
Clinical characteristics and determinants of mortality in coronavirus disease 2019 (COVID-19) patients on an intensive care unit—a retrospective explorative 1-year all-comers study
Introduction
Infection with severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) may cause coronavirus disease 2019 (COVID-19) and is associated with a high mortality in patients admitted to intensive care units (ICU) (1,2). Mortality risk has been linked with several parameters, including advanced age, male gender, body mass index (BMI), multi-organ failure, and associated laboratory abnormalities (3-5). Care of these critically ill patients is challenging (6,7). The goal of therapy is mainly to avoid complications, to prevent disease deterioration and to support recovery (1,2). Regarding respiratory support, the use of non-invasive ventilation (NIV) has evolved during the 1st and 2nd wave of the pandemic (8,9), with initially high rates of early intubation partly due to concerns of viral transmission to physicians and health care staff. At our hospital, NIV has early on been proposed as the main type of respiratory support to avoid intubation and mechanical ventilation (10,11). Therefore, many patients admitted to our hospital were initially referred to a “COVID-19 intermediate care unit” with high rates of NIV (10,11), unless they had concomitant diseases other than COVID-19 that necessitated ICU therapy. This led to identification of two distinct groups of patients, i.e., those with COVID-19 and those with SARS-CoV-2 infection as comorbidity, e.g., with sepsis, severe heart or renal failure, etc., as the leading cause for ICU admission. In some patients, respiratory function deteriorated and they were escalated to invasive mechanical ventilation (IMV) according to guidelines (1,12,13). Despite all efforts, mortality rates appeared to be high. It is therefore the aim of this exploratory study to examine the clinical course of patients admitted to our ICU in this setting and to identify predictors of intubation and mortality. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1713/rc).
Methods
Study design, data collection and ethics
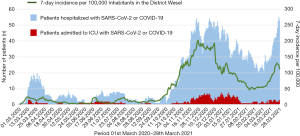
We conducted a single-center retrospective observational study that per protocol included all patients admitted to our ICU between March 2020 and March 2021 (Figure 1). All parameters reported in this study were measured as part of clinical routine. We searched our hospital database for all patients that were coded as having SARS-CoV-2 infection and that were admitted to our ICU. A SARS-CoV-2 infection was determined by polymerase chain reaction (PCR) and supported by clinical criteria, laboratory values, and chest computed tomography (CT). We distinguished patients admitted due to COVID-19, from those admitted with SARS-CoV-2 infection as comorbidity, i.e., primarily due to acute heart failure, renal failure, etc. without direct relation to SARS-CoV-2. Precautions of viral transmission and the same medical protocol were applied in all patients. The study complies with the Declaration of Helsinki (as revised in 2013), and was approved by the ethics committee of University Duisburg-Essen (#21-9911-BO). Due to the retrospective nature of the study and blinding of study data, the need for informed consent was waved.
Inclusion and exclusion criteria and admission criteria to ICU
All patients transferred to our ICU entered the database (Figure S1). We excluded one patient who was transferred to our clinic for weaning from intubation after recovery from COVID-19. Patients that acquired SARS-CoV-2 at our hospital after ICU stay were also excluded from the database. No other patient was excluded. Patients were admitted to ICU using established criteria such as hemodynamic or metabolic instability, including elevated troponin, N-terminal pro-B-type natriuretic peptide (NT-proBNP), and/or D-dimer values, sepsis, renal failure, reduced Glasgow coma scale (GCS), etc. Hypoxemic awake COVID-19 patients were transferred to ICU in case of respiratory failure with CO2-elevation despite NIV-therapy, or complications such as subcutaneous or mediastinal emphysema.
Study definitions and measurements
COVID-19 was present when patients developed respiratory failure and other symptoms typically associated with COVID-19 and required therapy. ARDS was determined as per Berlin criteria (14). Accordingly, ARDS categories are based upon worst partial pressure of oxygen (pO2)/inspiratory oxygen fraction (FiO2) values despite maximum respiratory support measured during ICU stay: mild: pO2/FiO2 =201–300 mmHg, moderate: pO2/FiO2 =101–200 mmHg, and severe: pO2/FiO2 ≤100 mmHg. NIV was coded irrespective of interface, mode and ventilator type employed. On ICU, ventilator settings were modified clinically depending on blood gas analysis and work of breathing. In patients on IMV, pO2 and FiO2 were measured. In patients on NIV (Stellar 100 or 150, Resmed, Germany or Evita XL or V600, Dräger, Germany), pO2 was measured. FiO2 was estimated based on established tabulation for converting oxygen insufflation to FiO2 (15-17) (see Table S1). Duration of NIV in days prior to ICU was determined based on date of first initiation of NIV during hospital admission until date of ICU admission. In patients with oxygen insufflation using oxygen cannula (ASID BONZ, Germany), FiO2 was estimated from O2-flow using established tabulation (18,19) (see Table S2). pO2 was measured by blood gas analysis. In few patients without arterial line, pO2 was estimated from pulse oximetry-based O2-staturation and converted to pO2 using tabulation (see Table S3). Septic shock was defined according to the 2016 Third International Consensus Definition for Sepsis and Septic Shock (20). Sepsis organ failure assessment (SOFA) score and Charlson Comorbidity Index (CCI) were calculated as published (21-23).
