
Older patients more likely to die from cancer-related diseases than younger with stage IA non-small cell lung cancer: a SEER database analysis
Introduction
Lung cancer is the leading cause of cancer-related death in the United States (1). It has been estimated that the number of individuals aged ≥65 who will be diagnosed with cancer between 2010 and 2030 will increase by 67% (2). So cancer is an age-related disease. AS we know, although older people have a higher incidence rate, it remains unclear whether they have a better prognosis compared with younger patients. Some reports about NSCLC in young patients have been published, but there is still opposite data regarding whether young people with non-small cell lung cancer (NSCLC) have a better or worse prognosis compared with older patients. According to research results published before 2008, younger patients with NSCLC had a worse prognosis than older ones and the interpretation of this result considers that younger patients have a greater delay in seeking thoracic surgical care (3-5); however, some research results after 2008 showed that older patients had a worse prognosis and the reason for this result is that younger patients receive more complete and aggressive treatment (6-8). Furthermore, due to the large time span and the lack of rigorous research standards and we do not have a good understanding of this phenomenon from the observed results.
An increase in age is associated with the increased probability of developing cardiovascular and cerebrovascular diseases. Cancer patients are a special group, especially older patients. Many different causes of death have been identified in older patients with lung cancer, including both lung cancer and non-lung cancer causes (9). Although Eguchi et al. revealed that non-cancer-specific mortality is a significant competing event that increases with age, the results may be biased due to retrospective analysis study design, coupled with the fact that no further debiased analysis was performed (10). Accordingly, in order to obtain more precise results, we analyzed the data using the propensity-score matching (PSM) method.
The aim of this study was to investigate the main cause of death in older patients with lung cancer and to perform a comparison with younger patients in order to observe the differences between these two cohorts. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-505/rc).
Methods
Patients
We collect data in the Surveillance, Epidemiology, and End Results-18 (SEER-18) database (1973–2016 varying) and analyze the data retrospectively. The database covers many U.S. regional cancer registries and most of the U.S. cancer population, making it possible to do some research using its large data. Patients with microscopically confirmed first primary stage I NSCLC ≤3 cm who underwent lobectomy and were diagnosed from Jan 2004 to Dec 2016 were selected. The tumor-node-metastasis (TNM) stage was manually adjusted according to the American Joint Committee on Cancer (AJCC) 8th edition criteria. To explore the causes of death at different ages, patients were stratified into three groups according to their age: ≤60, 61–70, and ≥71 years. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Inclusion and exclude criteria
The main inclusion criteria for this study included NSCLC and stage IA. The variables collected included Gender, grade, age, number of lymph nodes, insurance, and tumor size. Pathological type of NSCLC was defined according to International Classification of Diseased for Oncology-3 (ICD-O-3) and divided into three main categories: adenocarcinoma, squamous cell carcinoma and other NSCLC. The main exclude criteria for this study included received radiation therapy or chemotherapy (including neoadjuvant and adjuvant therapy), tumors located bilaterally and in the main bronchus, missing values of baseline characteristics and lymph nodes examined.
Outcomes
The causes of mortality were categorized as lung cancer, cardio-cerebrovascular, chronic obstructive pulmonary disease (COPD), other causes of cancer and other causes of non-cancer diseases. According to the International Classification of Diseased-10 (ICD-10) criteria, cardio-cerebrovascular and COPD-associated codes were I00-I78 and J40-J47, respectively. As defined by the SEER database, overall survival (OS) was defined as the period from the date of diagnosis to the last follow-up or death. Lung cancer-specific survival (LCSS) was calculated from the date of diagnosis to the date of death from lung cancer.
PSM
There are some biases in the retrospective data. We used the PSM method to minimize the potential selection biases. We chose six variables that may affect the long-term prognosis for the PSM, including sex, tumor grade, tumor histology, tumor size, lymph node examined, and insurance. In order to highlight the differences in the causes of death between older and younger patients, we only included two groups older ≥71 and ≤60 years for matching score analysis. A 1:1 match without replacement was conducted to pair each patient aged ≤60 years with one patient aged ≥71 years. The caliper size for matching was set at 0.001.
