
Afatinib 30 mg in the treatment of common and uncommon EGFR-mutated advanced lung adenocarcinomas: a retrospective, single-center, longitudinal study
Introduction
Epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) is the standard treatment for lung adenocarcinomas (LAD) with EGFR mutations (1). While all generations of EGFR-TKIs are applicable for EGFR-mutated LAD, the effectiveness may vary across different EGFR mutation subtypes. EGFR-TKI-sensitive mutations exon 19 deletions (19del) and exon 21 L858R (21L858R) are the most common mutations in non-small cell lung cancer (NSCLC) (2,3). However, uncommon EGFR are increasingly reported owing to the technical advances in genomic sequencing (4,5). These uncommon mutations primarily consisting of G719X in exon 18, S768I in exon 20, L861Q in exon 21, classical compound mutations, and exon 20 insertions (20ins) account for 10–20% of all EGFR mutations. Inconsistent efficacy to EGFR-TKIs has been observed in LAD patients with uncommon EGFR mutations and treatment strategies for this entity remain to be optimized (4).
Increasing evidence has shown favorable outcomes of LAD with uncommon EGFR mutations upon treatment with second-generation TKI afatinib which has an irreversible and broad inhibitory spectrum against the ErBB family (6-8). Afatinib gained similar or even better effectiveness to first-generation EGFR-TKIs in the treatment of NSCLC with EGFR exon 19del or 21L858R mutations (9,10). Moreover, afatinib is superior to the first-generation TKIs gefitinib and erlotinib for major uncommon mutations including G719X, S768I, L861Q, and classical compound mutations (7,11). A combined post-hoc analysis of LUX-Lung series trials demonstrated that afatinib treated NSCLC patients with G719X, S768I and L861Q had an objective response rate (ORR) of 77.8%, 100% and 56.3%, and a PFS of 13.8, 14.7 and 8.2 months, respectively (6). However, clinical benefit of EGFR-TKIs seemed to be inferior in patients with EGFR 20ins mutation (6).
The clinical application of afatinib may be limited due to its high frequency of adverse events (AEs), especially for Asian patients. The recommended initiating dose of afatinib at 40 mg/d was associated with up to 28% of grade 3 or greater treatment-related AEs in the LUX-Lung series studies (3,10,12-14). Asian patients seemed to be more susceptible to experiencing AEs caused by 40 mg afatinib compared with non-Asian patients (13,15). In the real-world studies, 29.6–38.3% of Asian patients receiving afatinib starting from 40 mg needed a dose reduction throughout the course of treatment (16,17). Recent studies demonstrated that afatinib at 30 mg/d was more tolerable than the dose of 40 mg/d, but favorable efficacy was maintained for Asian patients with NSCLC (18-20). However, evidence is sparse regarding the efficacy and safety of afatinib at 30 mg in the treatment of advanced LAD with uncommon EGFR mutations.
Hence, in the present study on EGFR-mutated LAD, we attempted to investigate the clinical outcomes of afatinib starting at 30 mg/d on different EGFR subtypes. We hypothesized that afatinib 30 mg would be effective and well-tolerable for either common or major uncommon EGFR mutations. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-507/rc).
Methods
Patients and treatment
This was a single-center, retrospective, longitudinal study. Medical records of advanced NSCLC patients with EGFR mutation who received afatinib at a starting dose of 30 mg from January 2017 to November 2021 at Shanghai Chest Hospital, China were retrospectively reviewed. The inclusion criteria were: (I) pathologically confirmed stage IIIB/IIIC/IV LAD according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual; (II) received afatinib as a first- or later-line therapy for at least 30 consecutive days; (III) with EGFR mutations including 19del, 21L858R, G719X, S768I, L861Q, classical compound mutations, and exon 20ins mutation. Patients without complete medical records or follow-up information were excluded.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Shanghai Chest Hospital approved the study (No. IS22027), and all the patients supplied written consent before treatment.
