
Lung recruitment after cardiac arrest during procurement of atelectatic donor lungs is a protective measure in lung transplantation
Introduction
The application of lung transplantation (LTx) as a therapy for end-stage lung disease is limited due to donor shortage (1). Marginal or extended donor lungs are widely used currently to solve this problem. Although long-term survival after LTx using extended donor lungs is acceptable, short-term outcomes, such as a higher grade of primary graft dysfunction (PGD) and longer intensive care unit and hospital stays, may be less acceptable (2,3). There are diverse potentially detrimental donor factors in LTx, but lung atelectasis (AT) is a common and definitively influential conditions occurring in donors. The lungs of brain-dead donors often collapse in the supine position under prolonged management with mechanical ventilation. In a previous study, approximately 33% marginal potential donors showed evidence of collapse/consolidation/pleural effusion on chest radiography (4). Lungs from extended donors are often successfully transplanted when AT is recovered through procurement procedure. Some studies have shown that donor lungs with large or long-term AT can be usable (5,6). However, atelectatic donor lungs are occasionally vulnerable to critical edema, triggered by lung-recruitment maneuver and cold flushing, which a major cause of severe PGD after LTx. According to a study by the Cleveland group, roughly 30% lungs from obese donors with AT were not recovered due to functional deterioration after the recruitment procedure (7).
When compression AT is reverted, rapid blood inflow with sudden alveolar distention causes a loss of surfactant protein and expression of inflammatory cytokines. The subsequent increase in pulmonary capillary pressure, hydrostatic pressure, and capillary permeability results in pulmonary edema and hypoxia (8,9). In the standard lung-procurement procedure, an elaborate lung recruitment maneuver is performed before aortic clamping to evaluate lung function and gross findings. However, this procedure can cause the pathology leading to lung edema in the bodies of cadaveric organ donors, especially those subject to the systemic inflammatory condition of brain death-related cytokine storms.
We hypothesized that lung injury can be mitigated if AT can be reverted under no blood-circulated conditions to avoid rapid blood inflow with alveolar distention. This situation can be achieved during the procurement procedure by scheduling the lung recruitment maneuver after aortic clamping (cardiac arrest), instead of before. This study aimed to investigate the potential benefit of the no blood-circulated recruitment (BCR) maneuver, eliminating AT without blood flow, using an animal-transplant model. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-226/rc).
Methods
Study design
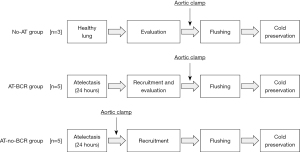
Thirteen pairs of Landrace domestic pigs were used as donors and recipients. All of these pigs were male conventional animal and were purchased from Okayama JA Chikusan Co. (Okayama, Japan). Ten donors were randomly divided with numbered container method into two atelectatic donor groups according to the procurement procedure: blood-circulated recruitment (AT-BCR) group (n=5) and no-BCR maneuver (AT-no-BCR) group (n=5). The remaining three donors were included in the non-atelectatic (No-AT) control LTx group (n=3) (Figure 1). Since the No-AT group plays a mere role to verify the baseline data as negative control, the sample size of the group was limited to minimize the number of animals from an ethical perspective. After a 24 hours intervention of experimentally modeled lung AT in each donor pig, its left lung was transplanted to a corresponding recipient pig. Lung graft function was evaluated for 6 hours after LTx and compared between the three groups. The animals received humane care according to the Principles of Laboratory Animal Care (formulated by the National Society for Medical Research) and the Policy on the Care and Use of Laboratory Animals, Okayama University. The study protocol was prepared before the study and approved by the Animal Care and Use Committee of Okayama University (No. OKU-2017 269).
Animal preparation and anesthesia
Ketamine chloride and atropine sulfate were injected intramuscularly as a premedication. The airway was secured orally during donor intervention for atelectatic modelling and through tracheostomy in procurement and transplant surgery. The tidal volume, respiratory rate, and end-expiratory pressure was set at 12 mL/kg, 12/min, and 5 cmH2O, respectively. General anesthesia was maintained with 100% oxygen, sevoflurane, and vecuronium bromide. Peak airway pressure, tidal volume, systemic blood pressure, pulmonary arterial pressure (PAP), and central venous pressure (CVP) were monitored continuously.