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 (SPSS, IBM, Chicago, IL, USA). χ2 tests and t-tests were used to compare differences between categorical and continuous variables, respectively. A Cox proportional hazards model including terms for age, sex, grade, histology, tumor size, lymph node examined, and insurance was fit for all subjects in this cohort to evaluate the effect of the different covariates on prognosis. Kaplan-Meier analysis with the log-rank test was used to plot survival curves for comparisons between all age groups. P<0.05 was considered statistically significant.
Results
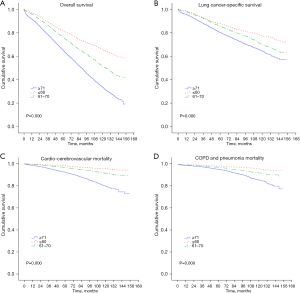
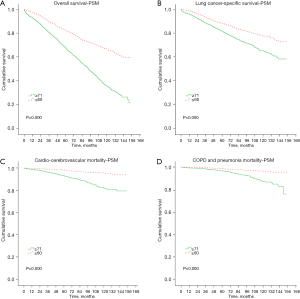
Among the 16,672 eligible NSCLC patients found in the SEER database, 3,930 were aged ≤60 years, 6,391 were aged 61–70 years, and 6,351 were aged ≥71 years. The clinicopathological characteristics of the patients in terms of age before and after PSM are summarized in Table 1. Before matching, the median survival time for patients aged ≤60 years was 120 months, the median survival time for those 61–70 years was 107 months, and that of patients aged ≥71 years was 88 months. After PSM of the ≤60- and ≥71-year-old patients using a ratio of 1:1, there were 3,130 patients in the ≤60 years group and 3,130 patients in the ≥71 years group. Significant differences were found in sex, the number of lymph nodes, insurance, histology, and tumor size between the two groups (all P<0.05; Table 1).
Table 1
| Covariate | Age group (years), n (%) | Age group (PSM) (years), n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤60 | 61–70 | ≥71 | P | ≤60 | ≥71 | P | ||
| Sex | 0.000 | 0.979 | ||||||
| Male | 1,587 (40.4) | 2,870 (44.9) | 2,769 (43.6) | 1,229 (39.3) | 1,230 (39.3) | |||
| Female | 2,343 (59.6) | 3,521 (55.1) | 3,582 (56.4) | 1,901 (60.7) | 1,900 (60.7) | |||
| Grade | 0.400 | 1.000 | ||||||
| I | 939 (23.9) | 1,429 (22.4) | 1,476 (23.2) | 768 (24.5) | 769 (24.5) | |||
| II | 1,890 (48.1) | 3,115 (48.7) | 3,050 (48.0) | 1,566 (50.0) | 1,565 (50.0) | |||
| III | 1,039 (26.4) | 1,766 (27.6) | 1,741 (27.4) | 778 (24.9) | 778 (24.9) | |||
| IV | 62 (1.6) | 81 (1.3) | 84 (1.3) | 18 (0.6) | 18 (0.6) | |||
| Lymph node examined | 0.002 | 0.992 | ||||||
| 0 | 96 (2.4) | 144 (2.3) | 210 (3.3) | 30 (1.0) | 31 (1.0) | |||
| 1–3 | 681 (17.3) | 1,063 (16.6) | 1,106 (17.4) | 469 (15.0) | 469 (15.0) | |||
| ≥4 | 3,153 (80.2) | 5,184 (81.1) | 5,035 (79.3) | 2,631 (84.1) | 2,630 (84.0) | |||
| Insurance | 0.000 | 0.946 | ||||||
| Yes | 2,976 (75.7) | 4,992 (78.1) | 4,863 (76.6) | 2,513 (80.3) | 2,514 (80.3) | |||
| No | 115 (2.9) | 52 (0.8) | 15 (0.2) | 5 (0.2) | 4 (0.1) | |||
| Unknown | 839 (21.3) | 1347 (21.1) | 1,473 (23.2) | 612 (19.6) | 612 (19.6) | |||
| Histology | 0.000 | 0.999 | ||||||
| Adenocarcinoma | 2,933 (74.6) | 4,347 (68.0) | 4,079 (64.2) | 2,439 (77.9) | 2,440 (77.9) | |||