Data collection and follow-up
Baseline clinicopathological data including age, gender, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS) score, clinical stage, treatment line, and metastatic site at initiation were collected from the medical record system. EGFR genotyping was performed at the hospital’s laboratory by polymerase chain reaction (PCR) or next-generation sequencing (NGS) as previously described (21). EGFR mutations were divided into the following three subtypes: (I) common mutations, including exon 19del and 21L858R; (II) uncommon mutations, including G719X in exon 18, L861Q in exon 21, S768I in exon 20, and classical complex mutations; and (III) exon 20ins.
Therapeutic and prognostic information was retrospectively collected. Efficacy was assessed according to the Response Evaluation Criteria in Solid Tumor (version 1.1) with complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) by computed tomography (CT) scans, nuclear magnetic resonance imaging (MRI) or abdominal ultrasound at every 4–8 weeks during treatment (22). ORR was defined as achieving CR or PR. Progression-free survival (PFS) was defined as the time from starting administration of oral afatinib to PD or last follow-up. AEs were graded using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median and interquartile range (IQR). Categorical variables were reported as number and percentage. The chi-square test was used to compare the subgroup differences in baseline characteristics. PFS was analyzed by the Kaplan-Meier survival curves and subgroup comparsion was conducted using a log-rank test. Multivariable Cox regression analysis was used to determine the estimated hazard ratio (HR) for PFS. Analyses were performed using SPSS 23.0 software. A two-sided P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Among 62 initially screened patients, four patients were excluded due to incomplete medical records or follow-up information. A total of 58 patients were finally included in this study. The median age was 62 (range, 41–78) years old. Male patients accounted for 53.4%, and 26 (44.8%) patients were ever or current smokers. Half of all patients received afatinib as the first-line regimen. Eleven (19.0%) patients had brain metastases. Most patients (98.3%) had an ECOG PS score of 0–1 except 1 patient had a score of 2. At the data cut-off date, the median follow-up duration was 22.9 months (Table 1).
Table 1
| Characteristics | 19del/21L858R (n=32), n (%) | G719X/L861Q/S768I/complex (n=19), n (%) | 20ins (n=7), n (%) | P value |
|---|---|---|---|---|
| Age (years) | 0.601 | |||
| <65 | 20 (62.5) | 12 (63.2) | 3 (42.9) | |
| ≥65 | 12 (37.5) | 7 (36.8) | 4 (57.1) | |
| Sex | 0.446 | |||
| Male | 15 (46.9) | 11 (57.9) | 5 (71.4) | |
| Female | 17 (53.1) | 8 (42.1) | 2 (28.6) | |
| Smoking history | 0.451 | |||
| No | 20 (62.5) | 9 (47.4) | 3 (42.9) | |
| Ever/current | 12 (27.5) | 10 (52.6) | 4 (57.1) | |
| Stage | 0.155 | |||
| IIIB/IIIC | 2 (6.3) | 4 (21.1) | 0 (0.0) | |
| IV | 30 (93.8) | 15 (78.9) | 7 (100.0) | |
| Treatment line | 0.852 | |||
| First-line | 15 (46.9) | 10 (52.6) | 4 (57.1) | |
| ≥ Second-line | 17 (53.1) | 9 (47.4) | 3 (42.9) | |
| Brain metastasis | 0.376 | |||
| No | 28 (87.5) | 14 (73.7) | 5 (71.4) | |
| Yes | 4 (12.5) | 5 (26.3) | 2 (28.6) | |
| Number of metastatic sites | 0.702 | |||
| <3 | 27 (84.4) | 15 (78.9) | 5 (71.4) | |
| ≥3 | 5 (15.6) | 4 (21.1) | 2 (28.6) |
EGFR, epidermal growth factor receptor; 19del, exon 19 deletions; 21L858R, exon 21 L858R; 20ins, exon 20 insertions.
The number of patients with EGFR common mutations of 19del or 21L858R was 32 (55.2%). The number of patients with EGFR uncommon mutations of G719X, L861Q, S768I, or complex mutations was 19 (32.8%), while the number of patients with EGFR 20ins was 7 (12.1%). The baseline characteristics among the three subtypes of EGFR mutations did not differ significantly (Table 1).