COVID-19 therapy
Standard therapy comprised i.v. dexamethasone in COVID-19 ARDS after May 2020. Anticoagulation regimen was modified according to recommendations considering comorbidities. High-flow nasal oxygen—if applied—was used alternating with NIV. Proning was routinely applied in intubated patients with pO2/FiO2 <150 mmHg and was repeated in responders. Awake NIV patients were placed in prone position depending on pO2/FiO2 and clinical judgement. Crystalloids were given restrictively and diuretics applied as hemodynamically tolerated. A macrolide was routinely prescribed and additional antibiotics given as needed. Intravenous opioids were administered to facilitate NIV tolerance as needed. National COVID-19 guidelines were applied in all ICU patients (1,12,13). Conscious vigilant normocapnic patients without need for catecholamines usually continued to receive NIV for initial respiratory support when admitted to ICU. Intubation and further escalation to extracorporeal membrane oxygenation (ECMO) therapy was a clinical decision of the treating physician.
Comorbidities, complications, follow-up
Comorbidities were defined as present, when being established prior to admission on ICU. Specifically, chronic kidney disease was defined as repeatedly measured glomerular filtration rate (GFR) <60 mL/min prior to present hospitalization. Chronic heart failure was defined as known left ventricular ejection fraction <50% or pre-existing heart failure diagnosis and medication. Chronic pulmonary disease was summarized by asthma, chronic obstructive pulmonary disease (COPD), silicosis, and asbestosis. Diabetes was defined as HbA1c ≥6.5% or antidiabetic medication. Hypertension was defined by previous prescription of antihypertensive medication or pre-existing diagnosis of hypertension. Atherosclerotic cardiovascular disease (ASCVD) summarized as established diagnoses of coronary artery disease, peripheral artery disease or cerebrovascular disease. Additional comorbidities had all been diagnosed prior to ICU admission and are listed in Table S4.
Complications were determined using established clinical criteria and include encephalopathy, increasing confusion and/or seizure; pulmonary embolus or other thrombo-embolic events including intestinal ischemia; septic shock, disseminated intravascular coagulopathy (DIC); acute renal failure; myocardial infarction; acute heart failure; pneumothorax; mediastinal or cutaneous emphysema; and cardio-pulmonary resuscitation (CPR).
For all-cause in-hospital mortality, database was closed after all included patient had either died or left hospital alive, including patients that had been transferred for ECMO therapy to tertiary specialized ECMO centers.
Statistical analysis
Continuous variables are reported as mean ± standard deviation (SD) or median and interquartile range (IQR). Discrete variables are given in frequency and percentiles. Baseline characteristics are specified for the overall cohort as well as stratified by patients admitted due to COVID-19 infection vs. patients with SARS-CoV-2 infection as comorbidity. In the subgroup of patients admitted due to COVID-19, continuous variables were compared using two-sided t-test or Man-Whitney U test and discrete variables using a two-sided Fischer’s exact test. Subgroups of patients with and without intubation and with and without mortality were compared using identical statistical methods in an exploratory analysis. Patients with missing values in subgroups analyses were excluded from these analyses. Univariate and multivariable logistic regression analyses were performed for the association of clinical characteristics with mortality. As lactate dehydrogenase (LDH) and interleukin-6 (IL-6) were not normally distributed, logarithmic transformation was performed. For continuous variables, odds ratio (OR) and confidence intervals (CI) were depicted per one SD increase. Multivariable model included all variables with significant association in univariate analysis. Given the high co-linearity, severe ARDS and intubation could not be included within the same model. Therefore, we provided separate analyses, including either severe ARDS or intubation into the model. All analyses were performed using SAS software (Version 9.4, SAS Institute Inc.). A P value of <0.05 indicated statistical significance.
Results
Study population
During the first year of the pandemic, i.e., between March 2020 and March 2021, 573 patients had been hospitalized and coded SARS-CoV-2-positive at any time during hospital stay. Of these, 61 patients (10.6%, 44.3% women) aged 66.4±13.3 (range, 17–92 years) were admitted to ICU. During the first (03/2020–09/2020) and second 6 months (10/2020–03/2021), n=11 (18%) and n=50 (82%) patients respectively, were admitted. Clinical characteristics and laboratory findings are shown in Tables 1,2. On admittance, ARDS was present in 79% of patients, with more than half of patients having severe ARDS. Lowest pO2/FiO2 during ICU stay averaged 123±89 mmHg. Comorbidities were present in 56 patients (92%). Half of the patients were intubated and overall mortality was 44.3% (Table 1). Maximum duration on ICU was 75 days.
Table 1
| Variables | All patients |
|---|---|
| N (%) | 61 (100.0) |
| Age (years) | 66.4±13.3 |
| Sex, n (% women) | 27 (44.3) |
| BMI (kg/m²) | 31.1±7.4 |
| Any comorbidity (n (%) | 56 (91.8) |
| Hypertension | 34 (55.7) |
| Diabetes | 27 (44.3) |
| ASCVD | 26 (42.6) |
| Chronic kidney disease | 11 (18.0) |
| SOFA score on admission | 5.6±2.9 |
| SOFA score after 24 h | 7.0±3.8 |
| Charlson Comorbidity Index | 3.9±2.4 |
| pO2/FiO2 (mmHg) | |
| On admission | 178±115 |
| After 24 h | 158±93 |
| Lowest value | 123±89 |
| Any ARDS, n (%) | 48 (78.7) |
| Mild | 3 (4.9) |
| Moderate | 12 (19.7) |
| Severe | 33 (54.1) |
| Any complication, n (%) | 43 (70.5) |
| CPR | 14 (23.0) |
| Encephalopathy/confusion | 17 (27.9) |
| Heart failure | 14 (23.0) |
| Acute renal failure | 12 (19.7) |
| Septic shock | 10 (16.4) |
| Length of ICU stay, median days (IQR) | 6 (2; 14) |
| Intubation, n (%) | 31 (50.8) |
| Mortality, n (%) | 27 (44.3) |
Data were presented as mean ± standard deviation if not otherwise specified. ICU, intensive care unit; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; BMI, body mass index; ASCVD, atherosclerotic cardiovascular disease; SOFA, sepsis organ failure assessment; pO2, partial pressure of oxygen; FiO2, inspiratory oxygen fraction; ARDS, acute respiratory distress syndrome; CPR, cardio-pulmonary resuscitation; IQR, interquartile range.