| Squamous | 563 (14.3) | 1,501 (23.5) | 1,746 (27.5) | 477 (15.2) | 477 (15.2) | |||
| Other NSCLC | 434 (11.0) | 543 (8.5) | 526 (8.3) | 214 (6.8) | 213 (6.8) | |||
| Tumor size, x ± s | 18.60±6.25 | 19.11±6.22 | 20.05±6.14 | 0.000 | 19.26±5.96 | 19.26±5.96 | 0.998 | |
| Causes of death | 0.000 | 0.000 | ||||||
| Alive | 3,046 (77.5) | 4,409 (69.0) | 3,517 (55.4) | 2,454 (78.4) | 1,860 (59.4) | |||
| Lung cancer | 527 (13.4) | 1,018 (15.9) | 1,235 (19.4) | 403 (12.9) | 584 (18.7) | |||
| Cardio-cerebrovascular disease | 95 (2.4) | 264 (4.1) | 555 (8.7) | 77 (2.5) | 235 (7.5) | |||
| COPD and pneumonia | 82 (2.1) | 234 (3.7) | 353 (5.6) | 58 (1.9) | 139 (4.4) | |||
| Other cancers | 26 (0.7) | 64 (1.0) | 77 (1.2) | 18 (0.6) | 28 (0.9) | |||
| Other non-cancer diseases | 154 (3.9) | 402 (6.3) | 614 (9.7) | 120 (3.8) | 284 (9.1) | |||
NSCLC, non-small cell lung cancer; COPD, chronic obstructive pulmonary disease; PSM, propensity score matching.
Before matching, 2,780 (16.7%) patients died of lung cancer, and 2,920 (17.5%) patients died of non-lung cancer diseases. The three main causes of non-lung cancer mortality were cardio-cerebrovascular disease [914 (5.5%)], COPD and pneumonia [669 (4.0%)], and other cancers [167 (1.0%)]. The number of deaths from lung cancer was 527 (13.4%), 1,018 (15.9%), and 1,235 (19.4%) in the ≤60, 61–70, ≥71 years age groups, respectively. Detailed cause of death in these three groups are shown in Table 1. Patients aged ≥71 years had slightly higher mortality rates of lung cancer, cardio-cerebrovascular disease, COPD and pneumonia, and other cancers (Table 1). Notably, lung cancer-related mortality rates were almost two times higher in the ≥71 years age groups compared with those ages ≤60 years (P<0.05).
After PSM, there were 987 (15.8%) patients who died of lung cancer and 959 (15.3%) who died of non-lung cancer diseases (Table 1). The three main causes of non-lung cancer mortality were cardio-cerebrovascular disease [312 (5.0%)], COPD and pneumonia [197 (3.1%)], and other cancers [46 (0.7%)]. The number of deaths from lung cancer was 403 (12.9%) and 584 (18.7%) in the ≤60 and ≥71 years age groups, respectively. Detailed causes of mortality in these two cohorts are shown in Table 1. Patients aged ≥71 years had slightly higher mortality rates of lung cancer, cardio-cerebrovascular disease, COPD and pneumonia, and other cancers.
In Cox proportional hazards regression model results, gender, grade, age, number of lymph nodes, pathological type, and tumor size could affect OS (P<0.05; Table 2). Sex, grade, age, number of lymph nodes, pathological type, and tumor size were also associated with patient OS after SPM (P<0.05; Table 2). Gender, grade, age, number of lymph nodes, insurance, and tumor size were able to influence patients’ LCSS (P<0.05; Table 3). Similar results were obtained in other areas except insurance after PSM (P<0.05; Table 3).