Efficacy evaluation
The overall median PFS was 9.83 [95% confidence index (CI): 5.76–13.91] months. A total of 46 (79.3%) patients experienced disease progression or died at the last follow-up and 12 (20.7%) patients were still under treatment.
Comparisons between subgroups showed that significant differences in PFS and ORR existed among patients with different subtypes of EGFR mutations. The median PFS for patients with EGFR common mutations (19del/21L858R), uncommon mutations (G719X/L861Q/S768I, or complex mutations), and EGFR 20ins was 13.97 (12.06–15.89), 8.48 (0.32–16.64), and 3.78 (1.93–5.64) months, respectively (P=0.002) (Figure 1A). The ORR for subgroups of common mutations, uncommon mutations, and EGFR 20ins was 43.8%, 63.2%, and 0.0%, respectively (P=0.016). The corresponding DCR was 84.4%, 94.7%, and 85.7%, respectively (P=0.537) (Table 2).
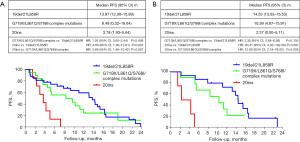
Table 2
| Characteristics | N | PFS | ORR | DCR | |||||
|---|---|---|---|---|---|---|---|---|---|
| PFS (95% CI) (months) | P value | N (%) | P value | N (%) | P value | ||||
| Age (years) | 0.140 | 0.710 | 0.692 | ||||||
| <65 | 35 | 9.44 (3.53–15.35) | 15 (42.9) | 30 (85.7) | |||||
| ≥65 | 23 | 13.25 (6.04–20.46) | 11 (47.8) | 21 (91.3) | |||||
| Sex | 0.114 | 0.125 | 1.000 | ||||||
| Male | 31 | 8.48 (3.56–13.41) | 11 (35.5) | 27 (87.1) | |||||
| Female | 27 | 11.38 (6.93–15.82) | 15 (55.6) | 24 (88.9) | |||||
| Smoking history | 0.194 | 0.380 | 1.000 | ||||||
| No | 32 | 11.38 (4.91–17.84) | 16 (50.0) | 28 (87.5) | |||||
| Ever/current | 26 | 8.48 (5.00–11.96) | 10 (38.5) | 23 (88.5) | |||||
| Stage | 0.425 | 0.393 | 1.000 | ||||||
| IIIB/IIIC | 6 | 12.13 (1.93–22.33) | 4 (66.7) | 6 (100.0) | |||||
| IV | 52 | 9.44 (5.81–13.06) | 22 (42.3) | 45 (86.5) | |||||
| EGFR mutation | 0.002 | 0.016 | 0.537 | ||||||
| 19del/21L858R | 32 | 13.97 (12.06–15.89) | 14 (43.8) | 27 (84.4) | |||||
| G719X/L861Q/S768I/complex mutations | 19 | 8.48 (0.32–16.64) | 12 (63.2) | 18 (94.7) | |||||
| 20ins | 7 | 3.78 (1.93–5.64) | 0 (0.0) | 6 (85.7) | |||||
| Treatment line | 0.464 | 0.035 | 0.102 | ||||||
| First-line | 29 | 11.38 (7.96–14.79) | 17 (58.6) | 28 (96.6) | |||||
| ≥ Second-line | 29 | 6.67 (0.67–12.68) | 9 (31.0) | 23 (79.3) | |||||
| Brain metastasis | 0.097 | 1.000 | 0.327 | ||||||
| No | 47 | 10.39 (6.68–14.10) | 21 (44.7) | 40 (85.1) | |||||
| Yes | 11 | 5.33 (1.74–8.91) | 5 (45.5) | 11 (100.0) | |||||
| Number of metastatic sites | 0.030 | 0.517 | 0.607 | ||||||
| <3 | 47 | 11.38 (4.94–17.81) | 20 (42.6) | 42 (89.4) | |||||
| ≥3 | 11 | 5.52 (0.00–11.62) | 6 (54.5) | 9 (81.8) | |||||
EGFR, epidermal growth factor receptor; LAD, lung adenocarcinomas; PFS, progression-free survival; ORR, objective response rate; DCR, disease control rate; CI, confidence index; 19del, exon 19 deletions; 21L858R, exon 21 L858R; 20ins, 20 exon insertions.