Table 2
| Variables (units) | Normal range | All patients |
|---|---|---|
| N (%) | – | 61 (100.0) |
| Lymphocytes (%) | 20–40 | 11.9±9.3 |
| CRP (mg/dL) | <0.5 | 13.2±9.1 |
| PCT (ng/mL) | <0.5 | 0.40 (0.15; 0.85) |
| IL-6 (pg/mL) | <7 | 148 (55; 303) |
| D-dimer (µg/mL) | <0.5 | 3.5±3.9 |
| hs-troponin (pg/mL) | <14 | 41.0 (21.5; 102.5) |
| NT-proBNP (pg/mL) | <125 | 1,702 (422; 4,430) |
| LDH (U/L) | <250 | 486 (412; 648) |
| Creatinine (mg/dL) | 0.5–0.9 | 1.7±1.8 |
Data were presented as mean ± standard deviation or median days (IQR) if not otherwise specified. ICU, intensive care unit; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin-6; hs, high sensitive; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LDH, lactate dehydrogenase; IQR, interquartile range.
Intubation, complications and mortality on ICU in the entire cohort
One patient had been intubated for 10 days prior to being transferred to our clinic. IMV was initiated at our ICU in n=30 patients (49.2%), i.e., on day 1 in n=9 patients (30.0%), on days 2–4 in n=13 (43.3%) and on day ≥5 in n=8 (26.7%) patients. Three patients were intubated on days 13, 15, and 20 at our ICU. pO2/FiO2 just prior to intubation averaged 94±65 mmHg. While on ICU, 43 patients (70.5%) experienced complications, mostly encephalopathy/confusion, heart failure, acute renal failure, or septic shock (Table 1). CPR was necessary in 14 patients (23.0%). Seven patients died with a do not intubate/do not resuscitate (DNI/DNR)-order, four with COVID-19 and three with SARS-CoV-2. These patients were included in the analysis because they initially wished to be and were treated, and DNI/DNR was consented at end stage disease. Mortality in the entire cohort was 44.3% (Table 1). Mortality on IMV in the entire cohort was 64.5% (n=20/31).
Patients admitted due to COVID-19 or with SARS-CoV-2 as comorbidity
We identified 50 patients (82%) that were admitted because of COVID-19, while 11 patients (18%) were admitted with SARS-CoV-2 as comorbidity (Table 3). The two sub-groups differed significantly in several clinically important aspects: patients admitted due to COVID-19 had lower CCIs, lower pO2/FiO2, and higher rates of ARDS and of intubation (Table 3). Mortality rates, SOFA scores and duration on ICU were similar (Table 3). Moreover, patients admitted due to COVID-19 had higher LDH and CRP values, and lower lymphocytes (Table 4). Among patients admitted due to COVID-19, the rate of intubation and mortality increased with increasing ARDS severity (Tables 5,6). In contrast, only 27% of patients with SARS-CoV-2 as comorbidity were intubated (Table 3).
Table 3
| Variables | Patients admitted due to COVID-19 | Patients with SARS-CoV-2 as comorbidity | P value |
|---|---|---|---|
| N (%) | 50 (82.0) | 11 (18.0) | – |
| Age (years) | 66.3±13.4 | 67.2±13.7 | 0.83 |
| Sex, n (% women) | 23 (46.0) | 4 (36.4) | 0.74 |
| BMI (kg/m²) | 31.4±7.4 | 30.1±7.7 | 0.61 |
| Any comorbidity, n (%) | 45 (90.0) | 11 (100.0) | 0.57 |
| Hypertension | 29 (58.0) | 5 (45.5) | 0.51 |
| Diabetes | 23 (46.0) | 4 (36.4) | 0.74 |
| ASCVD | 17 (34.0) | 9 (81.8) | 0.006 |
| Chronic kidney disease | 5 (10.0) | 6 (54.5) | 0.003 |
| SOFA score on admission | 5.6±2.7 | 5.5±3.7 | 0.92 |
| SOFA score after 24 h | 7.1±3.7 | 6.6±3.8 | 0.69 |
| Charlson Comorbidity Index | 3.7±2.2 | 5.2±2.6 | 0.051 |
| pO2/FiO2 (mmHg) | |||
| On admission | 151±93 | 302±127 | <0.0001 |
| After 24 h | 137±72 | 253±118 | 0.009 |
| Lowest value | 101±58 | 126±129 | 0.009 |
| Any ARDS, n (%) | 45 (90.0) | 3 (27.3) | <0.0001 |
| Mild | 3 (6.0) | 0 (0) | 1.00 |
| Moderate | 9 (18.0) | 3 (27.3) | 0.68 |
| Severe | 33 (66.0) | 0 (0) | <0.0001 |
| Any complication, n (%) | 35 (70.0) | 8 (72.7) | 1.00 |
| CPR | 12 (24.0) | 2 (18.2) | 1.00 |
| Encephalopathy/confusion | 13 (26.0) | 4 (36.4) | 0.48 |
| Heart failure | 10 (20.0) | 4 (36.4) | 0.26 |
| Acute renal failure | 8 (16.0) | 4 (36.4) | 0.20 |
| Septic shock | 6 (12.0) | 4 (36.4) | 0.07 |
| Length of ICU stay, median (IQR) (days) | 5.5 (2; 14) | 7 (2; 19) | 0.99 |
| Intubation, n (%) | 28 (56.0) | 3 (27.3) | 0.11 |
| Mortality, n (%) | 22 (44.0) | 5 (45.5) | 1.00 |
Data were presented as mean ± standard deviation if not otherwise specified. ICU, intensive care unit; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; BMI, body mass index; ASCVD, atherosclerotic cardiovascular disease; SOFA, sepsis organ failure assessment; pO2, partial pressure of oxygen; FiO2, inspiratory oxygen fraction; ARDS, acute respiratory distress syndrome; CPR, cardio-pulmonary resuscitation; IQR, interquartile range.