Table 2
| Covariate | OS | OS (PSM) | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| Sex | 0.716 (0.679–0.754) | 0.000 | 0.743 (0.679–0.813) | 0.000 | |
| Grade | 1.328 (1.279–1.379) | 0.000 | 1.422 (1.332–1.519) | 0.000 | |
| Age | 1.042 (1.039–1.045) | 0.000 | 1.041 (1.037–1.046) | 0.000 | |
| Lymph node examined | 0.829 (0.790–0.871) | 0.000 | 0.882 (0.795–0.979) | 0.018 | |
| Insurance | 1.016 (0.987–1.046) | 0.286 | 1.038 (0.987–1.092) | 0.147 | |
| Histology | 1.100 (1.056–1.146) | 0.000 | 1.102 (1.021–1.189) | 0.012 | |
| Tumor size | 1.015 (1.010–1.019) | 0.000 | 1.011 (1.003–1.019) | 0.005 | |
OS, overall survival; PSM, propensity score matching; CI, confidence interval.
Table 3
| Covariate | LCSS | LCSS (PSM) | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| Sex | 0.786 (0.730–0.845) | 0.000 | 0.833 (0.736–0.943) | 0.004 | |
| Grade | 1.439 (1.366–1.515) | 0.000 | 1.610 (1.471–1.763) | 0.000 | |
| Age | 1.025 (1.021–1.029) | 0.000 | 1.026 (1.021–1.032) | 0.000 | |
| Lymph node examined | 0.809 (0.756–0.867) | 0.000 | 0.857 (0.743–0.988) | 0.033 | |
| Insurance | 1.041 (1.000–1.084) | 0.048 | 1.048 (0.978–1.123) | 0.182 | |
| Histology | 1.040 (0.983–1.101) | 0.175 | 1.061 (0.957–1.177) | 0.259 | |
| Tumor size | 1.023 (1.017–1.029) | 0.000 | 1.022 (1.012–1.033) | 0.000 | |
LCSS, lung cancer-specific survival; PSM, propensity score matching; CI, confidence interval.
In the subsequent survival analysis, the OS of younger patients highlighted a significant survival advantage over older patients (P<0.05, Figure 1A). In terms of LCSS, older patients showed a worse survival than younger patients (P<0.05; Figure 1B). When stratified by the four main non-lung cancer causes of mortality, fewer younger patients died from cardio-cerebrovascular disease, COPD, and pneumonia than older patients (P<0.05; Figure 1C,1D). After PSM, the OS of patients aged ≤60 years illustrated a significant survival advantage compared to those aged ≥71 years (P<0.05; Figure 2A). In terms of LCSS, patients aged ≥71 years had a worse survival than those aged ≤60 years (P<0.05; Figure 2B). Also, fewer patients aged ≤60 years died from cardio-cerebrovascular disease, COPD, and pneumonia compared to patients aged ≥71 years (P<0.05; Figure 2C,2D).
Discussion
In the past 14 years ago, it was believed that NSCLC patients younger than 45 years had a significantly worse prognosis than older patients, as younger patients tended to seek thoracic surgical care with substantial delay, even when symptomatic (5). However, Dell’Amore et al. showed that young patients could achieve better survival regardless of this delay due to the more comprehensive and aggressive treatment (6). In order to investigate the impact of age on survival and the differences between the causes of death between different age groups, we excluded many interfering factors, such as the administration of radiotherapy and chemotherapy. Finally, this study with large population-based assessed long-term cause-specific mortality in a balanced cohort of 16,672 patients with stage IA NSCLC who underwent lobectomy. In our study, the OS and LCSS of younger patients had a better prognosis over older patients. Younger patients with NSCLC have a lower mortality rate, which is consistent with Subramanian et al. (11). Also, Tian et al. reported that younger with NSCLC had a better prognosis than older (12).