Donor atelectasis model
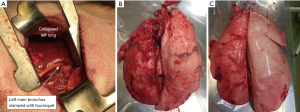
Muscle-sparing left lateral thoracotomy with 12–15 cm skin incision was performed on each donor pig after intravenous administration of amoxicillin. The left main bronchus was encircled with 1-0 silk and ligated using the tourniquet procedure. As for the setting of mechanical ventilator, the tidal volume was 8 mL/kg (body weight). Additionally, the chest cavity was filled with 150 mL of saline at 37 ℃. Following the confirmation of atelectatic change in the left lung (Figure 2A), the chest was tightly closed. After the recovery from anesthesia and administration of ketoprofen for analgesia, each pig was placed in a compartment cage and rested overnight.
Procurement surgery and lung recruitment procedures in each study group
After the 24 hours intervention of left lung AT, donor pigs were re-anesthetized. In the No-AT and AT-BCR groups, the lung recruitment maneuver was performed immediately after median sternotomy. In the AT-no-BCR group, the lung recruitment maneuver was performed between aortic clamping and cold flushing in no blood-circulated and normothermic states. In all the groups, elimination of AT with no barotrauma was achieved by careful manual ventilation procedures with the airway pressure of 25 cmH2O or less. Heparin (200 U/kg) was administered 10 minutes before aortic clamping in each group. Topical cooling of heart and lungs with crushed ice and cutoff of superior and inferior vena cava was performed at the same time as aortic clamping. These procedures resulted in prompt cardiac arrest and removal of blood from right heart system to prevent from perfusing pulmonary artery and lung. A liter of low potassium dextran glucose solution chilled at 4 ℃ was used to flush both lungs. After final lung recruitment and clamping of trachea, the semi-inflated heart-lung block was excised and stored in a refrigerator set at 4 ℃ until implantation.
Recipient operation and evaluation of post-transplant graft function
Each recipient pig underwent left pneumonectomy and single-LTx through the left fourth intercostal space using standard techniques. Data collection was started 30 minutes after reperfusion and was continued for 6 hours. To measure the gas exchange capacity of the lung graft as the primary outcome, the right main pulmonary artery was intermittently taped and blocked with a tourniquet for one minute at each measurement. Dynamic lung compliance was measured under double lung ventilation. After a 6 hours evaluation, the pig was euthanized, and the lung allograft was excised for tissue sampling.
Wet weight-to-dry weight (W/D) ratio
The W/D ratio of the graft tissue was analyzed to evaluate edema caused by reperfusion injury. The drying procedure involved placing the lung allograft in an oven at 80 ℃ for 2 weeks after acquiring tissue samples.
Cytokine assay of allograft tissue
A section of peripheral lung tissue was acquired from the basal edge of the left lower lobe at the end of the 6 hours evaluation period. The sample was frozen in liquid nitrogen and stored at −80 ℃. The levels of tissue interleukin (IL)-8 in the implanted lungs were measured to evaluate the extent of reperfusion injury. One hundred milligrams of tissue sample (wet mass) was homogenized and incubated in 800 µL of tissue lysis buffer [Cell extraction buffer (Thermo Fisher Scientific, Waltham, MA, USA)] and mixed with protease inhibitors [PMSF protease inhibitors (Thermo Fisher Scientific) and protease inhibitor cocktail powder (Sigma-Aldrich, St Louis, MO, USA)]. Tissue debris was discarded after centrifugation at 4 ℃ and 13,000 rpm for 10 minutes. IL-8 levels in the extracted solution were assayed using enzyme-linked immunoassay kits [IL-8 porcine ELISA kit (Thermo Fisher Scientific)]. The optical density (OD) of each well was read at 450 nm with a Flex Station 3 Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA, USA) at the Central Research Laboratory, Okayama University Medical School. The concentration of cytokines was calculated by converting the OD readings against a standard curve.
Histological evaluation
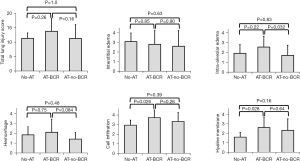
A peripheral lung tissue block collected from basal edge of the left lower lobe and apex of the left upper lobe was fixed in 10% buffered formalin and embedded in paraffin. Sectioned samples were stained with hematoxylin and eosin for histological evaluation under a light microscope. Two boarded pathologists evaluated these samples with lung injury score in randomized and blinded fashion. The lung injury score includes interstitial edema, intra-alveolar edema, hemorrhage, cell infiltration and hyaline membrane formation. The severity of these findings were graded as 0: absent, 1: trivial (1–20% of the field), 2: mild (20–40%), 3: moderate (40–60%) and 4: severe (>60%) (10,11).
