Factors that predict progression-free survival in Chinese lung adenocarcinoma patients treated with epidermal growth factor receptor tyrosine kinase inhibitors
Introduction
Targeted molecular therapy is playing an increasingly important role in the treatment of non-small cell lung cancer (NSCLC). In advanced disease settings, first-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib, erlotinib and icotinib have demonstrated good clinical efficacy.
Several studies with EGFR-TKIs have revealed that factors such as East-Asian ethnicity, female sex, adenocarcinoma histology, and a non-smoking history are predictors of a favorable response in advanced NSCLC (1-3). Subsequent studies have shown that EGFR-activating mutations such as deletions in exon 19 (19del) and L858R point mutation in exon 21 are strong predictors of a favorable response to EGFR-TKIs (4-7). Consequently, for NSCLC patients with activating EGFR mutations, EGFR-TKIs are now recommended as the standard first-line therapy (8-10). However, in China, the EGFR mutation status is often unknown for patients when they receive treatment. Firstly, sometimes small specimens acquired by biopsies (e.g., bronchoscopy or lung biopsy) cannot meet the requirements for both diagnosis and EGFR detection, and thus EGFR mutation status is yet available (11). Secondly, elderly people or patients with poor physical conditions are intolerable to biopsy or secondary biopsy for the purpose of diagnosis and EGFR detection. In addition, despite Chinese patients with lung adenocarcinoma having a higher EGFR mutation rate (about 60%) than people of other ethnicities, the detection rate of EGFR mutations is still only about 30% in China. Additionally, some patients with advanced stage whose tumor specimens have been sent for EGFR detection cannot wait to receive first-line chemotherapy because they believe the duration waiting for the results is too long. Therefore, identifying factors other than the EGFR mutation status that will predict greater efficacy and survival in Chinese lung adenocarcinoma populations is vital.
Currently, few studies have focused on clinical factors other than the EGFR mutation status that can potentially influence survival in Chinese patients with advanced lung adenocarcinoma treated with EGFR-TKIs (12). Therefore, exploring factors that may have major roles in determining survival in these patients would help clinicians determine appropriate treatment strategies. In this study, we retrospectively collected clinicopathologic data on Chinese lung adenocarcinoma patients treated with EGFR-TKIs to identify clinical factors that may predict progression-free survival (PFS).
Patients and methods
Patients
A total of 208 patients treated with EGFR-TKIs (gefitinib, erlotinib or icotinib) between July 01, 2010 and December 01, 2013 at the Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, were retrospectively registered in this study. All were histologically diagnosed and staged as clinically advanced (stage IIIB or stage IV) lung adenocarcinoma. Prior to initiation of therapy, all patients were evaluated by computed tomography (CT) of the thorax.
Age, gender, smoking status, EGFR mutation status, clinical stage, surgical history, differentiation, tumor location, pretreatment levels of serum tumor markers [including carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), cancer antigen 125 (CA125), squamous cell carcinoma antigen (SCC), cytokeratin-19 fragments (CYFRA21-1), and lactate dehydrogenase (LDH)], the Eastern Cooperative Oncology Group performance status (ECOG PS), and the treatment line with EGFR-TKIs (first-line or other line) were all analyzed, along with the patients’ PFS times.
The study was approved by the Ethics Committee of Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai [Approval number: K(P)15-04].
Detection methods
To determine the patients’ EGFR mutation status, we used the ADx EGFR Mutation Detection Kit (Amoy Diagnostics, Xiamen, China), which has been approved by China’s Food and Drug Administration (CFDA). The principle of amplification refractory mutation system (ARMS) was used in the kit. Serum tumor markers were detected by radioimmunoassay. The cut-off values for judging normal or high levels of CEA, NSE, CA125, SCC, CYFRA21-1 and LDH were: 5 ng/mL, 25 ng/mL, 35 U/mL, 1.5 µg/L, 5 ng/mL, and 250 U/L, respectively.
Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) treatment, response evaluation and follow-up
All patients received 1 of the 3 EGFR-TKIs in 28-day cycles. Gefitinib and erlotinib were administered in dosages of 250 and 150 mg once daily, respectively, while icotinib was administered in a dosage of 125 mg 3 times daily. The tumor response was assessed after the first cycle of therapy and subsequently after every 2 cycles using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 (13). CT scans were performed to assess the response to EGFR-TKIs as clinically indicated or until discontinuation of treatment. Patients continued to receive EGFR-TKIs as long as they did not have progressive disease (PD) or intolerable adverse effects. The final cutoff date for the study was April 01, 2015.
Statistical analysis
Pearson χ2 tests were used for comparing characteristics between patients with 19del and L858R mutations. PFS was defined as the time from the date EGFR-TKIs were first administered until the date of objective PD according to RECIST version 1.0 or until the death of a patient. The Kaplan-Meier method and log-rank tests were used to analysis PFS and select factors with P values less than 0.05 in different levels, and then a Cox proportional hazards model was used to further identify the independent prognostic factors associated with PFS. All confidence intervals reported were 2-sided, and P values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS® software, version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
The demographic and clinicopathologic characteristics of the 208 patients registered in the study are summarized in Table 1. The patients tended to be young (<60 years of age, 58.2%), female (57.7%), and never-smokers (64.4%). Most patients (73.6%) sought medical attention because of symptoms such as cough, expectoration, chest pain, tightness, and shortness of breath; 67 (32.2%) had a history of surgery for lung cancer, and the majority (73.6%) had peripheral tumors. Most patients (79.3%) had tumors that were poorly differentiated; 190 (91.3%) had clinical IV stage disease and 18 (8.7%) had clinical stage IIIB disease according to the International Association for the Study of Lung Cancer Staging Project (7th edition). EGFR mutations were identified in 128 patients (61.5%), 65 of whom harbored 19del mutations while 63 harbored L858R mutations; 8 patients (3.8%) had a negative EGFR status and 72 (34.6%) had an unknown status. Most patients (87.0%) were in good physical condition, with an ECOG PS of 0 to 1. The numbers of patients who received gefitinib, erlotinib, and icotinib were 107 (51.4%), 33 (15.9%) and 68 (32.7%), respectively.
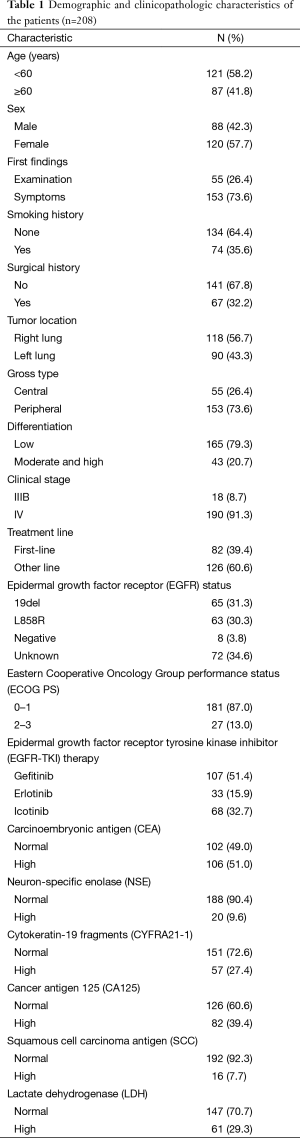
Full table
Response evaluation
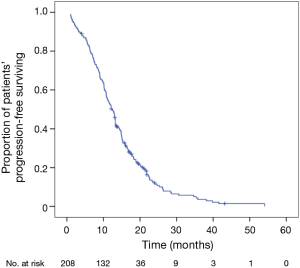
At the study date cutoff, 189 of the 208 patients (90.9%) met the RECIST criteria for disease progression, while 19 (9.1%) were found to have stable disease (SD). Six patients had to discontinue EGFR-TKIs treatment due to severe adverse effects (SAEs, 3 for AST or ALT elevation, 1 for vomiting, 1 for severe rash and 1 for interstitial lung disease). For these patients, we calculated the PFS from the day they were first administrated EGFR-TKIs to the day they were found SAEs. The median PFS for all 208 patients was 12.4 months (95% CI, 11.0-13.8 months) (Figure 1); 19 patients (9.1%) were censored at the study cutoff date.
