|---|---|---|---|
| Operative duration, min | 127.23±22.99 | 128.13±35.42 | 0.91 |
| Blood loss, median [IQR], mL | 50 [30–100] | 100 [50–100] | 0.70 |
| 30-day morbidity | 7 (23.33) | 10 (29.41) | 0.58 |
| Clavien I–II | 6 | 8 | – |
| Atrial fibrillation | 1 | 2 | – |
| Air leak | 1 | 1 | – |
| Pleural effusion | 1 | 0 | – |
| Wound infection | 0 | 1 | – |
| Pneumonia | 3 | 4 | – |
| Clavien III–IV | 1 | 2 | – |
| Air leak | 0 | 1 | – |
| Pleural effusion | 0 | 1 | – |
| Pneumonia | 1 | 0 | – |
| Readmission, n (%) | 1 | 0 | – |
| In-hospital mortality | 0 | 0 | – |
| 30-day mortality | 0 | 0 | – |
| Duration of drainage, median (IQR), d | 2.5 (2.0–3.0) | 2 (2.0–3.0) | 0.12 |
| PLOS, median (IQR), d | 4 (3.5–5.0) | 3.75 (3.0–4.75) | 0.71 |
Mean ± SD is used to describe continuous data, and n (%) is used to describe categoric data. RATS, robot-assisted thoracic surgery; VATS, video-assisted thoracic surgery; IQR, interquartile range; PLOS, postoperative length of stay.
Pathologic results
The pathologic results of patients in the RATS and VATS groups are presented in Table 4. An R0 resection was achieved in all patients. The most common pathological type was adenocarcinoma (93.33% vs. 84.38%; P=0.44). There is no significant difference in the parenchymal margins, and the distribution of the T stage was statistically comparable between two groups. As for the pulmonary lymph node, there were more N1 lymph nodes [3 (IQR, 2–5) vs. 2 (IQR, 1–2); P=0.002] and N1 stations [2.5 (IQR, 1–3) vs. 2 (IQR, 1–2); P=0.008] dissected in the RATS group compared to the VATS group, and the same results were found for the number [2 (IQR, 1–3) vs. 1 (IQR, 0–2); P=0.007] and the stations [2 (IQR, 1–3) vs. 1 (IQR, 0–2); P=0.009] of N2 lymph nodes dissected.
Table 4
| Characteristic | RATS (n=30) | VATS (n=32) | P value |
|---|---|---|---|
| Histology lung cancer | 0.44 | ||
| Adenocarcinoma | 28 (93.33) | 27 (84.38) | |
| Squamous-cell carcinoma | 0 | 1 (3.12) | |
| Other | 2 (6.67) | 4 (12.5) | |
| R0 resection | 30 (100.0) | 32 (100.0) | – |
| Parenchymal margins, median (IQR), cm | 2.15 (1.88–2.40) | 2 (1.8–2.18) | 0.30 |
| pT stage lung cancer | 0.13 | ||
| Benign | 1 (3.33) | 3 (9.38) | |
| Tis | 1 (3.33) | 4 (12.5) | |
| T1a | 21 (70.0) | 23 (71.88) | |
| T1b | 7 (23.34) | 2(6.24) | |
| pN stage lung cancer | – | ||
| N0 | 30 (100.0) | 32 (100.0) | |
| N1 | 0 | 0 | |
| LN1 stations, median [IQR] | 2.5 [1–3] | 2 [1–2] | 0.008 |
| LN2 stations, median [IQR] | 2 [1–3] | 1 [0–2] | 0.009 |
| No. of LN1, median [IQR] | 3 [2–5] | 2 [1–2] | 0.002 |
| No. of LN2, median [IQR] | 2 [1–3] | 1 [0–2] | 0.007 |
N (%) is used to describe categoric data. RATS, robot-assisted thoracic surgery; VATS, video-assisted thoracic surgery; IQR, interquartile range; LN, lymph node.
Analysis of cost
The comparative cost analysis between the two surgical approaches is presented in Table 5. There was a significantly higher mean total cost ($13,617.6±1,316.84 vs. $9,253.13±1,926.99; P<0.001) and mean indirect cost ($5,983.07±176.67 vs. $1,553.52±943.52; P<0.001) in the RATS CAS group compared to the VATS CAS group. Nevertheless, no significant difference was found in the mean of direct cost ($7,634.53±1,356.23 vs. $7,699.61±1,719.14; P=0.87) between the two groups.
Table 5
| Type of cost | RATS (n=30) | VATS (n=32) | P value |
|---|---|---|---|
| Total cost ($) | 13,617.6±1,316.84 | 9,253.13±1,926.99 | <0.001 |
| Direct cost ($) | 7,634.53±1,356.23 | 7,699.61±1,719.14 | 0.87 |
| Indirect cost ($) | 5,983.07±176.67 | 1,553.52±943.52 | <0.001 |
Mean ± SD is used to describe continuous data. RATS, robot-assisted thoracic surgery; VATS, video-assisted thoracic surgery.