Table 4
| Variables (units) | Normal range | Patients admitted due to COVID-19 | Patients with SARS-CoV-2 as comorbidity | P value |
|---|---|---|---|---|
| N (%) | – | 50 (82.0) | 11 (18.0) | – |
| Lymphocytes (%) | 20–40 | 10.8±8.6 | 19.0±11.6 | 0.028 |
| CRP (mg/dL) | <0.5 | 14.6±9.3 | 6.3±4.1 | 0.0001 |
| PCT (ng/mL) | <0.5 | 0.4 (0.2; 0.8) | 0.4 (0.1; 4.0) | 0.89 |
| IL-6 (pg/mL) | <7 | 147 (55; 355) | 224 (37; 267) | 0.80 |
| D-dimer (µg/mL) | <0.5 | 3.5±4.0 | 4.0±3.6 | 0.77 |
| hs-troponin (pg/mL) | <14 | 38.0 (20.5; 92.5) | 63.5 (25.0; 132.0) | 0.41 |
| NT-proBNP (pg/mL) | <125 | 1,681 (426; 3,965) | 3,664 (263; 7,326) | 0.58 |
| LDH (U/L) | <250 | 538 (424; 688) | 281 (199; 405) | 0.004 |
| Creatinine (mg/dL) | 0.5–0.9 | 1.7±1.9 | 1.7±0.9 | 0.98 |
Data were presented as mean ± standard deviation or median days (IQR) if not otherwise specified. ICU, intensive care unit; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin-6; hs, high sensitive; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LDH, lactate dehydrogenase; IQR, interquartile range.
Table 5
| Variables | All Patients | Intubation | Survival status | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | P value | Died | Survived | P value | |||
| N (%) | 50 (100.0) | 28 (56.0) | 22 (44.0) | – | 22 (44.0) | 28 (56.0) | – | |
| Age (years) | 66.3±13.4 | 66.3±10.4 | 66.2±16.7 | 0.99 | 69.1±12.0 | 64.1±14.2 | 0.19 | |
| Sex, n (% women) | 23 (46.0) | 8 (28.6) | 15 (68.2) | 0.01 | 8 (36.4) | 15 (53.6) | 0.26 | |
| BMI (kg/m²) | 31.4±7.4 | 31.7±7.2 | 30.9±7.9 | 0.70 | 32.6±7.8 | 30.4±7.1 | 0.29 | |
| ARDS, n (%) | ||||||||
| No ARDS | 5 (10.0) | 0 | 5 (22.7) | 0.001 | 0 | 5 (17.9) | 0.059 | |
| Mild ARDS | 3 (6.0) | 1 (3.6) | 2 (9.1) | 0.58 | 0 | 3 (10.7) | 0.25 | |
| Moderate ARDS | 9 (18.0) | 1 (3.6) | 8 (36.4) | 0.007 | 2 (9.1) | 7 (25.0) | 0.27 | |
| Severe ARDS | 33 (66.0) | 26 (92.9) | 7 (31.8) | <0.0001 | 20 (90.9) | 13 (46.4) | 0.001 | |
| pO2/FiO2 (mmHg) | ||||||||
| On admission | 151±93 | 114±68 | 198±102 | 0.002 | 107±43 | 185±108 | 0.001 | |
| After 24 h | 137±72 | 114±46 | 167±88 | 0.015 | 104±32 | 164±84 | 0.001 | |
| Lowest value | 101±58 | 79±40 | 128±66 | 0.004 | 75±22 | 120±70 | 0.003 | |
| SOFA score | ||||||||
| On admission | 5.6±2.9 | 6.2±2.7 | 4.8±2.5 | 0.06 | 6.2±2.8 | 5.1±2.5 | 0.17 | |
| After 24 h | 7.0±3.8 | 8.1±3.5 | 5.9±3.7 | 0.04 | 8.0±3.5 | 6.4±3.8 | 0.12 | |
| CCI | 3.9±2.4 | 3.7±2.1 | 3.6±2.5 | 0.85 | 4.1±2.3 | 3.3±2.2 | 0.18 | |
| NIV prior to ICU, n (%) | 23 (46.0) | 16 (57.1) | 7 (31.8) | 0.09 | 13 (59.1) | 10 (35.7) | 0.15 | |
| Days NIV prior to ICU | 4.7±4.9 | 5.1±5.4 | 3.6±3.7 | 0.35 | 6.3±5.9 | 2.5±2.0 | 0.046 | |
| Days NIV ≥5 prior to ICU, n (%) | 7 (14.0) | 5 (17.9) | 2 (9.1) | 0.44 | 6 (27.3) | 1 (3.6) | 0.035 | |
| Hospitalization prior to ICU, median (IQR) (days) | 1 (0; 4) | 2 (0; 4) | 0 (0; 5) | 0.32 | 2 (0; 7) | 0 (0; 3) | 0.083 | |
Data were presented as mean ± standard deviation if not otherwise specified. ICU, intensive care unit; COVID-19, coronavirus disease 2019; BMI, body mass index; ARDS, acute respiratory distress syndrome; pO2, partial pressure of oxygen; FiO2, inspiratory oxygen fraction; SOFA, sepsis organ failure assessment; CCI, Charlson Comorbidity Index; NIV, non-invasive ventilation; IQR, interquartile range.