Univariate survival analysis
The results of the univariate survival analysis by the Kaplan-Meier method are shown in Table 2. The analyses suggested that female sex (PFS 14.4 vs. 9.0 months for males; P<0.001), a non-smoking history (PFS 14.8 vs. 8.1 months for a history of smoking; P<0.001), a history of surgery for lung cancer (PFS 13.3 vs. 11.1 months for no surgical history; P=0.004), tumor located in the right lung (PFS 13.1 vs. 10.8 months for the left lung; P=0.027), first-line EGFR-TKI therapy (PFS 15.3 vs. 10.7 months for other lines; P<0.001), EGFR sensitive mutation status (PFS 14.9 vs. 3.2 months for a negative status; P<0.001), ECOG PS 0–1 (PFS 13.0 vs. 7.4 months for PS 2–3; P=0.001), and a high pretreatment CEA level (PFS 14.8 vs. 10.9 months for a normal level; P=0.044) were all predictors of a longer PFS. No statistically significant differences in PFS were found for age, the first finding (examination vs. symptoms), gross type, differentiation, clinical stage, EGFR-TKIs, and pretreatment serum levels of NSE, CYFRA21-1, CA125, SCC and LDH. We did not include the EGFR mutation status in the subsequent Cox multivariate regression analysis since the EGFR mutation status could influence the other two factors (sex and smoking history) in predicting PFS.
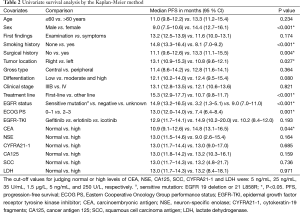
Full table
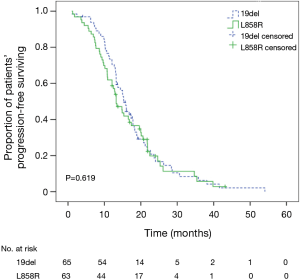
The demographic and clinicopathologic characteristics of 128 patients with EGFR sensitive mutations (19del or L858R) are shown in Table 3. Although PFS was longer in patients with 19del mutations (15.3 months; 95% CI, 13.6–17.0 months) compared with those with L858R mutations (13.2 months; 95% CI, 11.1–15.4 months), the difference between the 2 groups was not statistically significant (P=0.619, log-rank test) (Figure 2).
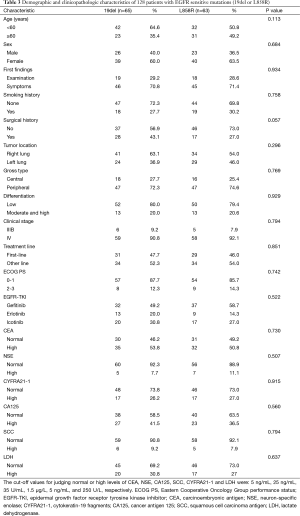
Full table
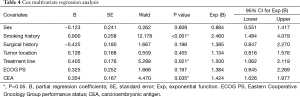
Cox multivariate regression analysis
In the multivariate analysis using a Cox proportional hazard model, a non-smoking history [hazard ratio (HR) =2.460; 95% CI, 1.484–4.079; P<0.001), first-line treatment (HR =1.500; 95% CI, 1.062–2.119; P=0.021), and a high pretreatment serum level of CEA (HR =1.424; 95% CI, 1.026–1.977; P=0.035) were independent predictors of a longer PFS with EGFR-TKI therapy. Female sex, tumor location in the right lung, a history of surgery for lung cancer, and ECOG PS 0–1 were not independent predictors of PFS (Table 4).
Full table
Discussion
The present study retrospectively analyzed clinical factors associated with PFS in Chinese patients with lung adenocarcinoma who were treated with EGFR-TKIs. We found that a non-smoking history, first-line EGFR-TKIs treatment and a high pretreatment serum level of CEA were independent predictors of a longer PFS by means of a univariate survival analysis and a Cox multivariate regression analysis. This suggests that in addition to patients with an EGFR mutation status, patients who receive first-line EGFR-TKIs, with a non-smoking history and a high pretreatment serum level of CEA may also benefit from treatment with EGFR-TKIs.