Statistical analysis
All data are expressed as mean ± standard deviation (SD). The error bars show SD. Baseline parameters, W/D ratio, tissue cytokine levels and lung injury score were compared using one-way analysis of variance (ANOVA). Repeated measures ANOVA were used for the analysis of serial measurements. Statistical significance was set at P<0.05. All statistical analyses were performed using Excel Statistics, v3.00, RRID: SCR_017294.
Results
Baseline parameters
Table 1 summarizes the baseline parameters of the animals used in this study. There was no significant difference in the baseline conditions of the donor and recipient pigs among the three groups. PPVO2 after AT indicates the oxygen partial pressure of the blood from the left donor pulmonary vein sampled immediately after the ligation of left main bronchus in preparation surgery making atelectatic models, representing the function of the collapsed left lung. PPVO2 after AT were equally low in both AT groups (AT-BCR group: 48.0±5.2, AT-no-BCR group: 49.5±11.3, P=0.208). In procurement surgery, PPVO2 just prior to aortic clamping was significantly lower in AT-BCR group than No-AT group. In AT-no-BCR group, PPVO2 prior to aortic clamping was extremely low because AT of left lung was not eliminated at this timing yet (No-AT group: 495.5±29.8 mmHg and AT-BCR group: 266.4±114.9 mmHg, AT-no-BCR group: 46.9±5.1 mmHg, P<0.001). These results verified that optimal atelectatic intervention was administered to the experimental AT groups. There was no significant difference in cold ischemic time among three groups (No-AT group: 302.0±13.1 minutes, AT-BCR group: 311.7±25.0 minutes, AT-no-BCR group: 319.7±7.9 minutes, P=0.457).
Table 1
| Parameters | No-AT (n=3) | AT-BCR (n=5) | AT-no-BCR (n=5) | P value |
|---|---|---|---|---|
| Donor | ||||
| Weight (kg) | 29.3±1.2 | 26.7±5.8 | 26.9±5.2 | 0.781 |
| PaO2/FiO2 (mmHg) | 497.6±18.7 | 481.0±93.2 | 521.5±34.9 | 0.675 |
| Mean pulmonary arterial pressure (mmHg) | 27.3±3.5 | 23.8±4.4 | 20.4±3.6 | 0.161 |
| Cardiac output (L/min) | 2.9±0.1 | 3.4±1.0 | 2.7±0.6 | 0.441 |
| PPVO2 after AT (mmHg) | – | 48.0±5.2 | 49.5±11.3 | 0.208 |
| PPVO2 prior to aortic clamp (mmHg) | 495.5±29.8 | 266.4±114.9 | 46.9±5.1 | <0.001 |
| Recipient | ||||
| Weight (kg) | 28.8±0.6 | 27.3±5.4 | 26.9±5.4 | 0.793 |
| PaO2/FiO2 (mmHg) | 535.5±22.4 | 497.3±19.4 | 473.6±54.8 | 0.193 |
| Mean pulmonary arterial pressure (mmHg) | 27.3±1.5 | 26.6±6.2 | 24.5±6.7 | 0.708 |
| Cardiac output (L/min) | 3.2±0.5 | 3.3±0.8 | 3.5±0.7 | 0.896 |
| Cold ischemic time (min) | 302.0±13.1 | 311.7±25.0 | 319.7±7.9 | 0.457 |
Values are presented as mean ± SD. AT, atelectasis; BCR, blood-circulated recruitment; SD, standard deviation.
Evaluation of graft function in the three groups
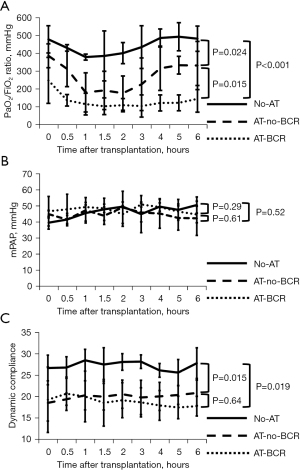
Macroscopic findings of the lung graft immediately after procurement in AT-BCR group and AT-no-BCR group are shown in Figure 2B,2C. The left lung graft procured with no-BCR maneuver was less edematous than the graft procured with conventional BCR maneuver. The graft function, represented by the gas exchange capacity within the first 6 hours after transplantation, is shown in Figure 3A. Immediately after reperfusion, the PaO2/FiO2 ratio was 479.0±76.3, 248.4±129.2, and 386.5±66.9 mmHg in the No-AT, AT-BCR, and AT-no-BCR groups, respectively. The AT-no-BCR group showed significantly better oxygenation than the AT-BCR group throughout the post-transplant observation time (P=0.015). Hemodynamics after transplantation were similar among three groups. Mean PAP of the graft with occluding right main pulmonary artery is shown in Figure 3B. Dynamic lung compliance was lower in both the AT groups than in the No-AT group. Although there was no statistical difference, lung compliance tended to decrease gradually in the AT-BCR group and remains stable in the AT-no-BCR group (Figure 3C).
