Chemotherapy for patients with advanced lung cancer receiving long-term oxygen therapy
Introduction
Lung cancer is the leading cause of cancer-related death in the world (1). Because most lung cancers are unfortunately diagnosed at an advanced stage, systemic chemotherapy (including molecular-targeted therapy) or best supportive care is the only treatment choice (2,3). A meta-analysis showed that chemotherapy improves overall survival in patients with advanced lung cancer (4). Good responses in patients with advanced non-small lung cancer (NSCLC) were recently observed for chemotherapy regimens including epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) or anaplastic lymphoma kinase tyrosine kinase inhibitors (ALK-TKIs) (5-8). However, physicians often encounter lung cancer patients with severe comorbidities, including cardiovascular disease, chronic obstructive pulmonary disease (COPD), and interstitial lung disease (ILD), and the prognosis of these patients remains poor (9,10). These patients sometimes experience chronic respiratory failure, and physicians commonly prescribe long-term oxygen therapy (LTOT) to support their daily activities (11).
In the 1980s, LTOT significantly improved the survival of patients with COPD and chronic hypoxemia (12,13). In Japan, LTOT has been increasingly prescribed for chronic respiratory diseases including lung cancer (14,15). Patients with advanced lung cancer who receive LTOT are sometimes in relatively good condition owing to their enhanced ability to perform daily activities. However, aggressive chemotherapy in patients receiving LTOT should be carefully considered by physicians because it has been associated with poor outcomes in patients with severe comorbidities (16). To our knowledge, no reports have addressed the use of chemotherapy in patients with advanced lung cancer who require LTOT. The aim of this study was to evaluate the effects of chemotherapy in such patients based on our clinical experience.
Methods
Study approval
This study was approved by the Institutional Review Board of the Osaka Prefectural Medical Center for Respiratory and Allergic Diseases. Because it was a retrospective chart review, informed consent was not required for approval. Written informed consent for chemotherapy was obtained from all eligible patients.
Patient selection
This study included patients with advanced lung cancer who received LTOT while undergoing at least 1 cycle of chemotherapy between January 2009 and December 2014 at our institution. Chemotherapy included cytotoxic agents, EGFR-TKIs, or ALK-TKIs. Patients receiving adjuvant or neoadjuvant chemotherapy were excluded from this study. All study participants were observed until death or April 30, 2015.
Indication for long-term oxygen therapy (LTOT)
Patients with a PaO2 level ≤60 mmHg or a SpO2 level ≤90% at rest or during exercise were indicated for LTOT. Non-hypoxemic patients receiving palliative oxygen therapy were excluded from this study. Oxygen was provided to patients via an oxygen concentrator (Hi-Sanso®, Teijin Pharma Ltd, Japan) and an oxygen cylinder. In patients with an underlying pulmonary disease (e.g., COPD or ILD), maximum medical therapy (e.g., use of bronchodilators or administration of inhaled or oral corticosteroids) was performed before prescribing LTOT. Oxygen rates were reevaluated every month. The first date of LTOT was defined as the date when continuous oxygen therapy was started in an outpatient or inpatient setting.
Treatment strategy
Treatments for lung cancer (e.g., surgery, radiotherapy, chemotherapy, or best supportive care) were determined via regular multidisciplinary team discussions at our institution. Moreover, board-certified oncologists and pulmonologists carefully considered the eligibility of each candidate for chemotherapy and selected the appropriate chemotherapy regimen (platinum-based, non-platinum-based, EGFR-TKI, or ALK-TKI).
Clinical review
The clinical history of the eligible patients was retrospectively reviewed. Baseline demographic information including age, sex, smoking status, underlying respiratory disease, histology, disease stage, and Eastern Cooperative Oncology Group performance status (PS) was obtained for each patient. Age and PS were determined at the beginning of the first chemotherapy cycle performed in conjunction with LTOT. Staging was based on the criteria of the seventh edition of the Tumor, Node, and Metastasis classification for lung cancer (17). The components and treatment line of the first chemotherapy regimen with LTOT, and the number of regimens performed with LTOT were also evaluated. Chemotherapy-related severe adverse events (non-hematological toxicity ≥ grade 3 or hematological toxicity ≥ grade 4) were examined during and immediately after the first chemotherapy regimen with LTOT.