Previous studies have shown that first-line therapy with first-generation EGFR-TKIs in patients harboring tumors with EGFR-activating mutations achieves a longer PFS, with acceptable toxicity, in comparison with standard platinum-based chemotherapy (9,14-16). Our results revealed that first-line EGFR-TKIs treatment was an independent predictor of a longer PFS. However, some studies hold that EGFR-TKIs showed similar efficacy in patients with EGFR mutation-positive adenocarcinoma in terms of PFS regardless of treatment timing (17). Taking the assurance on drug exposure, improvement in quality of life, better tolerance by patients with poor PS, and deferral of whole-brain radiation therapy for patients with brain metastasis into account, the general application of first-line EGFR TKIs was recommended (18). In addition, EGFR-TKIs are currently recommended as the standard first-line therapy for patients with activating EGFR mutations (8-10). In contrast, EGFR-TKIs are not suitable for patients with no EGFR sensitive mutations. The TORCH study revealed that if the EGFR mutation status is unknown or there is no mutation, administration of EGFR-TKIs instead of chemotherapy is associated with a deleterious effect in terms of the response rate, PFS, and overall survival (19).
In this study, we confirmed that an EGFR sensitive mutation status was a predictor of a longer PFS. However, our findings did not indicate a statistically significant difference in PFS between patients with EGFR 19del and L858R mutations. Other investigations have reported clinical differences in both the response rate and survival between patients with different EGFR mutations (5,20-22). These studies have shown that patients with the EGFR 19del mutation have a longer survival following treatment with gefitinib or erlotinib than those with the L858R mutation, indicating that the mutation itself could possibly have an effect on outcomes. This has been demonstrated in a study of patients with surgically-resected, early-stage NSCLC who did not receive EFGR-TKIs (6). Existing data also suggest that the survival advantage found after treatment with gefitinib or erlotinib reflects an even more significant alteration in the potential disease course for patients with the 19del mutation (5,22). However, the sample sizes of these studies were relatively small. While our study did not find a statistically significant difference in PFS with EGFR-TKI treatment between patients with the 19del and L858R mutations, we cannot be certain that the effect of the EGFR mutation on outcomes in patients with advanced lung adenocarcinoma will be the same as that observed in patients with surgically-resected, early-stage disease.
NSCLC patients with a smoking history may have a poorer response and shorter PFS with EGFR-TKI treatment (23-25). Consistent with these findings, our study showed that a non-smoking history was an independent predictor of a longer PFS. Specific reasons for this phenomenon remain unclear, but several mechanisms have been proposed to explain the poorer response to EGFR-TKIs in patients with a smoking history, such as cigarette smoking-induced EGFR post-translational changes, and activation of the nicotinic acetylcholine receptor by cigarette smoking-induced EGFR-TKI resistance (26,27).
Several studies have suggested that levels of serum tumor markers may affect the survival of patients treated with EGFR-TKIs (28-34). In a multivariate regression analysis, we found that patients with a high serum level of CEA before EGFR-TKI treatment achieved a longer PFS compared with those with a normal serum level of CEA. Previous studies have also reached this conclusion (28,29). A recent study revealed that pre-treatment higher CEA (>5 ng/mL) was associated significantly with a higher overall survival (30). The reason maybe that high serum CEA levels are possibly associated with sensitive mutations of the EGFR gene in patients with lung adenocarcinomas (34). In clinical practice, patients with poor PS are intolerance to invasive procedures such as bronchoscopy, thus their EGFR mutation status remains unknown. Additionally, EGFR detection was always limited by the insufficient tissue acquired by biopsies, especially for the advanced stage. Peripheral blood samples are easily obtained for all patients. Therefore, serum CEA levels of these people could be used as a reference of EGFR-TKIs treatment. For patients with a high serum CEA level, it is worth attempting EGFR-TKI treatment, as this may achieve a better clinical response and survival. In addition, one recent study showed that in patients with wild-type/unknown EGFR mutation status, CEA response was significantly correlated with disease control rate and resulted as a significant predictor of PFS (31). Hence, according to this study, CEA response after 1 month of EGFR-TKIs therapy could be used as an early predictor of PFS in EGFR wild-type/unknown NSCLC for which EGFR status is not available.
