
Clinical outcomes and quantitative CT analysis after bronchoscopic lung volume reduction using valves for advanced emphysema
Introduction
In recent decades, bronchoscopic lung volume reduction (BLVR) with one-way valves has been shown to improve lung function, exercise capacity, and quality of life in patients with severe emphysema (1-8).
BLVR can cause target lobe volume reduction (TLVR) (9,10), and a TLVR of 350 mL measured by quantitative high-resolution CT (HRCT) analysis is assumed to be clinically significant (11). Even though recent studies showed the cut-off value for TLVR should be higher (12,13). However, variability in clinical outcomes has been observed, which warrants further research to investigate the other mechanisms or predictors of BLVR. It is commonly believed that with reduction of volume in the target lobe, the compressed, less diseased lobes will expand correspondingly. Then, it is rational that the airway may be dilated with pleural cavity pressure reduction and the improvement of dynamic airway compression following successful BLVR. Unfortunately, there are few studies illustrated that. Could the airway structure change with the treatment of valve volume reduction? Answering this question will help clarify the mechanism of BLVR and may determine parameters to predict responsiveness to treatment. We have observed several patients treated with BLVR experiencing intraluminal area (LA) enlargement and percentage of wall area (WA%) attenuation in the non-target bronchi (14). Therefore, we hypothesized that the airway structures changed after BLVR, and these changes may contribute to clinical benefits.
The objective of the present study was to detect the bronchial changes after BLVR and find the relationship between these changes and clinical benefits. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1734/rc).
Methods
Study design and patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was retrospective, so no informed consent was signed. A total of all 24 patients with advanced emphysema who underwent BLVR with valves [both Zephyr endobronchial valve (EBV) and the intrabronchial valve (IBV) system] at Peking University First Hospital, Beijing, China, between January 2010 and June 2018 were included in the study. Inclusion criteria were age over 40 years, nonsmoker status for at least three months, severe airflow obstruction [post-bronchodilator forced expiratory volume in 1 s (FEV1) <50% of predicted], hyperinflation [total lung capacity (TLC) >100% of predicted and residual volume (RV) >150% of predicted], heterogeneous emphysema (heterogeneity compared to the ipsilateral lobe ≥15% difference), and an intact interlobar fissure (≥90% complete). Chartis system was conducted for patients who received EBV therapy during surgery to evaluate the absence of collateral ventilation (CV) (1,2,15). Patients with severe pulmonary hypertension, diffusion capacity less than 20%, or severe comorbidities were excluded from the BLVR. HRCT with consolidations other than the target lobe, pneumothorax, or pleural effusion were excluded. Data collection included medical history, symptoms, modified Medical Research Council (mMRC) dyspnea scale, pulmonary function tests, 6 min walk distance (6MWD), and HRCT at pre-operation and at 1 month, 3, 6, and 12 months of follow-up. The study design was approved by the ethics committee of the Peking University First Hospital (No. 201971).
Pulmonary function tests
Pulmonary function tests (spirometry and body plethysmography) for FEV1, FVC (post-bronchodilator), TLC, RV, and percentages of predicted values were performed according to ATS/ERS guidelines. The baseline pulmonary function tests were completed three days before BLVR.
3D-CT analysis
An automatic analysis software (SYNAPSE VINCENT; Fuji Film, Tokyo, Japan) was used to reconstruct and analyze the HRCT images performed at full inspiration.
Airway
All the second-(lobar), third-(segmental), and fourth-(sub-segmental) bronchi were evaluated. Five parameters, including wall thickness (WT) and percentage of wall thickness (WT%), LA, wall area (WA), and WA% at the midpoint of each level of airways were calculated automatically and then the median of these parameters were used to subsequent analysis (Figure 1). Bronchi with bronchiectasis were not calculated.
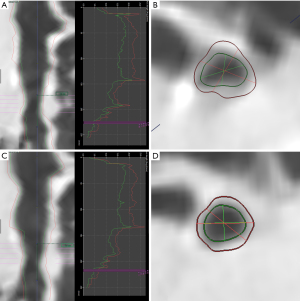
Emphysema and volume
The percentage of low attenuation area (LAA%) was used to evaluate the degree of emphysema, which was defined as the proportion below the CT density threshold of −950 Hounsfield units (HU). It was automatically calculated for the whole lung and for each lobe. Changes in the volume of all lobes, including the target lobe, non-target ipsilateral lobes, and contralateral lobes, were also quantified.