Survival time from diagnosis was calculated as the duration from the date of diagnosis to the date of death or final follow-up. Survival time with LTOT was calculated from the date of the first chemotherapy cycle with LTOT to the date of death or final follow-up. For patients alive at the end of the study period, the final follow-up date was April 30, 2015.
Chemotherapy at the end of life
Because of the toxicity of aggressive chemotherapy in patients with comorbidities, whether chemotherapy is terminated at the appropriate time should be determined in patients receiving LTOT. In this study, we evaluated the composition of the final chemotherapy regimen, the survival time (time between the date of the final chemotherapy and death), and the cause of death in patients who received chemotherapy during the last 30 days of their lives.
Statistical analysis
Values are presented as frequency, percentage, or median (range). Survival time was assessed via Kaplan-Meier survival analysis. For survival time with LTOT, differences between survival curves were assessed by using the log-rank test. Variables with a P value <0.05 in a univariate analysis were included in a multivariate analysis using Cox’s regression model to identify independent predictors of survival with LTOT. All analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is the graphical user interface of the R foundation for Statistical Computing (Vienna, Austria). All comparisons with a P value <0.05 were considered statistically significant.
Results
Overall, 40 patients received at least 1 cycle of chemotherapy along with LTOT between January 2009 and December 2014 at our institution. Patient characteristics are summarized in Table 1. The study population had a median age of 69.5 years and mostly consisted of men. Most patients were smokers (n=34) and/or had underlying respiratory diseases including COPD (n=14) and ILD (n=10). Thirty patients had NSCLC, either adenocarcinoma (n=24) or squamous cell carcinoma (n=6), and 10 had small cell lung cancer (SCLC). There was an EGFR mutation in 5 of the 24 patients with adenocarcinomas and an ALK translocation in 2. The disease stage was IIIA in 3 patients, IIIB in 4 patients, IV in 28 patients, and recurrent after surgery in 5 patients. The PS at the beginning of the first chemotherapy cycle with LTOT was 2 in 33 patients and 3 in 7 patients.
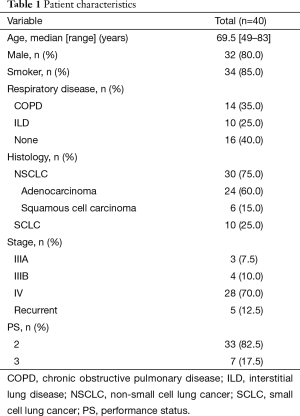
Full table
The characteristics of the chemotherapy regimens performed in conjunction with LTOT are summarized in Table 2. The first chemotherapy regimen that was accompanied by LTOT was the first-line treatment in 17 patients, the second-line treatment in 10 patients, the third- or fourth-line treatment in 8 patients, and the fifth- to eighth-line treatment in 5 patients. It was platinum-based in 12 patients, non-platinum-based in 22 patients, and consisted of an EGFR-TKI in 5 patients and an ALK-TKI in 1 patient. Seven (58.3%) of the 12 patients receiving a platinum-based regimen, 4 (18.2%) of the 22 patients receiving a non-platinum-based regimen and 3 (50%) of the 6 patients receiving TKIs experienced chemotherapy-related severe adverse events. Most patients (n=28, 70%) received only 1 regimen during the study period.
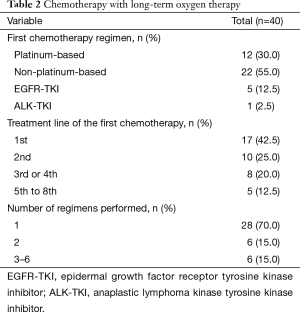
Full table
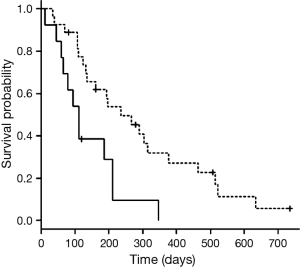
The median survival time from diagnosis was 679 days (95% confidence interval, 327 to 968 days), while median survival time with LTOT was 194 days (95% confidence interval, 112 to 266 days). In univariate analyses of survival time with LTOT, prognosis was better when respiratory disease (COPD or ILD) was present (236 and 134 days with and without respiratory disease, respectively; P=0.046) (Table 3) and when the first chemotherapy regimen with LTOT was the first- or second-line treatment (236 and 111 days for first/second-line and third-line or higher, respectively; P=0.005) (Table 3, Figure 1). None of the other variables (age, sex, histology, stage, PS, and composition of the first chemotherapy regimen with LTOT) were associated with better prognosis. In a multivariate analysis of survival time with LTOT, the only factor significantly associated with better prognosis was the treatment line (first or second) of the first chemotherapy with LTOT (hazard ratio, 0.42; 95% confidence interval, 0.18 to 0.94) (Table 3).