Previous studies have provided some possible explanations for these results. Our results revealed that older patients had less examined lymph nodes and a larger tumor size than younger patients, and these results can also explain some of the reasons. Dell’Amore et al. results showed that the median time until presentation for treatment was 3.9 months in younger patients vs. 2.6 months for older patients (P=0.03); however, the younger group were able to obtain more aggressive treatment and achieve better survival (6). It was found that the leading risk factor for NSCLC was tobacco smoking and older patients tend to have a longer history of smoking, so that could explain why tumors tend to occur in older people. However, young patients might have different genetic and molecular characteristics of NSCLC, especially those who had never smoked (11,13).
Although the above research can explain some of the reasons, the research flaws are also obvious. As older patients tend to be frailer and have multiple cardiac and respiratory comorbidities, younger patients are more likely to undergo more comprehensive, radical, aggressive, and combination treatment modalities than older patients. In this study, we used PSM to minimize patient selection bias, and found a worse long-term survival in older patients. After PSM, the 5-year OS in younger patients was 82% vs. 62% in older subjects (P=0.000). Also, lung cancer was the main cause of death among all the possible death causes. In the multivariate regression analysis, age was an unfavorable factor, which is consistent with some previous research results (9,14).
As we know, the physiological gets weaker in cardiovascular and pulmonary functions in older and morbidity and mortality after pulmonary resection may increase. Previous studies have reported a postoperative complication rate of approximately 30–50% in older NSCLC patients (15-17). In this study, the rate of older patients dying from heart and lung diseases was higher than that of younger patients. The aging process affects the function of most organs, including those regulating the immune system. This decline is often believed to increase the risks of adequate cancer therapy (18). In these cases, surgical procedures are often less aggressive, and chemotherapy doses may be lowered, or treatment is denied altogether.
Although toxicities are more common in older patients, most respond well to treatment (19). Nevertheless, it is a fact that older patients are frequently undertreated or excluded from therapy trials (20). Several groups have been evaluating the use of existing cancer treatment protocols in older patients with regards to prognostic factors, toxicities, quality of life, and cost of care. Specific prospective data on adjuvant chemotherapy in older patients is unavailable (20,21). A meta-analysis of the effect of age on adjuvant chemotherapy for completely resected NSCLC showed that the survival benefit of chemotherapy was almost the same among the three age groups (<65; 65–69; ≥70 years). We generally believed that older patients had poor physiological and couldn’t tolerate chemotherapy, so patients age ≥70 years got less chemotherapy, but the results were confirmed that the overall toxicity rates were similar between the different age groups (22). Specific recommendations should be developed, and physicians should be encouraged to make appropriate protocols available to their patients (23,24). Furthermore, the research of Willén et al. also suggests that older patients with early-stage lung cancer should be considered for curative treatment to a larger extent (25). Our results showed that the OS of older patients with early-stage NSCLC who did not receive adjuvant therapy was worse than that of younger patients. According to previous studies, radical surgical treatment and further treatment are required for older patients in order to improve their survival, so long as the older patient is physically eligible (22,25).
There are some limitations in this study that need to be addressed. Firstly, many data are not detailed due to the long-time span of the SEER database included in this study. Secondly, this study will have certain biases because it is a retrospective study. Finally, it cannot be ruled out that the results are biased even when the PSM method is used.
The prognosis for stage IA NSCLC is worse with increasing age, and lung cancer-related death also increases. Older patients are more likely to die from lung cancer. Adjuvant treatment is necessary despite the older age and the relatively poor physical condition. The needs of older persons with respect to cancer prevention, detection, and treatment are areas of concern to all oncologists. We hope that these results will have a significant effect on the longevity and quality of life of older individuals.
