Futile lobectomies following video-thoracoscopic exploration for indeterminate pulmonary nodules: a retrospective study
Introduction
Lung cancer screening has been of much interest since the end of the 1990’s. The Early Lung Cancer Action Project evaluated lung cancer screening by low-dose CT scan in patients at high risk of malignancy. An increase in small pulmonary nodules detection and possibly an increase of the detection of lung cancers at an early and curable stage have been reported (1). When a solitary pulmonary nodule is detected, the prevalence of malignancy ranges from 1.1% to 12% (2). Non-surgical biopsies can be helpful to make a diagnosis such as percutaneous CT-guided fine needle aspiration, transbronchial needle aspiration or endobronchial ultrasound guided transbronchial needle aspiration (3). Unfortunately, these techniques have an imperfect sensitivity and limited negative predictive value. Diagnostic yield of these techniques ranges from 48.5% to 93% depending on the lesion size and the technique itself (4-7). Thus, a negative biopsy cannot exclude the possibility of malignancy, and surgical resection remains a reliable option for a definitive diagnosis. The use of surgical minimally invasive techniques is recommended whenever possible along with wedge resection and frozen section analysis (4). If lung cancer is confirmed, oncologic resection with lobectomy and lymph node dissection are performed. Over the past few years, video-assisted thoracoscopic surgery (VATS) has been increasingly preferred in this indication allowing similar overall, cancer specific and disease-free survival for patients undergoing thoracoscopic lobectomy compared to open lobectomy for lung cancer (8-10). The admitted advantages of VATS are its lower incidence of post-operative complications, shorter hospital length of stay and faster recovery time compared with thoracotomy (11). VATS has now become a standard and common way lung cancer resections are performed, even if, sometimes, wedge resection is difficult even impossible due to a small or deep pulmonary nodule. The negative effect is that the thoracic surgeon could be tempted to perform a larger lung resection in order to avoid thoracotomy inconvenience. The aim of this study was to assess if VATS development increased the risk of lobectomy for benign lesions. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1789/rc).
Methods
Patient population
Retrospective analysis of medical charts was conducted for all patients who underwent pulmonary resection whether by video-assisted thoracoscopic surgery or thoracotomy, at Louis Pradel Hospital (Lyon, France), from January 2013 to January 2019, for a solitary pulmonary nodule or a mass suspicious for cancer. Patients who had a preoperative diagnosis of malignancy or benign lesion were excluded from the study. All patients benefited from complete preoperative and functional evaluation including computed tomography (CT) scan of the chest, positron emission tomography scan, CT scan of the brain and pulmonary function test. For each patient, surgical management of solitary pulmonary nodule or mass suspicious for cancer was validated by a multidisciplinary team. VATS consisted of a standard three-port procedure with a four-centimeter access port, and thoracotomy consisted of a postero-lateral one, placed in the fifth intercostal space. When non-small cell lung cancer was confirmed intra-operatively by frozen section analysis, lobectomy with systematic lymph node dissection was performed. The choice of thoracotomy or VATS approach was at the discretion of the operating surgeon. However, in our institution, we usually perform VATS for clinical N0 patients with a lesion of less than 7 cm. The following data were extracted: age, smoking history, type of surgery, frozen section analysis, conversion rate and causes for video-assisted thoracoscopic surgery, definitive pathologic diagnosis, post-operative classification of lung cancer, 90-day post-operative complications, Clavien classification of surgical complications (12) and hospital length of stay. Data were collected until October 2019. There was no missing data and no patient was lost to follow up. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and informed consent was taken from all the patients. In accordance with French legislation, submission to an ethics committee was not required owing to the observational nature of the study.
Statistical analysis
Qualitative data were described in terms of counts and percentages. Continuous variables were described with mean and standard deviation. Quantitative variables were compared with parametric t-test. Qualitative variables were compared with either the Chi-2 test (with or without Yates correction) or Fisher Test. A threshold of α=0.05 was considered for statistical significance. Statistical analysis was performed using R software version 3.5.2 and following packages were used: tidyr, epiDisplay, survival, epiR and base (13).