For the 29 patients who received first-line afatinib, the median PFS was 11.38 (7.96–14.79) months. Patients with EGFR 20ins had a significantly poorer PFS compared to patients with other EGFR common and uncommon mutations (2.37 vs. 14.53 vs. 10.39 months, P<0.001) (Figure 1B). Similarly, patients with EGFR 20ins had a remarkably lower ORR (0% vs. 60% vs. 80%, P=0.023) and DCR (75% vs. 100% vs. 100%, P=0.039) compared to patients with common and uncommon EGFR mutations (Table 3).
Table 3
| Characteristics | N | PFS | ORR | DCR | |||||
|---|---|---|---|---|---|---|---|---|---|
| PFS (95% CI) (months) | P value | N (%) | P value | N (%) | P value | ||||
| Age (years) | 0.707 | 0.876 | 0.483 | ||||||
| <65 | 15 | 11.18 (2.84–19.52) | 9 (60.0) | 15 (100.0) | |||||
| ≥65 | 14 | 13.25 (6.19–20.31) | 8 (57.1) | 13 (92.9) | |||||
| Sex | 0.212 | 0.176 | 1.000 | ||||||
| Male | 15 | 11.18 (5.85–16.51) | 7 (46.7) | 14 (93.3) | |||||
| Female | 14 | 12.13 (8.96–15.30) | 10 (71.4) | 14 (100.0) | |||||
| Smoking history | 0.233 | 0.219 | 0.448 | ||||||
| No | 16 | 12.13 (8.96–15.30) | 11 (68.8) | 16 (100.0) | |||||
| Ever/current | 13 | 11.18 (6.02–16.34) | 6 (46.2) | 12 (92.3) | |||||
| Stage | 0.782 | 1.000 | 1.000 | ||||||
| IIIB/IIIC | 5 | 12.13 (0.00–33.31) | 3 (60.0) | 5 (100.0) | |||||
| IV | 24 | 11.38 (7.34–15.41) | 14 (58.3) | 23 (95.8) | |||||
| EGFR mutation | <0.001 | 0.023 | 0.039 | ||||||
| 19del/21L858R | 15 | 14.53 (13.53–15.53) | 9 (60.0) | 15 (100.0) | |||||
| G719X/L861Q/S768I/complex mutations | 10 | 10.39 (4.87–15.91) | 8 (80.0) | 10 (100.0) | |||||
| 20ins | 4 | 2.37 (0.00–5.11) | 0 (0.0) | 3 (75.0) | |||||
| Brain metastasis | 0.043 | 0.683 | 1.000 | ||||||
| No | 21 | 13.25 (9.00–17.50) | 13 (61.9) | 20 (95.2) | |||||
| Yes | 8 | 5.33 (0.86–9.79) | 4 (50.0) | 8 (100.0) | |||||
| Number of metastatic sites | 0.238 | 0.622 | 1.000 | ||||||
| <3 | 25 | 12.13 (9.13–15.14) | 14 (56.0) | 24 (96.0) | |||||
| ≥3 | 4 | 5.52 (0.40–10.65) | 3 (75.0) | 4 (100.0) | |||||
PFS, progression-free survival; ORR, objective response rate; DCR, disease control rate; CI, confidence index; EGFR, epidermal growth factor receptor; 19del, exon 19 deletions; 21L858R, exon 21 L858R; 20ins, exon 20 insertions.