Acknowledgments
Funding: This study was funded by the Henan Key Science and Technology Research Project (No. 202102310025) and the Henan Key Medical Science and Technology Research Project (No. LHGJ20190671).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-505/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-505/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758-65. [Crossref] [PubMed]
- Bourke W, Milstein D, Giura R, et al. Lung cancer in young adults. Chest 1992;102:1723-9. [Crossref] [PubMed]
- Antkowiak JG, Regal AM, Takita H. Bronchogenic carcinoma in patients under age 40. Ann Thorac Surg 1989;47:391-3. [Crossref] [PubMed]
- Bryant AS, Cerfolio RJ. Differences in outcomes between younger and older patients with non-small cell lung cancer. Ann Thorac Surg 2008;85:1735-9; discussion 1739. [Crossref] [PubMed]
- Dell’Amore A, Monteverde M, Martucci N, et al. Surgery for non-small cell lung cancer in younger patients: what are the differences? Heart Lung Circ 2015;24:62-8. [Crossref] [PubMed]
- Higton AM, Monach J, Congleton J. Investigation and management of lung cancer in older adults. Lung Cancer 2010;69:209-12. [Crossref] [PubMed]
- Yang CJ, Kumar A, Deng JZ, et al. A National Analysis of Short-term Outcomes and Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Clinical Stage II Non-Small-Cell Lung Cancer. Ann Surg 2021;273:595-605. [Crossref] [PubMed]
- Wei S, Tian J, Song X, et al. Causes of death and competing risk analysis of the associated factors for non-small cell lung cancer using the Surveillance, Epidemiology, and End Results database. J Cancer Res Clin Oncol 2018;144:145-55. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Tian DL, Liu HX, Zhang L, et al. Surgery for young patients with lung cancer. Lung Cancer 2003;42:215-20. [Crossref] [PubMed]
- Kreuzer M, Kreienbrock L, Gerken M, et al. Risk factors for lung cancer in young adults. Am J Epidemiol 1998;147:1028-37. [Crossref] [PubMed]
- Smith CB, Wolf A, Mhango G, et al. Impact of Surgeon Volume on Outcomes of Older Stage I Lung Cancer Patients Treated via Video-assisted Thoracoscopic Surgery. Semin Thorac Cardiovasc Surg 2017;29:223-30. [Crossref] [PubMed]
- Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg 2013;16:819-23. [Crossref] [PubMed]
- Liu HC, Huang WC, Wu CL, et al. Surgery for elderly lung cancer. Ann Thorac Cardiovasc Surg 2013;19:416-22. [Crossref] [PubMed]
- Srisomboon C, Koizumi K, Haraguchi S, et al. Thoracoscopic surgery for non-small-cell lung cancer: elderly vs. octogenarians. Asian Cardiovasc Thorac Ann 2013;21:56-60. [Crossref] [PubMed]
- Ramsey SD, Howlader N, Etzioni RD, et al. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol 2004;22:4971-8. [Crossref] [PubMed]
- Altundag O, Stewart DJ, Fossella FV, et al. Many patients 80 years and older with advanced non-small cell lung cancer (NSCLC) can tolerate chemotherapy. J Thorac Oncol 2007;2:141-6. [Crossref] [PubMed]
- Gridelli C, Aapro M, Ardizzoni A, et al. Treatment of advanced non-small-cell lung cancer in the elderly: results of an international expert panel. J Clin Oncol 2005;23:3125-37. [Crossref] [PubMed]
- Lilenbaum RC, Herndon JE 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 2005;23:190-6. [Crossref] [PubMed]
- Früh M, Rolland E, Pignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol 2008;26:3573-81. [Crossref] [PubMed]
- Dodd GD. Cancer control and the older person. An overview. Cancer 1991;68:2493-5. [Crossref] [PubMed]
- Ahmed Z, Kennedy K, Subramanian J. The role for chemotherapy in 80 years and older patients with metastatic non-small cell lung cancer: A National cancer database analysis. Lung Cancer 2021;154:62-8. [Crossref] [PubMed]
- Willén L, Berglund A, Bergström S, et al. Are older patients with non-small cell lung cancer receiving optimal care? A population-based study. Acta Oncol 2022;61:309-17. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