Results
Patient characteristics
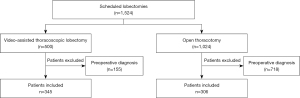
A total of 1,524 patients were scheduled for indeterminate pulmonary nodules surgical exploration at our institution. 873 patients had a preoperative diagnosis and were excluded from the study. 651 patients (42.7%) needed per-operative exploration of a solitary pulmonary nodule or mass with frozen section examination and were included in final analysis (Figure 1). 345 patients underwent VATS and 306 patients underwent thoracotomy. There were no significant differences in terms of age and smoking history between the two groups (Table 1). Patients in the VATS group underwent significantly more wedge resection (n=50 (14.5%) in the VATS group vs. n=25 (8.2%) in the thoracotomy group) and less lobectomy (n=295 (85.5%) in the VATS group vs. n=281 (91.8%) in the thoracotomy group) (P=0.012). Diagnosis of lung cancer was significantly more frequent in the thoracotomy group [n=270 (88.2%) in the thoracotomy group vs. n=280 (81.2%) in the VATS group, P<0.001].
Table 1
| Characteristics | VATS group (n=345), n (%) or mean (standard deviation) | Thoracotomy group (n=306), n (%) or mean (standard deviation) | P value |
|---|---|---|---|
| Age, years | 63.8 (10.3) | 63.0 (9.8) | 0.293 |
| Gender | 0.005 | ||
| Male | 205 (59.4) | 215 (70.3) | |
| Female | 140 (40.6) | 91 (29.7) | |
| History of smoking | 0.781 | ||
| Current smoker | 101 (29.3) | 95 (31.0) | |
| Former smoker | 180 (52.2) | 160 (52.3) | |
| Never smoker | 64 (18.6) | 51 (16.7) | |
| Number of packs/year | 37.2 (17.6) | 40.0 (19.1) | |
| Surgical resection | 0.012 | ||
| Lobectomy | 295 (85.5) | 281 (91.8) | |
| Wedge resection | 50 (14.5) | 25 (8.2) | |
| Lung cancer | 280 (81.2) | 270 (88.2) | <0.001 |
| Stage I | 218 (63.2) | 154 (50.3) | |
| Stage II | 40 (11.6) | 57 (18.6) | |
| Stage III | 22 (6.4) | 59 (19.3) | |
| Stage IV | 0 | 0 | |
| NA | 2 | 3 | |
| Histology | <0.001 | ||
| Final pathology | |||
| Adenocarcinoma | 206 (59.7) | 160 (52.3) | |
| Squamous cell carcinoma | 41 (11.9) | 63 (19.6) | |
| Large cell carcinoma | 7 (2.0) | 16 (5.2) | |
| Carcinoid tumors | 14 (4.1) | 15 (4.9) | |
| Others | 12 (3.5) | 16 (5.2) | |
| Benign lesion | 52 (15.1) | 31 (10.1) | |
| Hamartochondroma | 13 (3.8) | 5 (1.6) | |
| Fibro-inflammatory lésions | 7 (2.0) | 3 (1.0) | |
| Tuberculosis | 6 (1.7) | 5 (1.6) | |
| Organizing pneumonia | 4 (1.2) | 5 (1.6) | |
| Others | 22 (6.4) | 13 (4.2) |
VATS, video-assisted thoracoscopic surgery; NA, not available.
Surgical resection
In the video-assisted thoracoscopic surgery group (n=345), 47 patients (13.6%) required conversion to thoracotomy: 5 patients (10.6%) required conversion for a nodule that could not be found, 18 patients (38.3%) required conversion for a vascular accident, 12 patients (25.5%) for dissection difficulties, 8 patients (17%) for multiple adhesions and 4 patients (8.5%) for one-lung ventilation difficulties. Among the VATS group, 213 patients (61.7%) benefited from intra-operative diagnosis by frozen section analysis. 163 patients (47.2%) had a diagnosis of non-small cell lung cancer, 6 patients (1,7%) had a diagnosis of metastasis and 44 patients (12.7%) had a diagnosis of benign lesion (Table 2). For the latter 44 patients, surgery consisted of wedge resection only. 132 patients (38.3%) underwent lobectomy without prior diagnosis.
Table 2
| Final pathology | VATS group, n=345 | Thoracotomy group, n=306 | |||
|---|---|---|---|---|---|
| Patients evaluated with frozen section analysis, n=213 | Patients with no frozen assessment, n=132 | Patients evaluated with frozen section analysis, n=246 | Patients with no frozen assessment, n=60 | ||
| Lung cancer | 163 (76.5%) | 112 (84.8%) | 218 (88.6%) | 52 (86.7%) | |
| Metastasis | 6 (2.8%) | 7 (5.3%) | 3 (1.2%) | 0 | |
| Benign lesion | 44 (20.7%) | 13 (9.9%) | 25 (10.2%) | 8 (13.3%) | |
VATS, video-assisted thoracoscopic surgery.