Safety
AEs of any grade occurred in 22 patients (37.9%) and mainly included diarrhea (n=12, 20.7%), rash (n=10, 17.2%), stomatitis (n=6, 10.3%), and paronychia (n=6, 10.3%). Five patients (8.6%) developed diarrhea ≥ grade 3 but all recovered after symptomatic treatment. No patients discontinued afatinib treatment due to AEs (Table 4).
Table 4
| AEs | Any, n (%) | ≥ Grade 3, n (%) |
|---|---|---|
| Total | 22 (37.9) | 5 (8.6) |
| Diarrhea | 12 (20.7) | 5 (8.6) |
| Rash | 10 (17.2) | 0 (0.0) |
| Stomatitis | 6 (10.3) | 0 (0.0) |
| Paronychia | 6 (10.3) | 0 (0.0) |
| Fatigue | 5 (8.6) | 0 (0.0) |
| Nausea | 1 (1.7) | 0 (0.0) |
| Gingivitis | 1 (1.7) | 0 (0.0) |
AEs, adverse events.
Discussion
The current study demonstrated that afatinib at 30 mg/d was effective for EGFR-mutated advanced LAD with a favorable PFS of 9.8 months for the overall population. For common and uncommon EGFR mutations except for 20ins, afatinib 30 mg/d as the first-line regimen was associated with a PFS of 14.5 months and comparable 10.4 months, respectively. The corresponding ORR for common and uncommon subgroups was 60% and 80%, respectively. In addition, a well-tolerated safety profile was observed for afatinib starting from 30 mg/d.
Afatinib has been routinely recommended to start from 40 mg/d based on the results of the LUX-Lung trials. However, in the LUX-Lung trials and subsequent real-world studies, more than a quarter or even up to half of patients receiving afatinib 40 mg/d initially had to reduce the dose eventually due to AEs (2,3,13-15,23). The high frequency of AEs related to afatinib at 40 mg/d not only lowers the quality of life of patients but can also cause treatment discontinuation (24). On the contrary, afatinib starting from 30 mg/d may be more appropriate, especially for Asians patients with NSCLC (15,25). Recent studies demonstrated that an initial afatinib dose <40 mg or 30 mg/d resulted in a similar response and PFS to the dose of 40 mg/d but resulted in fewer serious AEs for EGFR-mutated LAD (25,26). The incidence of diarrhea as the most commonly experienced AE was only 41% at 30 mg/d compared with 100% at 40 mg/d (25). In the current study, we showed a similar favorable safety profile. Afatinib 30 mg/d was well tolerated, with an incidence of 37.9% for any-grade AEs and only 8.6% for manageable grade 3 diarrhea. More importantly, no patient discontinued treatment due to AEs, which may contribute to prolonged survival.
Afatinib had comparable efficacy with first-generation EGFR-TKIs in the treatment of LAD with EGFR common mutations. In the LUX-Lung 3 trial, the PFS for patients with EGFR 19del and 21L858R who received first-line afatinib at 40 mg was 13.6 months (13). A similar first-line PFS for LAD patients with common EGFR mutations treated with afatinib at 30 mg was observed in the current study. A previous study from Taiwan, which intended to investigate the efficacy of afatinib starting from 30 mg, also reported a similar PFS of 469 and 443 days for 19del and 21L858R mutations, respectively (25). Furthermore, afatinib at 30 mg was not only associated with non-inferior efficacy but a better safety profile compared with the higher dose of 40 mg. The low frequency of AEs and good tolerability in the present study was consistent with findings in the abovementioned study from Taiwan, China (25).