Table 6
| Variables | All patients | Intubation | No intubation | P value | Patients died | Patients survived | P value |
|---|---|---|---|---|---|---|---|
| Lymphocytes (%) | 10.8±8.6 | 8.9±8.3 | 13.1±8.6 | 0.10 | 9.4±8.4 | 11.9±8.7 | 0.33 |
| CRP (mg/dL) | 14.6±9.3 | 16.4±9.5 | 12.3±8.6 | 0.13 | 14.5±7.0 | 14.6±10.8 | 0.96 |
| PCT (ng/mL) | 0.4 (0.2; 0.8) | 0.4 (0.2; 0.9) | 0.4 (0.2; 0.8) | 0.83 | 0.4 (0.2; 0.6) | 0.4 (0.2; 0.9) | 0.29 |
| IL-6 (pg/mL) | 147 (55; 355) | 183 (89; 487) | 99 (33; 175) | 0.021 | 191 (139; 484) | 93 (26; 183) | 0.009 |
| D-dimer (µg/mL) | 3.5±4.0 | 3.8±3.2 | 3.0±4.9 | 0.52 | 3.7±3.1 | 3.3±4.6 | 0.77 |
| hs-troponin (pg/mL) | 38 (21; 93) | 34 (22; 72) | 57 (14; 149) | 0.76 | 35 (25; 61) | 51 (12; 160) | 0.82 |
| NT-proBNP (pg/mL) | 1,681 (426; 3,965) | 1,301 (379; 3,368) | 1,851 (469; 5,517) | 0.09 | 1,082 (279; 2,886) | 1,851 (727; 5,517) | 0.09 |
| LDH (U/L) | 538 (424; 688) | 629 (449; 771) | 470 (410; 545) | 0.026 | 640 (537; 796) | 449 (416; 607) | 0.008 |
| Creatinine (mg/dL) | 1.7±1.8 | 1.6±2.0 | 1.9±1.9 | 0.56 | 1.4±1.3 | 2.0±2.3 | 0.29 |
Data were presented as mean ± standard deviation or median days (IQR) if not otherwise specified. COVID-19, coronavirus disease 2019; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin-6; hs, high sensitive; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LDH, lactate dehydrogenase; IQR, interquartile range.
Rates and predictors of intubation and mortality in COVID-19 patients
Intubation and mortality rates are given in Tables 5,6. They increased with increasing ARDS severity as estimated by pO2/FiO2 (Figure 2). Among intubated patients with COVID-19, 64.3% (n=18/28) died, including all nine patients on ECMO or iLA-activve (Xenios, Germany).
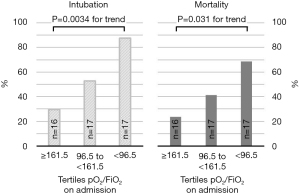
Patients who were intubated during ICU stay, had more often severe ARDS, lower pO2/FiO2 values on admission, after 24 h and at any time during ICU stay, and had slightly higher SOFA-scores after 24 h. These patients had also more often non-invasive respiratory support prior to ICU-admission than patients that were not intubated (Tables 5,6). CCI, duration of NIV and duration of hospital stay prior to ICU admission were slightly higher and longer (Tables 5,6). Intubated patients also differed in several laboratory findings in that they had higher IL-6 and LDH values on admission, but slightly lower NT-proBNP values (Tables 5,6).
Patients that died had more often severe ARDS (91%) and had lower pO2/FiO2 values on admission, after 24 h and lower values at any time of ICU stay than survivors (Tables 5,6). Patients that died were about 5 years older, and had been hospitalized longer, had more often NIV-therapy and longer duration of NIV therapy prior to ICU admission (Tables 5,6). Likewise, among patients who were escalated to intubation, those that died had longer total days on NIV, i.e., prior to ICU and in addition during ICU stay, than those who survived (7.8±5.6 vs. 4.1±3.0 days, P=0.063). Patients that died also differed in several laboratory findings: again, IL-6- and LDH values were much higher, and NT-proBNP values were slightly lower.
In univariate analysis, LDH, IL-6, presence of severe ARDS, intubation, and duration of NIV ≥5 days prior to ICU were associated with mortality. In multivariable logistic regression analysis, IL-6 and ≥5 days of NIV prior to ICU remained independent predictors of mortality (Table 7), but ORs for severe ARDS and LDH remained about 3-fold elevated.