Outcomes
The primary outcomes were changes in WT, WT%, LA, WA, WA% of 2nd- to 4th-generation airways in the target lobe, ipsilateral non-target lobes, and contralateral lobes from baseline to 1-, 3-, 6-, and 12-month follow-up.
The secondary outcomes were changes in target lobe volume (TLV), ipsilateral lobe volume (ILV), and contralateral lobe volume (CLV) from baseline to the 1-, 3-, 6-, and 12-month follow-up.
Statistical analyses
SPSS version 24 (IBM, USA) was used for the statistical analyses. Given the small sample size, data were expressed as median [interquartile range (IQR)] by missing the condition of normal distribution. The Wilcoxon signed-rank test and Wilcoxon rank sum test were used to test the in-group differences as appropriate. Statistical significance was set at P<0.05.
Results
Patients and procedural details
A total of 24 patients underwent BLVR with valves between January 2010 and June 2018. Five patients were excluded: 1 patient received valve removal due to a target lobe infection and 4 dropped out after treatment. Thus, 19 patients were included. Eleven of 19 patients were treated with EBV and 8 were treated with IBV. Patient characteristics were summarized in Table 1. FEV1 was 0.60 (0.47–0.79) L, FEV1(%Pred) was 24.50% (18.60–29.63)%, TLC (%Pred) was 138.90% (129.50–144.30)% and RV(%Pred) was 279.60% (256.50–295.80)% at baseline. Patients treated with EBV were more severe. Four pneumothorax and 5 acute exacerbations (AEs) occurred up to 12 months after BLVR.
Table 1
| Variables | Value | Changes after BLVR | |||
|---|---|---|---|---|---|
| 1 month (n=15) | P | 12 months (n=13) | P | ||
| Age, year | 63.00 (52.00 to 66.00) | ||||
| Male sex, No. (%) | 19 (100.00) | ||||
| Lung function | |||||
| FEV1 (L) | 0.60 (0.47 to 0.79) | 0.12 (0.04 to 0.25) | 0.005 | 0.00 (−0.09 to 0.07) | 0.969 |
| FEV1 (%Pred) | 24.50 (18.60 to 29.63) | 3.00 (0.80 to 8.10) | 0.017 | 0.01 (−2.95 to 5.20) | 0.625 |
| FVC (L) | 1.93 (1.63 to 2.44) | 0.47 (0.23 to 0.78) | 0.001 | 0.10 (−0.04 to 0.56) | 0.074 |
| FVC (%Pred) | 48.70 (36.80 to 65.30) | ||||
| FEV1/FVC, % | 30.71 (26.06 to 35.36) | ||||
| TLC (L) | 9.03 (7.97 to 9.43) | −0.66 (−0.98 to 0.82) | 0.426 | −0.12 (−0.71 to 0.33) | 0.552 |
| TLC (%Pred) | 138.90 (129.50 to 144.30) | ||||
| RV (L) | 6.74 (5.87 to 7.21) | −0.96 (−1.61 to −0.65) | 0.078 | −0.12 (−0.98 to 0.35) | 0.311 |
| RV (%Pred) | 279.60 (256.50 to 295.80) | ||||
| 6MWD (m) | 279.00 (170.00 to 384.00) | 120.00 (13.00 to 157.00) | 0.005 | 49.00 (−124.00 to 129.00) | 0.594 |
| mMRC scale | 3.00 (3.00 to 3.75) | −1.00 (−1.00 to 0.00) | 0.021 | 0.00 (−0.25 to 1.25) | 0.785 |
| TLV, mL | 1,837.45 (1,377.98 to 2,223.88) | ||||
| Target lobe LAA% | 34.40 (27.85 to 55.88) | ||||
| Valve | |||||
| EBV, No. (%) | 11 (57.89) | ||||
| IBV, No. (%) | 9 (47.37) | ||||
| No. per patient | 3.00 (3.00 to 4.00) | ||||
| Site, No. (%) | |||||
| RUL | 6 (31.58) | ||||
| RML | 3 (15.79) | ||||
| RLL | 1 (5.26) | ||||
| LUL | 6 (31.58) | ||||
| LLL | 3 (15.79) | ||||
Data are presented as median (Q1 to Q3) unless otherwise noted. BLVR, bronchoscopic lung volume reduction; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; RV, residual volume; 6MWD, 6-min walk distance; mMRC, modified Medical Research Council; TLV, target lobe volume; LAA%, percentage of low attenuation area; EBV, endobronchial valve; IBV, intrabronchial valve; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
Paired data analyses showed that FEV1, FVC, 6MWD, and mMRC improved significantly at 1 month after therapy. However, these benefits faded at 12 months (see Table 1). Patients who treated with EBV had a better 6MWD improvement than patients treated with IBV (see Tables S1,S2).