Discussion
Rapid advances in minimally invasive thoracic surgery have resulted in the clinical application of robot-assisted systems, which has facilitated the development of anatomical pulmonary dissection. Previous research (18) has shown that robotic segmentectomies are safe, effective, and offer excellent perioperative results. Our team has recently conducted a multi-institutional retrospective analysis (13) comparing the early results of segmentectomy via robot-assisted and thoracoscopic-assisted thoracic surgery for the patients with early-stage lung cancer and reported comparable results and oncologic safety in the short term. Here, we report the first analysis to compare the short-term results of CAS using robotic and thoracoscopic approaches for patients with lung cancer at an early stage.
To the best of our knowledge, there are only a few publications (7,8,19,20) focusing on the application of VATS CAS and only our previous research (12) focusing on the use of robot-assisted systems for CAS. In this study, the short-term comparative results of RATS and VATS CAS for patients with lung cancer were prudently analyzed for the first time. Our outcomes demonstrated that for early NSCLC, RATS CAS offers perioperative results similar to those of VATS CAS. No significant difference was observed in operative duration, intraoperative blood loss, rate of overall complications, mean duration of drainage and length of in-hospital stay. Our experience also suggests that surgeons may be able to obtain plenty of benefits from arm 3 in the robotic system when performing CAS. Specifically, control of small bleeders using arm 3 is more straightforward and convenient, leaving arms 1 and 2 available for operative procedures elsewhere (12).
Another significant finding was that RATS CAS might contribute to the potential improvement of N1 and N2 lymph node retrieval in early-stage NSCLC. Assessment of lymph nodes (LNs) includes the numbers of individual lymph nodes and stations dissected in sublobectomy for lung cancer (19,21). In this work, higher numbers of N1 and N2 LNs and stations were dissected in the RATS group. Our findings concur with those of recent studies (13,22-24). Li et al. (22) reported that RATS, compared with VATS, is associated with more lymph nodes stations examined and a higher number of LNs harvested. This could be attributed to the three-dimensional imaging, high-definition visualization, better maneuverability, and improved dexterity provided by the robot-assisted system, which endows the surgeon with better dissection capabilities for LNs around vessels and bronchi (23,24). Although all lymph nodes dissected from both groups of patients in our study were negative for metastasis, there may be a potential tendency to understage patients if adequate lymph node dissection is not performed. The sizes of the dissected lymph nodes are of great importance for precise and accurate pathological staging, as there may be latent neglected positive lymph node metastasis. The latest National Comprehensive Cancer Network guidelines also emphasize the importance of adequate lymph node dissection in sublobar resection. Extended segmental resection not only involves the removal of the affected and adjacent segments but also includes aggressive dissection of LNs surrounding the bronchi of the affected subsegment, as well as the hilum and mediastinum (25). However, in this study, the patient’s survival benefit from the additional number of LNs and stations dissected from the RATS group may have been negligible. Dezube et al. (26) recently reported a correlation between the number of LNs harvested and long-term survival according to the National Cancer Database, and they found that, for lobectomy, the optimal number of LNs dissected is four, with no survival benefit when additional LN sampling was performed. Further studies, preferably randomized controlled trials, are required to clarify the clinical relevance between the improved LNs harvest and long-term survival in the RATS group.
One of the major concerns regarding the application of RATS is its economic viability. Some studies (27,28) have compared the cost of RATS and VATS and found that the cost of robotic surgery is consistently higher, while others reported no significant difference in cost (29). Musgrove et al. (30) recently reported that the direct costs of robotic segmental resection and thoracoscopic segmental resection were comparable, although the total cost and indirect cost of the robot group were higher. Similar outcomes were observed in our study, with a significantly higher mean total cost and mean indirect cost in the RATS CAS group compared to the VATS CAS group. Additionally, no significant difference was observed in the mean direct cost between the groups. The Chinese’s National Medical Insurance System can cover part of the cost comes from these two types of surgery in the perioperative period. However, no operation cost was covered in RATS CAS. This means the indirect cost of RATS CAS is approximately $4,000 more than that of the VATS group, which mainly includes replenishment and depreciation of robot specifications.
This study has some limitations. First, the non-randomized retrospective design of this study may have created bias in patient selection. Second, the application of robotic systems is based on the patients’ economic levels and preferences, which may also lead to selection bias. Furthermore, although the difference in N1 and N2 lymph node dissection may reveal the potential superiority of robotic systems in the dissection of pulmonary LN, to some extent, the caution of surgeons in identifying or labeling these pulmonary lymph nodes also affects the outcomes. Finally, the purpose of this retrospective study was to obtain perioperative results in the short term with a small number of patients treated; a longer follow-up and larger sample population is needed to compare the efficacy of these two methods. Nevertheless, this study provides highly encouraging results regarding the potential benefits of RATS for CAS.
In conclusion, the perioperative results of RATS and VATS CAS in patients with early-stage NSCLC were compared. RATS CAS may contribute to the potential improvements in N1 and N2 lymph node retrieval.
Acknowledgments
We would like to thank Sabrina Anne from Editage for her help in polishing our paper.