Table 7
| Variables | Univariate | Multivariable Model 1 | Multivariable Model 2 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Age (per 1 SD) | 1.51 (0.81–2.80) | 0.16 | – | – | – | – | ||
| Male sex | 2.02 (0.64–6.33) | 0.23 | – | – | – | – | ||
| CCI (per 1 SD) | 1.52 (0.82–2.84) | 0.18 | – | – | – | – | ||
| LDH (log-values per 1 SD) | 3.85 (1.37–10.80) | 0.011 | 2.72 (0.65–11.40) | 0.17 | 3.25 (0.80–13.24) | 0.10 | ||
| IL-6 (log-values per 1 SD) | 3.18 (1.32–7.69) | 0.010 | 4.08 (1.16–14.33) | 0.028 | 4.13 (1.21–14.10) | 0.024 | ||
| Presence of severe ARDS | 11.5 (2.26–59.0) | 0.003 | 3.35 (0.26–42.97) | 0.35 | – | – | ||
| Intubation | 8.10 (2.14–30.65) | 0.002 | – | – | 2.57 (0.44–14.89) | 0.29 | ||
| Duration NIV ≥5 days prior to ICU | 10.1 (1.1–91.8) | 0.040 | 42.20 (1.22 to >999) | 0.038 | 32.76 (1.02 to >999) | 0.049 | ||
Model 1: including severe ARDS, LDH (log-transformed), IL-6 (log-transformed), and duration NIV ≥5 days prior to ICU admission; Model 2: including intubation, LDH (log-transformed), IL-6 (log-transformed), and duration NIV ≥5 days prior to ICU admission. COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval; SD, standard deviation; CCI, Charlson Comorbidity Index; LDH, lactate dehydrogenase; IL-6, interleukin-6; ARDS, acute respiratory distress syndrome; NIV, non-invasive ventilation; ICU, intensive care unit.
Discussion
At our institution, 10.6% of patients with COVID-19 or SARS-CoV-2 infection were admitted to ICU. Among COVID-19 patients on ICU, intubation rate was 57%, and 45% of patients died. These numbers are in line with previous reports as summarized in current guidelines (1,3). Roedl et al. reported a comparatively favorable overall ICU mortality of 35% in patients included until early June 2020 in a German cohort, but outcome was not reported for ARDS categories separately (24). Mortality of intubated patients was 44% (24), again lower compared to our study (i.e., 64.3%), possibly attributable to later intubation in our cohort of patients with mostly severe ARDS.
In our retrospective explorative study, severe ARDS estimated by pO2/FiO2, (hyper-)inflammation reflected in elevated IL-6, and long disease duration and poor recovery from respiratory failure evidenced by long NIV-therapy prior to and during ICU stay were associated with intubation and death. Within the time course of clinical SARS-CoV-2 manifestation from early viral infection to severe hyperinflammatory ARDS (25), our intubation and mortality rates are subject to confounding by indication but may also in part reflect comparatively long NIV-therapy in our patient cohort.
ARDS severity and IL-6 have early on been established as being prognostically relevant (3). Our study extends these findings in that long duration on NIV prior to ICU admission was also associated with mortality and may need to be added to the list of prognostically relevant parameters. Karagiannidis et al. report a mortality of 50% in patients that were initially treated with NIV, which is similar to the 52% mortality observed on IMV (8). This finding is consistent with a meta-analysis in almost 9,000 critically ill COVID-19 patients, where use of high flow nasal oxygen (HFNO) or NIV and timing of intubation had little effect if any on morbidity and mortality (26). However, Karagiannidis et al. also observed that mortality increased continuously the longer patients were on NIV before requiring IMV (8). Mortality was as high as 75% in patients on NIV for 5 days or longer prior to intubation, which is consistent with our findings. Unfortunately, markers of inflammation and indices of ARDS severity were not reported. A caution regarding NIV in moderate or severe ARDS also derives from the Lung Safe Study, where patients on NIV with pO2/FiO2 <150 mmHg had a higher ICU mortality compared to patients on IMV (27). Recently, Wendel Garcia et al. studied outcome in different strategies of early respiratory support in critically ill COVID-19 patients. Their data also suggest that NIV should be avoided due to an elevated ICU mortality risk (28). In contrast, Daniel et al. report a reduced mortality when NIV was employed as the initial intervention in COVID-19 patients (29). Yet again, patients that were escalated to intubation had a (non-significant) 39% increase in mortality compared to patients that were initially intubated (29). Our data thus add to the currently limited and heterogeneous evidence on NIV in moderate to severe COVID-19 ARDS.
There are at least two possible explanations for our findings. First, NIV may have aggravated disease progression in our patients attributable to “patient self-inflicted lung injury” (P-SILI) (30,31). This concept suggests that increased respiratory drive and breathing effort induces lung injury due to uncontrolled swings in transpulmonary pressure and hence increase lung stress in the aerated compartment of the baby lung. The positive end-expiratory pressure (PEEP) that is applied during NIV may not prevent injury caused by recurrent alveolar (hyper-)inflation and deflation (32). In addition, a marked decrease in pleural pressure may increase vascular transmural pressure and vascular permeability, contributing to alveolar and interstitial pulmonary oedema (32), beyond the increased vascular permeability caused by SARS-CoV-2 itself (1,2). Especially patients with pO2/FiO2 <200 mmHg may be at risk of NIV failure (16,32), which is consistent with our findings. On the other hand, Tobin et al. argue in a series of publications that there is insufficient evidence for the concept of P-SILI (33-35). They question its role for the progression of respiratory failure in COVID-19 and caution against pre-emptive liberal use of intubation and mechanical ventilation (33-35). Indeed, pressure support via NIV reduces respiratory effort, relieves dyspnea, and ameliorates oxygenation, especially in high PEEP settings (32,36), but prospective outcome studies in COVID-19 patients comparing NIV and IMV are limited.