Although some studies have shown that patients with a high CYFRA 21-1 level have a significantly shorter PFS (31,33), we found that the serum level of CYFRA21-1 was not a predictor for PFS. However, the previous studies were retrospective in design and had small sample sizes, and the patients either received chemotherapy before EGFR-TKI treatment or had EGFR mutations, which differs from our study. We hypothesize that these factors may, to some extent, have influenced the outcome.
It is worth noting that female sex was not a significant factor in predicting a longer PFS in the multivariate analysis of our study, although it is a well-known clinical factor for predicting a better PFS with EGFR-TKIs. In a recent large sample study analyzing gender-based impact of EGFR mutation in NSCLC, gender were not independent prognostic factors of 2-year overall survival (35). The reason may be that in Chinese adenocarcinoma patients, gender may not be an independent factor associated with EGFR mutation status, and thus, was not an independent predictor of PFS in this study.
Our study has some limitations. Firstly, it was a retrospective study conducted at a single center. Due to its retrospective nature, three EGFR-TKIs were not randomly assigned to patients and this may introduce some bias to the study. Most of the registered patients (107 cases) received gefitinib, while 33 patients received erlotinib. Although the three drugs may have a balanced efficacy and some analogous toxic profiles, heterogeneities exist among them. In addition, single center studies might show larger treatment effects than multicenter ones (36). Secondly, the sample size of the study was relatively small, and the associations reported as statistically significant require validation in larger patient cohorts in future studies. Thirdly, the results should be interpreted with caution as many patients with an unknown EGFR mutation status (34.6% of the population evaluated) were registered in the study. In addition, it should be noted that treatments administered before EGFR-TKI therapy may have influenced the EGFR mutation status, and influenced the survival of patients subsequently treated with EGFR-TKIs (37,38). Many of the patients registered had received chemotherapy and radiotherapy before EGFR-TKIs treatment, and not all of them had EGFR mutations that were detected only a short time before receiving EGFR-TKIs. This may have introduced some confounding factors into the study.
In conclusion, the findings of this study indicate that a non-smoking history, first-line EGFR-TKIs treatment and a high pretreatment serum level of CEA are independent predictors of a longer PFS in Chinese lung adenocarcinoma patients treated with EGFR-TKIs. However, due to the study’s limitations, prospective, multicenter analyses with larger sample sizes are needed to confirm these findings.
Acknowledgements
Editorial assistance with the manuscript was provided by Content Ed Net, Shanghai Co. Ltd.