Primary outcomes
Changes in airway structures
The target lobe bronchi could not be extracted effectively because of atelectasis after treatment. Thus, only the non-target lobes were measured. The WT, WT%, LA, WA and WA% of 2nd- to 4th-generation bronchi were showed in Table 2. After BLVR, in ipsilateral non-target lobes, WT and WA showed a decrease, but the significance was only observed in the bronchi of 3rd- and 4th-generation at 1 month. WT% was reduced significantly in 3rd-generation bronchi at 1 month [−2.00% (−9.50 to 2.00)%], 3 months [−3.00% (−8.00 to 2.00)%], and 6 months [−6.00% (−9.00 to −2.00)%], as did their WA% at 1 month [−5.00% (−13.00 to 2.50)%] and 6 months [−8.00% (−14.00 to −2.00)%], respectively. Changes in LA were ambiguous and varied at different time points without statistical significance (see in Table 3).
Table 2
| Variables | Ipsilateral airway | Contralateral airway | |||||
|---|---|---|---|---|---|---|---|
| 2nd- | 3rd- | 4th- | 2nd- | 3rd- | 4th- | ||
| WT (mm) | 1.15 (0.90 to 1.75) | 0.94 (0.73 to 1.17) | 0.74 (0.61 to 1.01) | 1.03 (0.78 to 2.07) | 0.86 (0.70 to 1.12) | 0.69 (0.59 to 0.89) | |
| WT% | 22.00 (16.00 to 26.75) | 29.00 (24.00 to 35.50) | 33.00 (30.00 to 36.00) | 21.00 (16.00 to 29.00) | 30.00 (24.00 to 35.00) | 33.00 (30.00 to 35.00) | |
| LA (mm2) | 65.30 (51.10 to 88.60) | 18.00 (11.85 to 29.10) | 7.65 (5.13 to 13.58) | 76.60 (48.10 to 90.90) | 15.85 (10.63 to 26.75) | 7.50 (4.90 to 10.75) | |
| WA (mm2) | 38.80 (20.70 to 67.05) | 18.55 (11.70 to 24.40) | 9.40 (6.35 to 16.70) | 37.30 (19.00 to 77.90) | 15.90 (11.40 to 23.05) | 8.70 (6.10 to 13.15) | |
| WA% | 38.50 (29.00 to 45.25) | 49.50 (43.00 to 58.00) | 55.00 (51.00 to 59.00) | 37.00 (30.00 to 48.00) | 51.00 (42.00 to 57.75) | 54.50 (51.00 to 58.25) | |
Data are presented as median (Q1 to Q3). WT, wall thickness; WT%, percentage of wall thickness; LA, intraluminal area; WA, wall area; WA%, percentage of wall area.