W/D ratio of the lung graft excised after 6 hours reperfusion
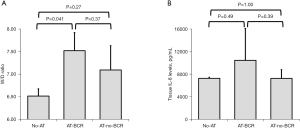
The mean W/D ratio was 6.51±0.16, 7.52±0.40 and 7.09±0.54 in the No-AT, AT-BCR, and AT-no-BCR groups, respectively. Although the difference between the AT-BCR group and the AT-no-BCR group does not reach statistical significance, the AT-BCR group showed significantly higher value than the No-AT group and there was no significant difference between the AT- no-BCR group and the No-AT group (Figure 4A).
Tissue IL-8 expression
The tissue IL-8 levels in the AT-no-BCR group and the No-AT group were comparable. The IL-8 levels in the AT-BCR group (10,466.0±5,658.9 pg/mL) were higher than those in the other groups; however, they were not significantly different from the levels in the AT- no-BCR group (No-AT: 7,263.6±185.0 pg/mL, P=0.49; AT-no-BCR: 7,254.0±1,550.3 pg/mL, P=0.39; Figure 4B).
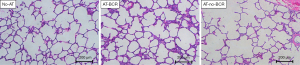
Histological findings and lung injury score
Representative histological findings in the graft tissue (Figure 5) and comparison of lung injury score among the three groups at the end of the 6 hours evaluation (Figure 6) are shown. Lung injury scores in the AT-no-BCR group and the No-AT group were comparable. The overall lung injury score in the AT-BCR group (13.75±4.46) tended to be higher than those in the other groups although the difference does not reach statistical significance (No-AT: 11.33±8.88, P=0.26; AT-no-BCR: 11.30±4.78, P=0.16). Looking at each single scoring factor, however, the score of intra-alveolar edema in the AT-BCR group was significantly higher than the AT-no-BCR group (AT-BCR group: 2.55±1.10, AT-no-BCR group: 1.70±1.03, P=0.032). Furthermore, the scores of cell infiltration and hyaline membrane formation in the AT-BCR group were significantly higher than the No-AT group (No-AT group: 2.92±0.52 and 1.58±0.52, AT-BCR group: 3.70±0.81 and 2.60±1.10, P=0.026 and 0.028).
Discussion
Donor lungs with broad AT often result in problematic pulmonary edema during lung procurement procedures. This is one of the major reasons for discarding donor lungs. This study focused on the lung recruitment maneuver in procurement surgery as a potential method of ameliorating injury from AT and increasing organ-utilization rates. The results demonstrated that lung recruitment prior to aortic clamping induced lung graft injuries, as seen in the AT-BCR group. Conversely, aortic clamping prior to recruitment, as seen in the experimental AT-no-BCR group, demonstrated better post-transplant lung function and lower grade of tissue reperfusion injury than the conventional procedure. Thus, this study shows that no blood-circulated conditions can potentially ameliorate the harmful effects of lung recruitment maneuvers on atelectatic donor lungs in the LTx setting.
In this study, the AT-BCR group exhibited substantially lower lung function than the control group immediately after reperfusion following transplantation. The conditions of cold static preservation were set equally among all groups. This implies that lung injury in the AT-BCR group had occurred inside the donor body where the atelectatic lungs were inflated under blood-circulated conditions. This scenario mimics the phenomenon found in re-expansion pulmonary edema (RPE). Several studies have reported that inflammatory and pro-inflammatory cytokines play important roles in the development of RPE (12-14). The complex inflammatory communication triggered by RPE prompts the development of severe ischemia-reperfusion injury (15). Tumor necrosis factor (TNF)-α is a typical early-phase pro-inflammatory cytokine that induces apoptosis and organ injury and triggers cytokine network activation (16). IL-8 is upregulated by TNF-α and IL-1β affecting over several hours of activity (17,18). The locally produced IL-8 leads to neutrophil infiltration and tissue injury (19). Inflammatory cytokine storm conditions are also found in brain-dead donor organs. It was reported that IL-8 levels in perfusate is related to post transplant outcome in clinical ex vivo lung perfusion (EVLP) cases (20). The levels of IL-8 in the lung tissue excised after 6 hours of evaluation in this study were also elevated in the AT intervention groups.