Different EGFR-targeted drugs have shown variable efficacy for uncommon mutations within EGFR exon 18–21 (27,28). Previous studies demonstrated that patients with uncommon EGFR mutations treated with afatinib might have a better prognosis than those treated with first-generation TKIs (4,7,29,30). Afatinib at 40 mg for LAD with major uncommon EGFR mutations (G719X, L861Q, and S768I) resulted in a varied PFS of 10.7–17.1 months and an ORR around 50–74% (6,31,32). However, little evidence has been released concerning the efficacy of afatinib at 30 mg in these patients. Our findings confirmed that uncommon exon 18–21 mutations except exon 20ins were sensitive to afatinib even at 30 mg (6,26,29). It is noteworthy that real-world studies often enroll patients usually excluded from clinical trials. For example, our study included approximately one-fifth of patients with brain metastases who had a dismal PFS of merely 5 months. Furthermore, while uncommon EGFR mutations account for 10–20% of all EGFR mutations, nearly half of our population had uncommon EGFR mutations. Afatinib applied as the first-line treatment was associated with a favorable PFS, which we speculate was linked to improved tolerance from the adjusted initiation dose and promising efficacy for major uncommon mutations as well. EGFR exon 20ins are generally associated with de novo resistance to first- or second-generation EGFR-TKIs (6,33). Our result confirmed this concept, with a median PFS of only 2–3 months for this special subgroup (34).
This study has a few limitations. First, the limited sample size may affect the results. Considering the small population size and genetic diversity of patients with uncommon EGFR mutations, it is difficult to collect a large enough sample and large-scale randomized trials on the current topic also seem to be infeasible. Second, we did not include EGFR T790M and other rare mutations. Third, this was an observational study without setting a control group of afatinib 40 mg/d, although previous studies have demonstrated similar efficacy of 30 mg/d compared to 40 mg/d (25,26). As an observational retrospective study, the descriptive data of efficacy and safety profile, although partly in line with previous studies, is necessary to confirm in the future large-scale studies.
In conclusion, our findings confirmed previous findings that afatinib was not only effective for LAD with common EGFR mutations but potent for those with EGFR G719X/S768I/L861Q and classical compound mutations. More importantly, a starting dose of afatinib 30 mg instead of 40 mg was used in this study and favorable toxicity was observed. Taken together, we believe that afatinib at 30 mg/d may be an option for the treatment of EGFR-mutated LAD especially for those with major uncommon EGFR mutations.
Acknowledgments
Funding: The study was supported by Shanghai Medical Research Program for the Outstanding Expert (No. TG20191101).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-507/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-507/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-507/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Shanghai Chest Hospital approved the study (No. IS22027), and all the patients supplied written consent before treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin JJ, Cardarella S, Lydon CA, et al. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J Thorac Oncol 2016;11:556-65. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 2016;107:1179-86. [Crossref] [PubMed]
- Evans M, O'Sullivan B, Smith M, et al. Large-Scale EGFR Mutation Testing in Clinical Practice: Analysis of a Series of 18,920 Non-Small Cell Lung Cancer Cases. Pathol Oncol Res 2019;25:1401-9. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Shen YC, Tseng GC, Tu CY, et al. Comparing the effects of afatinib with gefitinib or Erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer 2017;110:56-62. [Crossref] [PubMed]
- Nelson V, Ziehr J, Agulnik M, et al. Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. Onco Targets Ther 2013;6:135-43. [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Kim Y, Lee SH, Ahn JS, et al. Efficacy and Safety of Afatinib for EGFR-mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib. Cancer Res Treat 2019;51:502-9. [Crossref] [PubMed]