Table 3
| Variables | 1-month | P | 3-month | P | 6-month | P | 12-month | P |
|---|---|---|---|---|---|---|---|---|
| ΔWT (mm) | ||||||||
| 2nd- | −0.04 (−0.27 to 0.46) | 0.904 | −0.11 (−0.56 to 0.31) | 0.435 | −0.36 (−1.19 to −0.01) | 0.069 | −0.20 (−0.89 to 0.36) | 0.388 |
| 3rd- | −0.06 (−0.29 to 0.04) | 0.005 | −0.03 (−0.38 to 0.15) | 0.145 | −0.14 (−0.35 to 0.09) | 0.015 | −0.06 (−0.36 to 0.15) | 0.201 |
| 4th- | −0.07 (−0.28 to 0.09) | 0.010 | 0.03 (−0.21 to 0.13) | 0.988 | 0.02 (−0.11 to 0.13) | 0.918 | −0.04 (−0.14 to 0.09) | 0.386 |
| ΔWT% | ||||||||
| 2nd- | 0.00 (−3.00 to 5.00) | 0.704 | −2.00 (−6.00 to 2.50) | 0.169 | −3.50 (−11.25 to 1.00) | 0.091 | −1.50 (−9.00 to 5.50) | 0.533 |
| 3rd- | −2.00 (−9.50 to 2.00) | 0.012 | −3.00 (−8.00 to 2.00) | 0.049 | −6.00 (−9.00 to −2.00) | 0.006 | −2.50 (−8.75 to 4.75) | 0.223 |
| 4th- | −1.00 (−3.00 to 2.00) | 0.317 | 0.00 (−2.00 to 3.00) | 0.838 | 0.00 (−6.50 to 2.00) | 0.426 | 0.50 (−3.00 to 7.25) | 0.271 |
| ΔLA (mm2) | ||||||||
| 2nd- | 2.70 (−11.60 to 15.00) | 0.778 | 4.30 (−17.45 to 27.30) | 0.463 | −10.40 (−36.08 to 10.40) | 0.401 | −2.15 (−23.53 to 7.85) | 0.695 |
| 3rd- | −1.00 (−4.55 to 5.00) | 0.936 | 1.50 (−2.25 to 6.25) | 0.211 | 1.30 (−0.90 to 9.70) | 0.156 | −2.35 (−4.55 to 3.73) | 0.808 |
| 4th- | −1.00 (−2.95 to 0.78) | 0.060 | 0.00 (−2.30 to 2.90) | 0.833 | 0.15 (−2.78 to 4.03) | 0.933 | −0.65 (−6.48 to 2.55) | 0.204 |
| ΔWA (mm2) | ||||||||
| 2nd- | −0.20 (−12.30 to 18.20) | 0.904 | −2.40 (−22.35 to 17.55) | 0.619 | −12.65 (−62.75 to −0.80) | 0.123 | −10.65 (−37.40 to 11.83) | 0.272 |
| 3rd- | −2.4 (−6.45 to 1.60) | 0.011 | −1.10 (−9.25 to 4.85) | 0.219 | −2.50 (−7.10 to 5.20) | 0.363 | −1.40 (−9.68 to 5.50) | 0.458 |
| 4th- | −1.20 (−4.73 to 1.63) | 0.028 | −0.10 (−3.30 to 3.60) | 0.992 | 0.25 (−3.43 to 3.53) | 0.991 | −1.15 (−6.18 to 2.03) | 0.124 |
| ΔWA% | ||||||||
| 2nd- | −1.00 (−4.00 to 6.00) | 1.000 | −5.00 (−10.00 to 3.00) | 0.130 | −6.00 (−16.75 to 0.00) | 0.046 | −3.00 (−14.00 to 8.00) | 0.479 |
| 3rd- | −5.00 (−13.00 to 2.50) | 0.011 | −4.00 (−12.00 to 3.50) | 0.058 | −8.00 (−14.00 to −2.00) | 0.005 | −3.50 (−11.75 to 7.50) | 0.224 |
| 4th- | 0.00 (−4.75 to 3.00) | 0.417 | 0.00 (−3.00 to 5.00) | 0.872 | 0.00 (−8.25 to 3.00) | 0.338 | 0.50 (−4.00 to 9.50) | 0.383 |
Data are presented as median (Q1 to Q3). BLVR, bronchoscopic lung volume reduction; WT, wall thickness; WT%, percentage of wall thickness; LA, intraluminal area; WA, wall area; WA%, percentage of wall area.
Compared with ipsilateral bronchi, changes of contralateral bronchi structures were slight (see Table 4). The WT% of 4th-generation decreased significantly by −1.00% (−5.00 to 2.00)% and WA% of 4th-generation decreased by −2.00% (−6.00 to 3.00)% at 6 months after BLVR but raised back at 12 months. It was worth noting that LA of 3rd-generation showed continuous enlargement after BLVR and achieved the largest level at 6 months [increased by 3.60 (−2.60 to 7.80) mm2].