Second, the observation of impaired prognosis in patients being longer on NIV may indicate poor recovery from ARDS. Escalation of respiratory support from NIV to IMV is usually referred to as “NIV-failure”. The terminology “NIV-failure” in this context emphasizes the type of respiratory support on outcome. However, it should be recognized that “late failure” in COVID-19 also occurs on IMV, as reflected by long intubation times, escalation to ECMO therapy, and death due to respiratory failure. At present it is unclear if patients who have an unfavourable clinical course on NIV, should be regarded as “patients with NIV-failure” or as “patients with delayed or no recovery from lung failure”. The focus should currently be on both, i.e., (I) on the type of ventilation and (II) on disease severity and recovery from it. To date, prospective trials in COVID-19 patients in moderate or severe ARDS comparing timing of NIV and IMV are lacking. Several trials on the prognostic role of NIV in COVID-19 have been initiated (37), but results are pending.
Considering our data, evidence from other studies, and published opinion, it appears safe to state that patients with worsening respiratory failure and pO2/FiO2 <200 mmHg, that do not improve or even deteriorate clinically after 4–5 days of NIV, are candidates for very careful ICU surveillance. Whether outcome in these patients is better with intubation and IMV, remains to be shown.
An additional finding unrelated to ventilation support is that COVID-19 patients differed from the 18% SARS-CoV-2 comorbid patients in several clinically relevant aspects, despite similar mortality. Mortality in our patients without COVID-19 may have been worsened due to SARS-CoV-2 infection, as in other cohorts (38). From such few patients, it is difficult to draw conclusions for clinical practice. Yet, they appear to deserve special attention regarding diagnostic work up, surveillance and therapy. Patients with SARS-CoV-2 as comorbidity have previously not explicitly been excluded or mentioned (24,39,40). A separate analysis may help to better understand determinants of outcome in patients with both SARS-CoV-2 or COVID-19-induced ARDS.
This work has some limitations. It is a comparatively small single-center study. Therefore, absolute numbers as well as intubation and mortality rates in the different categories must be interpreted with caution. It is an advantage, though, that we had clinical, respiratory and laboratory values for each individual patient in a cohort with high NIV rates and late intubation, and all patients had completed ICU stay.
We could not include data of patients that were treated outside our ICU. It is thus expected that overall hospital mortality in each category of ARDS severity is lower than reported here. Our findings should nonetheless be relevant to ICU physicians treating patients in similar settings.
In patients on NIV, FiO2 could not be measured but had to be estimated from tabulations. However, the tables used in this study have also been used in large trials (16,17), and have been validated (15). Using alternative estimates would have overestimated FiO2 and hypoxemia severity (15).
NIV, that comprises CPAP, bilevel CPAP, HFNO, NIV via helmet or face mask, etc., was coded irrespective of interface, mode and ventilator type employed. We used NIV via face-mask in most and HFNO in few patients (10,11). Our data can therefore not be transferred to other modes of non-invasive respiratory support.
We have not reported details on ventilator settings and supporting medication. These data are stored automatically in our hospital Krankenhaus Informationssystem (KIS) (ORBIS KIS, Dedalus Health Care, Bonn, Germany). Yet, in previous studies, this information contained little prognostic information (25), and principles of lung-protective ventilation taking into account specific COVID-19 pathophysiology, were applied (1,6,7,12,13).
During the pandemic, treatment recommendations have been modified (1,12,13). For the period of this study, the modifications mainly pertained to ventilation support strategies, corticoid therapy and anticoagulation. Throughout the study period, we were restrictive with IMV, and used NIV below pO2/FiO2 thresholds suggested in guidelines. Early on, we used i.v. corticosteroids in COVID-19 patients with ARDS, as suggested by Surviving Sepsis Campaign guidelines (41). Even though anticoagulation was not mentioned in the initial guidelines (12), all patients were individually treated with prophylactic, half-therapeutic or full-dose unfractionated or low molecular weight heparin depending on risk factors such as overweight or elevated D-dimers (1,7,13). The degree to which such variation in therapy over time may have affected outcome is difficult to estimate. Interestingly, a decrease of early IMV from 75% in the first period to 37% in the second period of the pandemic did not reduce overall mortality (8).
In summary, in this 1-year all comers study, we found a clinical difference between patients with SARS-CoV-2 as comorbidity and COVID-19 patients. This should be considered in future analyses. In COVID-19 patients, prognosis appears to be largely determined by ARDS severity and the degree of accompanying inflammation. Especially patients ≥5 days on NIV appear to have a very poor prognosis. Our data indicate that patients with severe COVID-19 hypoxemia that do not improve on NIV during the first days, should be considered candidates for invasive ventilation to reduce work of breathing and maybe P-SILI. Yet, it is currently unclear if long duration on NIV aggravates the disease process or if it indicates lack of recovery, or both. The prognostic role of NIV and best timing of intubation for outcome should be clarified in prospective trials.