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897-907. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Goss GD, Cobo M, Lu S, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung: Final analysis of the randomised phase 3 LUX-Lung 8 trial. EClinicalMedicine 2021;37:100940. [Crossref] [PubMed]
- Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 2016;27:2103-10. [Crossref] [PubMed]
- Liu CY, Wang CL, Li SH, et al. The efficacy of 40 mg versus dose de-escalation to less than 40 mg of afatinib (Giotrif) as the first-line therapy for patients with primary lung adenocarcinoma harboring favorable epidermal growth factor mutations. Oncotarget 2017;8:97602-12. [Crossref] [PubMed]
- Liang SK, Hsieh MS, Lee MR, et al. Real-world experience of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma. Oncotarget 2017;8:90430-43. [Crossref] [PubMed]
- Ninomiya T, Nogami N, Kozuki T, et al. Survival of chemo-naïve patients with EGFR mutation-positive advanced non-small cell lung cancer after treatment with afatinib and bevacizumab: updates from the Okayama Lung Cancer Study Group Trial 1404. Jpn J Clin Oncol 2021;51:1269-76. [Crossref] [PubMed]
- Ko R, Shukuya T, Imamura CK, et al. Phase I study of afatinib plus bevacizumab in patients with advanced non-squamous non-small cell lung cancer harboring EGFR mutations. Transl Lung Cancer Res 2021;10:183-92. [Crossref] [PubMed]
- Ninomiya T, Nogami N, Kozuki T, et al. A phase I trial of afatinib and bevacizumab in chemo-naïve patients with advanced non-small-cell lung cancer harboring EGFR mutations: Okayama Lung Cancer Study Group Trial 1404. Lung Cancer 2018;115:103-8. [Crossref] [PubMed]
- Wang Y, Li J, Zhou Y, et al. Tumor genomics and response to chemotherapy in advanced non-small cell lung cancer with exon 20 insertion epidermal growth factor receptor mutations. Ann Transl Med 2020;8:1297. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Park K, Kim JS, Kim JH, et al. An open-label expanded access program of afatinib in EGFR tyrosine kinase inhibitor-naïve patients with locally advanced or metastatic non-small cell lung cancer harboring EGFR mutations. BMC Cancer 2021;21:802. [Crossref] [PubMed]
- Takahashi T, Terazono H, Suetsugu T, et al. High-Trough Plasma Concentration of Afatinib Is Associated with Dose Reduction. Cancers (Basel) 2021;13:3425. [Crossref] [PubMed]
- Yang CJ, Tsai MJ, Hung JY, et al. The clinical efficacy of Afatinib 30 mg daily as starting dose may not be inferior to Afatinib 40 mg daily in patients with stage IV lung Adenocarcinoma harboring exon 19 or exon 21 mutations. BMC Pharmacol Toxicol 2017;18:82. [Crossref] [PubMed]
- Brückl WM, Reck M, Griesinger F, et al. Afatinib as first-line treatment in patients with EGFR-mutated non-small cell lung cancer in routine clinical practice. Ther Adv Med Oncol 2021;13:17588359211012361. [Crossref] [PubMed]
- Saxon JA, Sholl LM, Jänne PA. EGFR L858M/L861Q cis Mutations Confer Selective Sensitivity to Afatinib. J Thorac Oncol 2017;12:884-9. [Crossref] [PubMed]
- Banno E, Togashi Y, Nakamura Y, et al. Sensitivities to various epidermal growth factor receptor-tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: What is the optimal epidermal growth factor receptor-tyrosine kinase inhibitor? Cancer Sci 2016;107:1134-40. [Crossref] [PubMed]
- Zhang T, Wan B, Zhao Y, et al. Treatment of uncommon EGFR mutations in non-small cell lung cancer: new evidence and treatment. Transl Lung Cancer Res 2019;8:302-16. [Crossref] [PubMed]
- Tan J, Hu C, Deng P, et al. The Predictive Values of Advanced Non-Small Cell Lung Cancer Patients Harboring Uncommon EGFR Mutations-The Mutation Patterns, Use of Different Generations of EGFR-TKIs, and Concurrent Genetic Alterations. Front Oncol 2021;11:646577. [Crossref] [PubMed]
- Yang JC, Schuler M, Popat S, et al. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J Thorac Oncol 2020;15:803-15. [Crossref] [PubMed]
- Li T, Wang S, Ying J, et al. Afatinib treatment response in advanced lung adenocarcinomas harboring uncommon mutations. Thorac Cancer 2021;12:2924-32. [Crossref] [PubMed]
- Tu HY, Ke EE, Yang JJ, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer 2017;114:96-102. [Crossref] [PubMed]
- Huang CH, Ju JS, Chiu TH, et al. Afatinib treatment in a large real-world cohort of nonsmall cell lung cancer patients with common and uncommon epidermal growth factor receptor mutation. Int J Cancer 2022;150:626-35. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