Table 4
| Variables | 1-month | 3-month | 6-month | 12-month |
|---|---|---|---|---|
| ΔWT (mm) | ||||
| 2nd- | 0.06 (−0.20 to 0.37) | 0.03 (−0.32 to 0.35) | −0.07 (−0.65 to 0.06) | −0.01 (−0.23 to 0.12) |
| 3rd- | 0.02 (−0.18 to 0.33) | 0.05 (−0.14 to 0.26) | −0.02 (−0.24 to 0.19) | 0.09 (−0.13 to 0.34) |
| 4th- | 0.02 (−0.14 to 0.11) | −0.01 (−0.12 to 0.12) | −0.02 (−0.24 to 0.12) | −0.01 (−0.17 to 0.13) |
| ΔWT% | ||||
| 2nd- | 0.00 (−2.75 to 4.25) | −1.00 (−6.00 to 4.50) | −1.00 (−4.50 to 1.50) | 0.00 (−4.50 to 2.75) |
| 3rd- | 0.00 (−5.25 to 7.00) | 0.00 (−3.00 to 7.25) | −2.00 (−7.00 to 5.00) | 2.00 (−5.00 to 8.00) |
| 4th- | 0.00 (−4.00 to 4.00) | 0.00 (−3.00 to 4.00) | −1.00 (−5.00 to 2.00)* | 1.00 (−3.75 to 3.00) |
| ΔLA (mm2) | ||||
| 2nd- | 0.70 (−7.75 to 5.40) | −3.10 (−14.65 to 6.45) | −3.70 (−19.00 to 3.90) | −0.40 (−5.40 to 8.95) |
| 3rd- | 0.15 (−4.28 to 4.45) | 1.00 (−3.03 to 5.38) | 3.60 (−2.60 to 7.80)* | 0.40 (−6.10 to 5.70) |
| 4th- | 0.10 (−1.82 to 2.22) | 0.00 (−1.80 to 1.50) | 0.30 (−2.00 to 3.20) | −0.15 (−1.78 to 1.38) |
| ΔWA (mm2) | ||||
| 2nd- | 1.90 (−8.35 to 9.73) | 3.10 (−19.85 to 15.90) | −1.80 (−38.30 to 1.45) | 0.10 (−9.25 to 3.18) |
| 3rd- | −0.40 (−4.40 to 8.10) | 1.15 (−3.88 to 7.33) | −1.10 (−5.10 to 5.20) | 2.00 (−2.20 to 8.80) |
| 4th- | 0.00 (−2.43 to 2.50) | −0.10 (−2.80 to 2.40) | 0.10 (−4.00 to 2.30) | −0.50 (−3.78 to 1.85) |
| ΔWA% | ||||
| 2nd- | 0.00 (−4.00 to 7.50) | −2.00 (−9.00 to 6.50) | −1.00 (−7.50 to 2.00) | 0.00 (−6.50 to 4.50) |
| 3rd- | 1.00 (−7.25 to 10.25) | 0.00 (−4.00 to 7.25) | −2.00 (−9.00 to 4.00) | −1.00 (−6.00 to 12.00) |
| 4th- | 0.00 (−5.00 to 5.00) | 0.00 (−4.00 to 5.00) | −2.00 (−6.00 to 3.00)* | 1.50 (−3.75 to 4.00) |
Data are presented as median (Q1 to Q3). *, indicate P<0.05. WT, wall thickness; WT%, percentage of wall thickness; LA, intraluminal area; WA, wall area; WA%, percentage of wall area.
Both EBV and IBV groups showed a tendency to decrease in WT, WT%, WA (except at 6 months), and WA% of 3rd-generation up to 6 months of follow-up in ipsilateral bronchi. However, these changes in the EBV group were more significant at 1 month while in the IBV group at 6 months (see in Table S3).
Subgroup analysis
The minimum clinically important difference (MCID) for FEV1 and 6MWD in the treatment of patients with severe emphysema has been established in previous studies (16,17). An improvement of 15% in FEV1 and 26 m in 6MWD was assumed to be clinically significant. In our study, patients who met both criteria were defined as responders (responder group, n=9), otherwise as non-responders (non-responder group, n=10). To evaluate whether the airway structures changes contribute to the responsiveness of therapy, subgroup analysis of 2nd- to 4th-generation airways of non-target lobes was conducted based on the primary endpoint results.