Funding: This project was supported by the National Nature Science Foundation of China (Grant No. 81472175) and Shanghai Municipal Commission of Health and Family Planning Key Projects (Grant No. 20134007).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med 2005;353:133-44. [PubMed]
- Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol 2005;23:2513-20. [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [PubMed]
- Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:839-44. [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [PubMed]
- Yang CH, Yu CJ, Shih JY, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol 2008;26:2745-53. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 2008;26:2442-9. [PubMed]
- Wu CY, Hou LK, Ren SX, et al. High feasibility of liquid-based cytological samples for detection of EGFR mutations in Chinese patients with NSCLC. Asian Pac J Cancer Prev 2014;15:7885-9. [PubMed]
- Park JH, Kim TM, Keam B, et al. Tumor burden is predictive of survival in patients with non-small-cell lung cancer and with activating epidermal growth factor receptor mutations who receive gefitinib. Clin Lung Cancer 2013;14:383-9. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Koo DH, Kim KP, Choi CM, et al. EGFR-TKI is effective regardless of treatment timing in pulmonary adenocarcinoma with EGFR mutation. Cancer Chemother Pharmacol 2015;75:197-206. [PubMed]
- Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line--is there a difference? J Clin Oncol 2013;31:1081-8. [PubMed]
- Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 2012;30:3002-11. [PubMed]
- Won YW, Han JY, Lee GK, et al. Comparison of clinical outcome of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations. J Clin Pathol 2011;64:947-52. [PubMed]
- Sun JM, Won YW, Kim ST, et al. The different efficacy of gefitinib or erlotinib according to epidermal growth factor receptor exon 19 and exon 21 mutations in Korean non-small cell lung cancer patients. J Cancer Res Clin Oncol 2011;137:687-94. [PubMed]
- Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:3908-14. [PubMed]
- Fukuhara T, Maemondo M, Inoue A, et al. Factors associated with a poor response to gefitinib in the NEJ002 study: smoking and the L858R mutation. Lung Cancer 2015;88:181-6. [PubMed]
- Kim MH, Kim HR, Cho BC, et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer 2014;84:196-202. [PubMed]
- Lee JK, Shin JY, Kim S, et al. Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory study. Ann Oncol 2013;24:2080-7. [PubMed]
- Filosto S, Becker CR, Goldkorn T. Cigarette smoke induces aberrant EGF receptor activation that mediates lung cancer development and resistance to tyrosine kinase inhibitors. Mol Cancer Ther 2012;11:795-804. [PubMed]
- Wang S, Takayama K, Tanaka K, et al. Nicotine induces resistance to epidermal growth factor receptor tyrosine kinase inhibitor by α1 nicotinic acetylcholine receptor-mediated activation in PC9 cells. J Thorac Oncol 2013;8:719-25. [PubMed]
- Zhang Y, Jin B, Shao M, et al. Monitoring of carcinoembryonic antigen levels is predictive of EGFR mutations and efficacy of EGFR-TKI in patients with lung adenocarcinoma. Tumour Biol 2014;35:4921-8. [PubMed]
- Yang ZM, Ding XP, Pen L, et al. Analysis of CEA expression and EGFR mutation status in non-small cell lung cancers. Asian Pac J Cancer Prev 2014;15:3451-5. [PubMed]
- Romero-Ventosa EY, Blanco-Prieto S, González-Piñeiro AL, et al. Pretreatment levels of the serum biomarkers CEA, CYFRA 21-1, SCC and the soluble EGFR and its ligands EGF, TGF-alpha, HB-EGF in the prediction of outcome in erlotinib treated non-small-cell lung cancer patients. Springerplus 2015;4:171. [PubMed]
- Facchinetti F, Aldigeri R, Aloe R, et al. CEA serum level as early predictive marker of outcome during EGFR-TKI therapy in advanced NSCLC patients. Tumour Biol 2015;36:5943-51. [PubMed]
- Barlési F, Tchouhadjian C, Doddoli C, et al. CYFRA 21-1 level predicts survival in non-small-cell lung cancer patients receiving gefitinib as third-line therapy. Br J Cancer 2005;92:13-4. [PubMed]
- Tanaka K, Hata A, Kaji R, et al. Cytokeratin 19 fragment predicts the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitor in non-small-cell lung cancer harboring EGFR mutation. J Thorac Oncol 2013;8:892-8. [PubMed]
- Pan JB, Hou YH, Zhang GJ. Correlation between EGFR mutations and serum tumor markers in lung adenocarcinoma patients. Asian Pac J Cancer Prev 2013;14:695-700. [PubMed]
- Chang CH, Lee CH, Ho CC, et al. Gender-based impact of epidermal growth factor receptor mutation in patients with nonsmall cell lung cancer and previous tuberculosis. Medicine (Baltimore) 2015;94:e444. [PubMed]
- Dechartres A, Boutron I, Trinquart L, et al. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med 2011;155:39-51. [PubMed]
- Luo YH, Chen YM. Influence of chemotherapy on EGFR mutation status. Transl Lung Cancer Res 2013;2:442-4. [PubMed]
- Bai H, Wang Z, Chen K, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol 2012;30:3077-83. [PubMed]