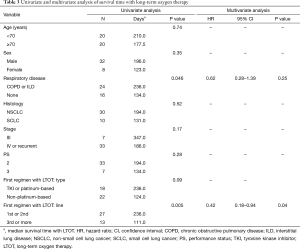
Full table
Ten patients (25%) received chemotherapy during the last 30 days of their lives (Table 4). The histological type was adenocarcinoma in 7 patients, SCLC in 2 patients, and squamous cell carcinoma in 1 patient. Survival time from the first chemotherapy with LTOT to death varied from 10 to 522 days, while survival time after the final chemotherapy to death varied from 3 to 23 days. The final chemotherapy regimen contained an EGFR-TKI in 4 patients and was platinum-based in 2 patients and non-platinum-based in 4 patients. It was third-line or more in 5 patients. Four patients received more than 1 regimen (2 to 6 regimens) with LTOT, and oxygen therapy was continued until death in all the patients. The cause of death was lung cancer in 7 patients and chemotherapy-related in 2 patients (ILD caused by the EGFR-TKI in 1 patient and febrile neutropenia in 1 patient). Sudden death at home presumably due to acute myocardial infarction occurred in 1 patient. In 1 of the 2 patients who died of chemotherapy-related adverse events, the final chemotherapy regimen administered was third-line.

Full table
Discussion
LTOT is sometimes prescribed for advanced lung cancer patients with chronic respiratory failure who might be candidates for chemotherapy. The present study describes our clinical experience with 40 advanced lung cancer patients who underwent systemic chemotherapy while receiving LTOT between January 2009 and December 2014. Two important clinical observations were made. First, chemotherapy with LTOT had an acceptable survival benefit, especially for patients for whom the first chemotherapy with LTOT was the first- or second-line treatment. Secondly, 10 of the 40 patients received chemotherapy during the last months of their lives, and chemotherapy-induced death was observed in 2 of the 40 patients (5%).
Patients with chronic respiratory failure requiring LTOT are usually ineligible for clinical trials; therefore, there is no standard chemotherapy regimen for these patients. The PS score at the start of chemotherapy with LTOT was 2 or 3 in our study population. Compared with lower values, PS scores ≥2 are associated with lower response rates to chemotherapy, shorter times to treatment failure, and shorter progression-free survival times (18,19). In recent clinical trials of patients with NSCLC with a PS of 2, overall survival ranged from 2.9 to 6.9 months (20-22). In the present study, the median survival time with LTOT was similar (194 days), suggesting that chemotherapy for patients with advanced lung cancer who receive LTOT might be beneficial.
Despite diversity in the tumor types, tumor stages, and chemotherapy regimens of the patients in our study, none of these variables significantly affected survival time with LTOT in univariate analyses. In the multivariate analysis, the treatment line (first or second) of the first chemotherapy with LTOT was the only factor associated with longer survival times. New effective therapeutic agents have been developed, especially for patients with NSCLC (6,7,23). First- and second-line chemotherapy with sufficient evidence of benefits may be acceptable even for advanced lung cancer patients receiving LTOT.
Although the eligible patients in the present study were carefully chosen via multidisciplinary team discussions, it has been reported that medical oncologists tend to overprescribe chemotherapy (24). Interestingly, the administration of chemotherapy to patients with advanced cancer at the end of their lives has increased recently (25,26). Twenty-five percent of the patients in our study received chemotherapy in their last month of life, 2 of whom died of chemotherapy-related adverse events. In the study by Näppä et al. (27), chemotherapy in the last month of life was associated with shorter survival, more hospital admissions, and fewer deaths at home. We did not evaluate quality of life in this study; however, the quality of life in patients receiving chemotherapy at the end of their lives might decline considerably. Consistent with the results of the present study, late-line chemotherapy in NSCLC patients with a poor PS has been shown to be ineffective (28,29). We also found that the final chemotherapy was third-line in 1 of the 2 patients who died of chemotherapy-related adverse events. Chemotherapy should be withheld for advanced lung cancer patients receiving LTOT if it is third-line or greater.