In responder group, WT% and WA% of 3rd-generation ipsilateral bronchi at 1, 3, and 6 months decreased (Figure 2), as well as the WT, WT%, WA, and WA% in 4th-generation bronchi at 1 month (Figure 3), whereas such changes were not observed in non-responder group. LA of 3rd-generation bronchi decreased especially at 1 month in non-responder group (Figure 2C). In contralateral bronchi, WT% and WA% showed a significant decrease only in 4th-generation at 6 months after therapy (see Table S4).
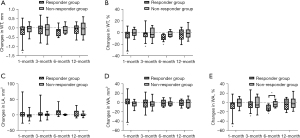
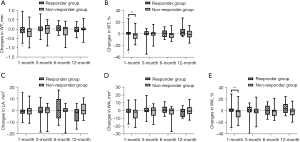
Secondary outcomes
After BLVR, the target lobe volume decreased −613.70 (−1,451.78 to −154.40) mL at 1 month, −913.50 (−1,462.08 to −338.18) mL at 3 months, −1,163.00 (−1,525.90 to −525.30) mL at 6 months, and −715.30 (−1,525.90 to −378.50) mL at 12 months. Meanwhile, the volume of ipsilateral lobes increased moderately consistent with them and achieved best improvements at 6 months (median change: 470.40 mL). The contralateral lobes also showed slight amelioration but there was no statistical significance (see in Table 5).
Table 5
| Variables | 1-month (N=14) | 3-month (N=14) | 6-month (N=13) | 12-month (N=11) |
|---|---|---|---|---|
| ΔTLV (mL) | −613.70 (−1,451.78 to −154.40) | −913.50 (−1,462.08 to −338.18) | −1,163.00 (−1,525.90 to −525.30) | −715.30 (−1,525.90 to −378.50) |
| P | 0.003 | 0.001 | 0.018 | 0.004 |
| ΔILV (mL) | 108.05 (−52.55 to 303.40) | 90.10 (16.20 to 335.70) | 470.40 (123.65 to 1,056.90) | 88.70 (−128.40 to 525.00) |
| P | 0.157 | 0.044 | 0.028 | 0.101 |
| ΔCLV (mL) | 131.10 (26.43 to 254.73) | 116.45 (−169.70 to 301.63) | 126.70 (−456.85 to 943.45) | 169.80 (−519.45 to 359.35) |
| P | 0.064 | 0.433 | 0.327 | 0.638 |
Data are presented as median (Q1 to Q3). TLV, target lobe volume; ILV, ipsilateral lobes volume; CLV, contralateral lobes volume.
Discussion
Our study showed that BLVR can cause mechanical changes in patients with severe emphysema. In addition to inducing a volume shift from the target lobe to non-target lobes, bronchial thickness and lumen area can be ameliorated bilaterally. Responsiveness to valve therapy correlates with changes in the bronchial structure.
Initially, it was believed that the clinical benefits in BLVR with valves for advanced emphysema came mainly from the results of volume reduction in the diseased lobe and thus provide more space to more functional lobes. It has been proven that patients with lobar atelectasis, even those with pneumothorax, had a higher response rate in lung function parameters (1,3,11,18). In recent years, more studies have focused on other potential mechanisms and predictors of BLVR. In 2013, Argula found that baseline regional perfusion could affect responsiveness to BLVR (19). Patients with low target lobe perfusion at baseline showed a greater improvement in exercise capacity with EBV therapy. Correspondingly, Thomsen revealed that patients with high ipsilateral non-target lobe perfusion demonstrated greater improvements in the 6MWD (20). In contrast to these earlier findings, the airway structures before and after therapy has not been studied previously. Although it is rational that volume reduction may induce compressed airway dilation, there is no direct evidence for this. We had reported the results of quantitative CT assessment after BLVR on chest congress but with only 4 cases included (14). In the 4 patients, LA enlargement and WA% attenuation were observed in the non-target bronchi.