The results of this animal study suggest that the process of recovery from AT in the donor lung can be more critically influential under blood-circulated conditions than generally recognized. This finding is supported by previous research and clinical reports. In an EVLP circuit using a controlled donation-after-circulatory-death (DCD) donor model, Lindstedt et al. reported that elimination of AT without perfusion reduces lung injury and lung edema (21). Furthermore, no blood-circulated inflation procedures in lung procurement from a DCD donor potentially contribute to the reported favorable post-transplant outcomes of this LTx category. The survival outcome of controlled DCD LTx was better than expected and comparable to that of the standard LTx using brain-dead donor lungs in previous studies (22,23). We speculate that this unexpectedly satisfactory clinical outcome can be partly attributed to the DCD procurement process in which lung recruitment is performed after circulatory arrest. Overall, we believe that the lung recruitment procedure under no blood-circulated conditions is substantially more protective of donor lungs than the BCR procedure under unstable, inflammatory donor-systemic conditions.
When applying the no-BCR approach in lung procurement surgery, there would be some debate regarding the assessment of donor lungs. The consensus document by the International Society for Heart and Lung Transplantation recommends that lung graft function should be validated after elimination of AT in the procurement surgery (24). Lung procurement procedure with no-BCR procedure, as described in this study, makes it difficult to conduct blood gas analysis in the no-AT condition for final evaluation. However, even if full blood gas assessment cannot be performed, the usability of lungs explanted through the no-BCR procedure can be adequately verified by an experienced surgeon using multiple-donor clinical parameters such as donor’s background information, laboratory assessments, bronchoscopic and radiological findings, gross inspection, and those transitional changes in the process. In addition, EVLP could be also an alternative powerful tool as needed and used to examine and improve usability of lungs with favorable clinical outcomes (25,26). Another point of potential arguments may be the actual no-BCR procedure in clinical setting that can interrupt the process of heart retrieval. However, lung recruitment procedure does not take so long time that it can be completed along with the cold flushing process for heart.
This study has several limitations. Being an animal study, it did not fully represent the actual clinical practice of LTx. Although total donor lung collapse was not common in clinical setting, we applied a completely atelectatic donor model to avoid the influence of healthy lobe on the experimental results and explore the definitive effect of no-BCR on the atelectatic donor lungs. Moreover, invasive thoracotomy was performed to establish the experimental AT donor model. Although the AT intervention was adequately and sufficiently performed in the experimental donor model, the condition of brain death was not reproduced in this study. It is unclear whether and how the brain-death condition in itself affects donor-lung AT and recruitment conditions.
In conclusion, the recruitment maneuver for collapsed lung allografts might be better performed following aortic clamping and under no blood-circulated conditions to ameliorate injury from re-expansion edema. Clinical research is warranted to verify the protective effect of the no-BCR procedure and the potential to increase the organ utilization rate. It might be preferable to apply EVLP to reinforce the critical assessment of the recovered donor lungs for clinical safety and ethical perspectives.
Acknowledgments
The authors thank Mr. Tetsuo Kawakami for his technical assistance.