This study had two limitations. First, it was a retrospective single-institution study with a small study population. Second, PaO2 was not routinely checked; therefore, the indication for LTOT might be arbitrary. A prospective multicenter study is needed in the future.
In conclusion, chemotherapy for patients with advanced lung cancer who receive LTOT may be acceptable if it is the first- or second-line treatment. We should be mindful of the potential overuse of chemotherapy and its negative impact on quality of life.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Hagerty RG, Butow PN, Ellis PM, et al. Communicating with realism and hope: incurable cancer patients' views on the disclosure of prognosis. J Clin Oncol 2005;23:1278-88. [PubMed]
- Spiro SG, Porter JC. Lung cancer--where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med 2002;166:1166-96. [PubMed]
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010;(5):CD007309. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol 2014;25:1044-52. [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Kravchenko J, Berry M, Arbeev K, et al. Cardiovascular comorbidities and survival of lung cancer patients: Medicare data based analysis. Lung Cancer 2015;88:85-93. [PubMed]
- Grose D, Morrison DS, Devereux G, et al. The impact of comorbidity upon determinants of outcome in patients with lung cancer. Lung Cancer 2015;87:186-92. [PubMed]
- Kim KH, Park TY, Kim ES, et al. Clinical features of patients on home oxygen therapy due to chronic respiratory failure at one university hospital. Korean J Intern Med 2012;27:311-6. [PubMed]
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981;1:681-6. [PubMed]
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980;93:391-8. [PubMed]
- Kawakami Y. Current status and research on chronic respiratory failure in Japan. Intern Med 1996;35:436-42. [PubMed]
- Kida K, Motegi T, Ishii T, et al. Long-term oxygen therapy in Japan: history, present status, and current problems. Pneumonol Alergol Pol 2013;81:468-78. [PubMed]
- Asmis TR, Ding K, Seymour L, et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol 2008;26:54-9. [PubMed]
- Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4-9. [PubMed]
- Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a phase II trial in patients with metastatic nonsmall cell lung carcinoma . Cancer 2001;92:2639-47. [PubMed]
- Lilenbaum R, Villaflor VM, Langer C, et al. Single-agent versus combination chemotherapy in patients with advanced non-small cell lung cancer and a performance status of 2: prognostic factors and treatment selection based on two large randomized clinical trials. J Thorac Oncol 2009;4:869-74. [PubMed]
- Kosmidis PA, Dimopoulos MA, Syrigos K, et al. Gemcitabine versus gemcitabine-carboplatin for patients with advanced non-small cell lung cancer and a performance status of 2: a prospective randomized phase II study of the Hellenic Cooperative Oncology Group. J Thorac Oncol 2007;2:135-40. [PubMed]
- Langer C, Li S, Schiller J, et al. Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small-cell lung cancer patients: ECOG 1599. J Clin Oncol 2007;25:418-23. [PubMed]
- Hainsworth JD, Spigel DR, Farley C, et al. Weekly docetaxel versus docetaxel/gemcitabine in the treatment of elderly or poor performance status patients with advanced nonsmall cell lung cancer: a randomized phase 3 trial of the Minnie Pearl Cancer Research Network. Cancer 2007;110:2027-34. [PubMed]
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [PubMed]
- Liu TW, Chang WC, Wang HM, et al. Use of chemotherapy at the end of life among Taiwanese cancer decedents, 2001-2006. Acta Oncol 2012;51:505-11. [PubMed]
- Sezgin Goksu S, Gunduz S, Unal D, et al. Use of chemotherapy at the end of life in Turkey. BMC Palliat Care 2014;13:51. [PubMed]
- Lee HS, Chun KH, Moon D, et al. Trends in receiving chemotherapy for advanced cancer patients at the end of life. BMC Palliat Care 2015;14:4. [PubMed]
- Näppä U, Lindqvist O, Rasmussen BH, et al. Palliative chemotherapy during the last month of life. Ann Oncol 2011;22:2375-80. [PubMed]
- Choi YW, Ahn MS, Jeong GS, et al. Is fourth-line chemotherapy routine practice in advanced non-small cell lung cancer? Lung Cancer 2015;87:155-61. [PubMed]
- Ying Geng Z, Chang Jiao S, Cui Liu S, et al. Third-line therapy in advanced non-small cell lung cancer. J BUON 2013;18:899-907. [PubMed]