In the present study, we observed meaningful changes in airways measured using 3D-CT images after BLVR. The wall of 2nd- to 4th-generation airways tended to become thinner after the procedure. In the responder group, all parameters of ipsilateral non-target 3rd- and 4th-generation bronchial WT decreased significantly after 1 month as well as WT% and WA% at 3 and 6 months, whereas no significant changes occurred in the non-responder group. However, further correlation analysis was not conducted given that only less than 15 patients were included in the follow up. Thickening of small airway walls, one of the most important features of chronic obstructive pulmonary disease (COPD), is thought to be a combination of inflammatory changes, constrictions, and remodeling. It remained unclear how airway structure changes after BLVR. One possible reason was that the compressed and less diseased ipsilateral lobes expanded after BLVR (as shown in Table 5), bronchi then changed correspondingly, which could lead to mechanic improvements. The bronchi measured in our study were larger airways (2nd- to 4th-generation). Earlier findings proved the relationship between the morphology of the central airways and distal small airways, which were the sites of airway obstruction in COPD (21). Recently, several studies have examined the relationship between the wall or intraluminal area of 3rd-to 6th-generation airways on CT and clinical features. Grydeland demonstrated that airway wall thickening was positively correlated with respiratory symptoms (22). Mohamed reported that airway WT was independently associated with a lower FEV1 after an average 3-year follow-up period (23). Karayama showed that both WT and intraluminal area were correlated with FEV1 (24). A noteworthy finding was that the effect was not only present at the ipsilateral lobes of the treatment but was also observed in the contralateral lobes. This implied that BLVR may have pan-pulmonary effects. Thus, valve therapy may play a significant role in alleviating local or pan-pulmonary disturbances in mechanics. Additionally, the reduction of the bronchial wall may have other mechanisms, such as the reduction of smooth muscles and the inhibition of edema or hyperplasia of the mucus membrane by inflammation reduction, although there was no evidence. More rigorous studies would be needed to provide further insights.
An important negative finding was that LA showed no consistent enlargement in our study. One possible explanation is that 2nd- to 4th-generation bronchi have cartilages, which may limit their enlargement to some extent. As small airways lack cartilage, we could expect that non-supported bronchi or bronchioles might be dilated with the reduction of pleural pressure, and thus airflow limitation was improved. As a limitation of the methodology, we could not analyze structural changes in these smaller airways. In the future, more sensitive imaging methodologies should be used to elucidate changes in precision.
We also assessed the volume changes in target lobes, non-target ipsilateral lobes, and contralateral lobes. Coxson reported a volume decrease in the treated upper lobe and an increase in the untreated non-upper lobes after IBV therapy at 6 months (9). The Endobronchial Valve for Emphysema Palliation Trial (VENT) study showed similar changes in patients with EBV therapy (1). Our analysis supports the observations of previous studies. It was showed that nontarget ipsilateral lobes acquired the largest increase in volume. This complies with the rationale for the lung volume reduction procedures. For the contralateral lobes, we only observed varying but insignificant increases. In addition, a decrease in target lobes was observed up to 12 months after treatment (Table 5).
Furthermore, we found that EBV seems to have a more rapid effect on bronchial wall thinning than IBV. This may be partly explained by the study design. The EBV data were obtained from patients in a real hospital setting. These patients seemed to be more severe, with lower FEV1, shorter distance of 6MWD, and higher mMRC scale, even than those reported in most published studies (1,2,4,25). IBV data were obtained from the REACH study, which is a randomized controlled trial. Thus, the baseline parameters in EBV group were worse than those in IBV group. Another noticeable reason is the possible difference in the mechanism between EBV and IBV. However, there was no convincing evidence given that the sample size was too small, and the baseline characters did not match. It is necessary to conduct further head-to-head trials with larger sample size to evaluate whether there are any differences in responses between EBV and IBV.
Our study has some limitations. Firstly, the small sample size resulted in insufficient power to explain the outcomes. Secondly, all measurement and analysis of quantitative CT were based on normal inspiratory CT, rather end-inspiratory CT guided by spirometer. Given that patients with severe emphysema would present dyspnea in all probability, the degree of emphysema and bronchitis in CT would be underestimate or overestimate. Furthermore, the techniques we used could only identify and construct airways with inner diameters greater than 2 mm, which include the 5th generation or larger bronchi. Even if it was feasible, the analysis of such is more challenging than in advanced emphysema because bronchi in these patients are frequently twisted.