Funding: This study was supported by the Japan Society for Promotion of Science KAKENHI (grant number JP17K10785 to KM).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-226/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-226/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-226/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-226/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Animal Care and Use Committee of Okayama University (No. OKU-2017 269). This work was performed in compliance with the Principles of Laboratory Animal Care (formulated by the National Society for Medical Research) and the Policy on the Care and Use of Laboratory Animals, Okayama University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2020 Annual Data Report: Lung. Am J Transplant 2022;22:438-518. [Crossref] [PubMed]
- Shepherd HM, Gauthier JM, Puri V, et al. Advanced considerations in organ donors. J Thorac Dis 2021;13:6528-35. [Crossref] [PubMed]
- Mulligan MJ, Sanchez PG, Evans CF, et al. The use of extended criteria donors decreases one-year survival in high-risk lung recipients: A review of the United Network of Organ Sharing Database. J Thorac Cardiovasc Surg 2016;152:891-898.e2. [Crossref] [PubMed]
- Gabbay E, Williams TJ, Griffiths AP, et al. Maximizing the utilization of donor organs offered for lung transplantation. Am J Respir Crit Care Med 1999;160:265-71. [Crossref] [PubMed]
- Bansal A, Shigemura N, Toyoda Y, et al. Successful lung transplantation from a donor with persistent lobar atelectasis. Ochsner J 2014;14:266-9. [PubMed]
- Tanaka S, Miyoshi K, Sugimoto S, et al. Successful Lung Transplantation Using a Deceased Donor Mechanically Ventilated for Ten Months. Ann Thorac Surg 2017;104:e177-9. [Crossref] [PubMed]
- Okamoto T, Omara M, Ahmad U, et al. Utilization of Marginal Lung Donors With Low PaO2/FiO2 Ratio and High Body Mass Index. Ann Thorac Surg 2020;109:1663-9. [Crossref] [PubMed]
- Genofre EH, Vargas FS, Teixeira LR, et al. Reexpansion pulmonary edema. J Pneumologia 2003;29:101-6. [Crossref]
- Sohara Y. Reexpansion pulmonary edema. Ann Thorac Cardiovasc Surg 2008;14:205-9. [PubMed]
- Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant 2009;9:2262-9. [Crossref] [PubMed]
- Nishina K, Mikawa K, Takao Y, et al. ONO-5046, an elastase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg 1997;84:1097-103. [Crossref] [PubMed]
- Nakamura M, Fujishima S, Sawafuji M, et al. Importance of interleukin-8 in the development of reexpansion lung injury in rabbits. Am J Respir Crit Care Med 2000;161:1030-6. [Crossref] [PubMed]
- Sakao Y, Kajikawa O, Martin TR, et al. Association of IL-8 and MCP-1 with the development of reexpansion pulmonary edema in rabbits. Ann Thorac Surg 2001;71:1825-32. [Crossref] [PubMed]
- Funakoshi T, Ishibe Y, Okazaki N, et al. Effect of re-expansion after short-period lung collapse on pulmonary capillary permeability and pro-inflammatory cytokine gene expression in isolated rabbit lungs. Br J Anaesth 2004;92:558-63. [Crossref] [PubMed]
- Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant 2016;21:246-52. [Crossref] [PubMed]
- Ward PA. Role of complement, chemokines, and regulatory cytokines in acute lung injury. Ann N Y Acad Sci 1996;796:104-12. [Crossref] [PubMed]
- Fujishima S, Hoffman AR, Vu T, et al. Regulation of neutrophil interleukin 8 gene expression and protein secretion by LPS, TNF-α, and IL-1β. J Cell Physiol 1993;154:478-85. [Crossref] [PubMed]
- Standiford TJ, Kunkel SL, Basha MA, et al. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest 1990;86:1945-53. [Crossref] [PubMed]
- Sekido N, Mukaida N, Harada A, et al. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature 1993;365:654-7. [Crossref] [PubMed]
- Sage AT, Richard-Greenblatt M, Zhong K, et al. Prediction of donor related lung injury in clinical lung transplantation using a validated ex vivo lung perfusion inflammation score. J Heart Lung Transplant 2021;40:687-95. [Crossref] [PubMed]
- Lindstedt S, Pierre L, Ingemansson R. A Short Period of Ventilation without Perfusion Seems to Reduce Atelectasis without Harming the Lungs during Ex Vivo Lung Perfusion. J Transplant 2013;2013:729286. [Crossref] [PubMed]
- Van Raemdonck D, Keshavjee S, Levvey B, et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant 2019;38:1235-45. [Crossref] [PubMed]
- Ehrsam JP, Benden C, Immer FF, et al. Current status and further potential of lung donation after circulatory death. Clin Transplant 2021;35:e14335. [Crossref] [PubMed]
- Copeland H, Hayanga JWA, Neyrinck A, et al. Donor heart and lung procurement: A consensus statement. J Heart Lung Transplant 2020;39:501-17. [Crossref] [PubMed]
- Divithotawela C, Cypel M, Martinu T, et al. Long-term Outcomes of Lung Transplant With Ex Vivo Lung Perfusion. JAMA Surg 2019;154:1143-50. [Crossref] [PubMed]
- Wallinder A, Riise GC, Ricksten SE, et al. Transplantation after ex vivo lung perfusion: A midterm follow-up. J Heart Lung Transplant 2016;35:1303-10. [Crossref] [PubMed]