Acknowledgments
We gratefully acknowledge the hard work of all nursing and physician staff on our ICU in their care for COVID-19 and non-COVID-19 patients alike. We are indebted to Dr. Jeibmann (Bethanien Hospital Moers) who contributed the data for Figure 1.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1713/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1713/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1713/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1713/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study complies with the Declaration of Helsinki (as revised in 2013), and was approved by the ethics committee of University Duisburg-Essen (#21-9911-BO). Due to the retrospective nature of the study and blinding of study data, the need for informed consent was waved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kluge S, Janssens U, Welte T, et al. Empfehlungen zur stationären Therapie von Patienten mit COVID-19. AWMF-Register-Nr. 113/001. (S3-Leitlinie, 02/21) Available online: https://www.grc-org.de/files/ArticleFiles/document/113-001l_S3_Empfehlungen-zur-stationaeren-Therapie-von-Patienten-mit-COVID-19__2021-02.pdf
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (last accessed 02 April 2021)
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934-43. [Crossref] [PubMed]
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020;323:2052-9. [Crossref] [PubMed]
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846-8. [Crossref] [PubMed]
- Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46:1099-102. [Crossref] [PubMed]
- Möhlenkamp S, Thiele H. Ventilation of COVID-19 patients in intensive care units. Herz 2020;45:329-31. [Crossref] [PubMed]
- Karagiannidis C, Hentschker C, Westhoff M, et al. Observational study of changes in utilization and outcomes in mechanical ventilation in COVID-19. PLoS One 2022;17:e0262315. [Crossref] [PubMed]
- Crimi C, Noto A, Cortegiani A, et al. Noninvasive respiratory support in acute hypoxemic respiratory failure associated with COVID-19 and other viral infections. Minerva Anestesiol 2020;86:1190-204. [Crossref] [PubMed]
- Voshaar T, Dellweg D, Hetzel M. Empfehlung zur Behandlung respiratorischer Komplikationen bei akuter Virusinfektion außerhalb der Intensivstation. Published by Verband Pneumologischer Kliniken. Available online: https://www.vpneumo.de/fileadmin/pdf/VPK_Empfehlung_neu_21.03.2020.pdf
- Voshaar T, Stais P, Köhler D, et al. Conservative management of COVID-19 associated hypoxaemia. ERJ Open Res 2021;7:00134-2021. [Crossref] [PubMed]
- Kluge S, Janssens U, Welte T, et al. Recommendations for critically ill patients with COVID-19. Med Klin Intensivmed Notfmed 2020;115:175-7. [Crossref] [PubMed]
- Kluge S, Janssens U, Spinner CD, et al. Clinical Practice Guideline: Recommendations on Inpatient Treatment of Patients with COVID-19. Dtsch Arztebl Int 2021; Epub ahead of print. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Coudroy R, Frat JP, Girault C, et al. Reliability of methods to estimate the fraction of inspired oxygen in patients with acute respiratory failure breathing through non-rebreather reservoir bag oxygen mask. Thorax 2020;75:805-7. [Crossref] [PubMed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. [Crossref] [PubMed]
- SRLF Trial Group. Hypoxemia in the ICU: prevalence, treatment, and outcome. Ann Intensive Care 2018;8:82. [Crossref] [PubMed]
- Deutsche Sepsis Gesellschaft (DSG). Available online: https://www.sepsis-gesellschaft.de/ sepsisdefinition-und-kodierung/ (last query on August 20, 2021)
- Deutsche Sepsis-Gesellschaft (DSG). “Sepsis 3” und Kodierung der "Sepsis" gemäß ICD-10-GM 2020. Available online: https://www.sepsis-gesellschaft.de/sepsisdefinition-und-kodierung/
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Lambden S, Laterre PF, Levy MM, et al. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019;23:374. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Roedl K, Jarczak D, Thasler L, et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: A multicentric study in Germany. Aust Crit Care 2021;34:167-75. [Crossref] [PubMed]
- Pfeifer M, Ewig S, Voshaar T, et al. Position Paper for the State-of-the-Art Application of Respiratory Support in Patients with COVID-19. Respiration 2020;99:521-42. [Crossref] [PubMed]
- Papoutsi E, Giannakoulis VG, Xourgia E, et al. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care 2021;25:121. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am J Respir Crit Care Med 2017;195:67-77. [Crossref] [PubMed]
- Wendel Garcia PD, Aguirre-Bermeo H, Buehler PK, et al. Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. Crit Care 2021;25:175. [Crossref] [PubMed]
- Daniel P, Mecklenburg M, Massiah C, et al. Non-invasive positive pressure ventilation versus endotracheal intubation in treatment of COVID-19 patients requiring ventilatory support. Am J Emerg Med 2021;43:103-8. [Crossref] [PubMed]
- Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med 2017;195:438-42. [Crossref] [PubMed]
- Windisch W, Weber-Carstens S, Kluge S, et al. Invasive and Non-Invasive Ventilation in Patients With COVID-19. Dtsch Arztebl Int 2020;117:528-33. [Crossref] [PubMed]
- Grieco DL, Menga LS, Eleuteri D, et al. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol 2019;85:1014-23. [Crossref] [PubMed]
- Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care 2020;10:78. [Crossref] [PubMed]
- Tobin MJ, Jubran A, Laghi F. Noninvasive strategies in COVID-19: epistemology, randomised trials, guidelines, physiology. Eur Respir J 2021;57:2004247. [Crossref] [PubMed]
- Tobin MJ. Basing Respiratory Management of COVID-19 on Physiological Principles. Am J Respir Crit Care Med 2020;201:1319-20. [Crossref] [PubMed]
- L'Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005;172:1112-8. [Crossref] [PubMed]
- Dobler CC, Murad MH, Wilson ME. Noninvasive Positive Pressure Ventilation in Patients With COVID-19. Mayo Clin Proc 2020;95:2594-601. [Crossref] [PubMed]
- Doglietto F, Vezzoli M, Gheza F, et al. Factors Associated With Surgical Mortality and Complications Among Patients With and Without Coronavirus Disease 2019 (COVID-19) in Italy. JAMA Surg 2020;155:691-702. [Crossref] [PubMed]
- Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019. Crit Care Med 2020;48:e799-804. [Crossref] [PubMed]
- Doidge JC, Gould DW, Ferrando-Vivas P, et al. Trends in Intensive Care for Patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med 2021;203:565-74. [Crossref] [PubMed]
- Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med 2020;46:854-87. [Crossref] [PubMed]