Funding: The study was supported by the grant from National Natural Science Foundation of China (No. 81871882); Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (No. 20172005); and Shanghai Municipal Commission of Health and Family Planning Outstanding Academic Leaders Training Program (No. 2017BR055).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1895/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1895/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1895/prf
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1895/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Investigation Review Board of Ruijin Hospital (No. KY201996). Because of its retrospective nature, informed patient consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schuchert MJ, Normolle DP, Awais O, et al. Factors influencing recurrence following anatomic lung resection for clinical stage I non-small cell lung cancer. Lung Cancer 2019;128:145-51. [Crossref] [PubMed]
- Song CY, Sakai T, Kimura D, et al. Comparison of perioperative and oncological outcomes between video-assisted segmentectomy and lobectomy for patients with clinical stage IA non-small cell lung cancer: a propensity score matching study. J Thorac Dis 2018;10:4891-901. [Crossref] [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; Discussion 762-4. [Crossref] [PubMed]
- Koike T, Koike T, Sato S, et al. Lobectomy and limited resection in small-sized peripheral non-small cell lung cancer. J Thorac Dis 2016;8:3265-74. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 472. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Wu W, He Z, Xu J, et al. Anatomical Pulmonary Sublobar Resection Based on Subsegment. Ann Thorac Surg 2021;111:e447-50. [Crossref] [PubMed]
- Yoshimoto K, Nomori H, Mori T, et al. Combined subsegmentectomy: postoperative pulmonary function compared to multiple segmental resection. J Cardiothorac Surg 2011;6:17. [Crossref] [PubMed]
- Chang CC, Yen YT, Lin CY, et al. Single-port video-assisted thoracoscopic surgery subsegmentectomy: The learning curve and initial outcome. Asian J Surg 2020;43:625-32. [Crossref] [PubMed]
- Wu WB, Xia Y, Pan XL, et al. Three-dimensional navigation-guided thoracoscopic combined subsegmentectomy for intersegmental pulmonary nodules. Thorac Cancer 2019;10:41-6. [Crossref] [PubMed]
- Li C, Han Y, Han D, et al. Robotic Approach to Combined Anatomic Pulmonary Subsegmentectomy: Technical Aspects and Early Results. Ann Thorac Surg 2019;107:1480-6. [Crossref] [PubMed]
- Zhang Y, Chen C, Hu J, et al. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: A multi-institutional propensity score-matched analysis. J Thorac Cardiovasc Surg 2020;160:1363-72. [Crossref] [PubMed]
- Veronesi G. Robotic surgery for the treatment of early-stage lung cancer. Curr Opin Oncol 2013;25:107-14. [Crossref] [PubMed]
- Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. [Crossref] [PubMed]
- Toker A, Ayalp K, Uyumaz E, et al. Robotic lung segmentectomy for malignant and benign lesions. J Thorac Dis 2014;6:937-42. [PubMed]
- Okamoto K, Hanaoka J. Surgical outcome of combined subsegmentectomy in the right upper lobe for GGO -dominant early stage lung cancer: Analysis of 7 cases. Respir Med Case Rep 2019;26:123-5. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016;8:S710-5. [Crossref] [PubMed]
- Nakada T, Akiba T, Inagaki T, et al. Thoracoscopic anatomical subsegmentectomy of the right S2b + S3 using a 3D printing model with rapid prototyping. Interact Cardiovasc Thorac Surg 2014;19:696-8. [Crossref] [PubMed]
- Li C, Zheng B, Yu Q, et al. Augmented Reality and 3-Dimensional Printing Technologies for Guiding Complex Thoracoscopic Surgery. Ann Thorac Surg 2021;112:1624-31. [Crossref] [PubMed]
- Li JT, Liu PY, Huang J, et al. Perioperative outcomes of radical lobectomies using robotic-assisted thoracoscopic technique vs. video-assisted thoracoscopic technique: retrospective study of 1,075 consecutive p-stage I non-small cell lung cancer cases. J Thorac Dis 2019;11:882-91. [Crossref] [PubMed]
- Mungo B, Hooker CM, Ho JS, et al. Robotic Versus Thoracoscopic Resection for Lung Cancer: Early Results of a New Robotic Program. J Laparoendosc Adv Surg Tech A 2016;26:243-8. [Crossref] [PubMed]
- Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann Surg 2022;275:295-302. [Crossref] [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961. [Crossref] [PubMed]
- Dezube AR, Mazzola E, Bravo-Iñiguez CE, et al. Analysis of Lymph Node Sampling Minimums in Early Stage Non-Small-Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2021;33:834-45. [Crossref] [PubMed]
- Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest 2014;146:1505-12. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Nelson DB, Mehran RJ, Mitchell KG, et al. Robotic-Assisted Lobectomy for Non-Small Cell Lung Cancer: A Comprehensive Institutional Experience. Ann Thorac Surg 2019;108:370-6. [Crossref] [PubMed]
- Musgrove KA, Hayanga JA, Holmes SD, et al. Robotic Versus Video-Assisted Thoracoscopic Surgery Pulmonary Segmentectomy: A Cost Analysis. Innovations (Phila) 2018;13:338-43. [Crossref] [PubMed]