Thoracotomy was performed in 306 patients. Intra-operative diagnosis by frozen section analysis was performed for 246 patients (80.4%). 218 patients (71.2%) had an intra-operative diagnosis of non-small cell lung cancer, 3 patients (1%) had a diagnosis of metastasis and 25 patients (8.2%) had a diagnosis of benign lesion. For the latter 25 patients, surgery consisted of wedge resection only. Sixty patients (19.6%) underwent lobectomy without prior diagnosis. There were significantly more lobectomies performed without prior diagnosis in the VATS group compared with the thoracotomy group (P<0.001).
In total, 83 patients (12.7%) had a final pathology of a benign lesion. Regarding patients whose frozen section examination revealed a benign lesion, 5 patients (11.4%) in the VATS group and 2 patients (8%) in the thoracotomy group had a final pathologic assessment of cancer (Table 3).
Table 3
| Final pathology | Frozen section examination | ||||||
|---|---|---|---|---|---|---|---|
| VATS group, n=213 | Thoracotomy group, n=246 | ||||||
| Lung cancer | Metastasis | Benign lesion | Lung cancer | Metastasis | Benign lesion | ||
| Lung cancer | 162 | 1 | 5 | 216 | 0 | 2 | |
| Metastasis | 1 | 5 | 0 | 2 | 3 | 0 | |
| Benign lesion | 0 | 0 | 39 | 0 | 0 | 23 | |
VATS, video-assisted thoracoscopic surgery.
Among patients who underwent lobectomy without prior diagnosis, the proportion of benign lesions was equivalent in both groups (3.8% in the VATS group vs. 2.6% in the thoracotomy group, P=0.16).
Post-operative outcomes
90-day post-operative complications occurred in 117 patients (33.9%) of the VATS group and in 114 patients (37.3%) of the thoracotomy group. There were no significant differences in terms of overall post-operative complications between the two groups (P=0.3738), but pneumonias were significantly more frequent in the thoracotomy group (P=0.0039) compared to the VATS group (Table 4). Atrial fibrillation (29 patients (8.4%) in the VATS group, 28 patients (8.1%) in the thoracotomy group) and prolonged air leak (32 patients (9.3%) in the VATS group, 19 patients (6.2%) in the thoracotomy group) were also observed. Most of post-operative complications were grade II of Clavien classification of surgical complications (12) (19.4% in the VATS group vs. 22.2% in the thoracotomy group). The mean hospital length of stay was significantly higher in the thoracotomy group (P<0.0001). Mean hospital stay was 8.5 days for the thoracotomy group while it was 6.6 days in the VATS group. 90-day post-operative mortality was 0.6% in the VATS group vs. 0.3% in the thoracotomy group.
Table 4
| Characteristics | VATS group (n=345), N (%*) |
Thoracotomy group (n=306), N (%*) | P value | VATS for benign lesion (n=52) | Thoracotomy for benign lesion (n=30) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobectomy without prior diagnosis (n=13), N (%*) | Wedge resection with frozen section (n=39), N (%*) | P value | Lobectomy without prior diagnosis (n=8), N (%*) | Wedge resection with frozen section (n=22), N (%*) | P value | |||||||
| Post-operative complications | 117 (33.9) | 114 (37.3) | 0.3738 | 2 (15.4) | 3 (7.7) | 0.589 | 0 | 3 (13.6) | 0.545 | |||
| Prolonged air leak | 32 (9.3) | 19 (6.2) | 0.1462 | 0 | 1 (2.6) | 0 | 0 | |||||
| Atrial fibrillation | 29 (9.5) | 28 (8.1) | 0.5397 | 0 | 0 | 0 | 1 (4.5) | |||||
| Pneumonia | 35 (10.1) | 55 (18.0) | 0.0039 | 2 (15.4) | 1 (2.6) | 0 | 1 (4.5) | |||||
| Pleural effusion | 8 (2.3) | 1 (0.3) | 0.0663 | 0 | 0 | 0 | 0 | |||||
| Recurrent paralysis | 5 (1.4) | 3 (1.0) | 0.8528 | 0 | 0 | 0 | 0 | |||||
| Other | 13 (3.8) | 17 (5.6) | 0.2776 | 0 | 1 (2.6) | 0 | 1 (4.5) | |||||
| Clavien classification | 0.2230 | 0.115 | 1 | |||||||||
| 0 | 229 (66.4) | 119 (62.7) | 11 (84.6) | 37 (94.9) | 8 (100.0) | 19 (86.4) | ||||||
| I | 23 (6.7) | 24 (7.8) | 0 | 1 (2.6) | 0 | 1 (4.5) | ||||||
| II | 67 (19.4) | 68 (22.2) | 2 (15.4) | 0 | 0 | 2 (9.1) | ||||||
| IIIa | 16 (4.6) | 12 (3.9) | 0 | 0 | 0 | 0 | ||||||