In conclusion, patients with severe emphysema could benefit from BLVR. In addition to volume reduction, bronchial structures, especially in 3rd- and 4th-generation show changes after the therapy. Our report provided a preliminary basis for BLVR mechanics. Further studies are needed to elucidate the mechanisms and structural changes of the smaller bronchi.
Acknowledgments
Part of the result of our study was reported in ATS2020 entitled “Different Airway Structure Changes on Computed Tomography and Efficacy after Bronchoscopic Lung Volume Reduction Using Two Different Valves Respectively for Advanced Chronic Obstructive Pulmonary Disease”: https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A5042.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1734/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1734/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1734/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1734/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study design was approved by the ethics committee of the Peking University First Hospital (No. 201971). This study was retrospective, so no informed consent was signed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. [Crossref] [PubMed]
- Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J 2012;39:1319-25. [Crossref] [PubMed]
- Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Hartman JE, Klooster K, Slebos DJ, et al. Improvement of physical activity after endobronchial valve treatment in emphysema patients. Respir Med 2016;117:116-21. [Crossref] [PubMed]
- Gompelmann D, Benjamin N, Bischoff E, et al. Survival after Endoscopic Valve Therapy in Patients with Severe Emphysema. Respiration 2019;97:145-52. [Crossref] [PubMed]
- Li S, Wang G, Wang C, et al. The REACH Trial: A Randomized Controlled Trial Assessing the Safety and Effectiveness of the Spiration® Valve System in the Treatment of Severe Emphysema. Respiration 2019;97:416-27. [Crossref] [PubMed]
- Coxson HO, Nasute Fauerbach PV, Storness-Bliss C, et al. Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur Respir J 2008;32:1443-50. [Crossref] [PubMed]
- Mata J, Altes T, Truwit J, et al. Characterization and detection of physiologic lung changes before and after placement of bronchial valves using hyperpolarized helium-3 MR imaging: preliminary study. Acad Radiol 2011;18:1195-9. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J 2013;41:302-8. [Crossref] [PubMed]
- Gompelmann D, Kontogianni K, Schuhmann M, et al. The minimal important difference for target lobe volume reduction after endoscopic valve therapy. Int J Chron Obstruct Pulmon Dis 2018;13:465-72. [Crossref] [PubMed]
- Welling JBA, Hartman JE, van Rikxoort EM, et al. Minimal important difference of target lobar volume reduction after endobronchial valve treatment for emphysema. Respirology 2018;23:306-10. [Crossref] [PubMed]
- Huang JF, Rui W, Qiu JX, Quantitative CT. Assessment of Bronchoscopic Lung Volume Reduction With Valve. Chest 2014;145:361A. [Crossref]
- Gompelmann D, Eberhardt R, Slebos DJ, et al. Diagnostic performance comparison of the Chartis System and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology 2014;19:524-30. [Crossref] [PubMed]
- Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008;31:416-69. [Crossref] [PubMed]
- Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J 2011;37:784-90. [Crossref] [PubMed]
- Gompelmann D, Benjamin N, Kontogianni K, et al. Clinical and radiological outcome following pneumothorax after endoscopic lung volume reduction with valves. Int J Chron Obstruct Pulmon Dis 2016;11:3093-9. [Crossref] [PubMed]
- Argula RG, Strange C, Ramakrishnan V, et al. Baseline regional perfusion impacts exercise response to endobronchial valve therapy in advanced pulmonary emphysema. Chest 2013;144:1578-86. [Crossref] [PubMed]
- Thomsen C, Theilig D, Herzog D, et al. Lung perfusion and emphysema distribution affect the outcome of endobronchial valve therapy. Int J Chron Obstruct Pulmon Dis 2016;11:1245-59. [PubMed]
- Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 2005;171:142-6. [Crossref] [PubMed]
- Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med 2010;181:353-9. [Crossref] [PubMed]
- Mohamed Hoesein FA, de Jong PA, Lammers JW, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J 2015;45:644-51. [Crossref] [PubMed]
- Karayama M, Inui N, Mori K, et al. Respiratory impedance is correlated with morphological changes in the lungs on three-dimensional CT in patients with COPD. Sci Rep 2017;7:41709. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]