| IIIb | 8 (2.3) | 4 (1.3) | 0 | 0 | 0 | 0 | ||||||
| IVa | 0 (0.0) | 5 (1.6) | 0 | 0 | 0 | 0 | ||||||
| IVb | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | ||||||
| V | 2 (0.6) | 1 (0.3) | 0 | 0 | 0 | 0 | ||||||
| Mean hospital length of stay, mean (SD) | 6.6 (5.7) | 8.5 (6.3) | <0.0001 | 5.2 (2.2) | 4.3 (2.5) | 0.0411 | 6.0 (2.2) | 5.8 (1.9) | 0.8050 | |||
*, percentage calculated in relation to the sub group population. VATS, video-assisted thoracoscopic surgery; SD, standard deviation.
Among patients who had a final diagnosis of benign lesion, there were no significant differences in terms of number, type and grade of post-operative complications between patients who had lobectomy compared with patients who underwent wedge resection only (P=0.589), independently of the surgical approach (Table 4). Most of the post-operative complications in these patients were pneumonias and grade I and II of Clavien classification of surgical complications (12). However, mean hospital stay in patients who underwent lobectomy without prior diagnosis seems to be longer than that of patients who underwent wedge resection only.
Discussion
Although the benefit of lung cancer screening and diagnosing early stage lung cancer has largely been demonstrated (14), some authors consider the risk of over-diagnosis. Some indolent tumors would not impact the patients’ life if left untreated (15) and some authors focus on additional cost, anxiety and morbidity associated with cancer diagnosis and treatment. 4.2% of patients, in whom a pulmonary nodule has been detected, underwent surgery (16). We demonstrate in this investigation that 12.7% of surgeries for indeterminate pulmonary nodules were performed for benign lesions. Our aim was to find out whether the development of VATS generated more lobectomies for benign lesions compared to thoracotomy. In our study, there was a trend toward more surgeries for benign lesions in the VATS group but the proportion of lobectomies performed for a benign lesion was equivalent in both groups.
One of the solutions to avoid surgery for benign lesions is to make a preoperative diagnosis. Non-surgical biopsy is suggested for solid nodules measuring >8 mm when probability of malignancy is low to moderate (4). Many techniques are available to obtain the diagnosis of an indeterminate pulmonary nodule, but their diagnostic yield is not 100% (4-7). Therefore, many patients undergo surgery without prior diagnosis. We report 42.7% of patients who underwent surgery without preoperative diagnosis. Our rate is at the lower limit of what has already been published the literature (17,18). Operating on patients without prior diagnosis leads inevitably to perform surgery for benign lesions and sometimes to perform avoidable surgeries. 12.7% of patients underwent surgery for a benign lesion in our study. This observation is compatible with data in the literature where benign lesion resection ranges from 9 to 35.6% (17,19,20) and this is a particular issue for patients enrolled in lung cancer screening trial (21).
Over the past two decades, video-assisted thoracoscopic surgery has been increasingly used worldwide (22) and, as other centers, we registered a distinct increase of VATS in our institution. Like other teams (23), we observed more resections for benign lesions in the VATS group compared to the thoracotomy group (15.1% versus 10.1%), with significantly more wedge resections in the VATS group. In our study, despite a significantly higher proportion of lobectomies without prior diagnosis in the VATS group, the number of lobectomies performed for benign lesions was equivalent in both groups, as also reported by Kuo et al. (23). Multidisciplinary decision for surgical exploration associated with the per-operative clinical judgment of the operating surgeon led to a reasonable number of benign lesion resection. In addition, benign lesion resection was not correlated with an excessive morbidity rate and did not cause any death in our study. Resecting a benign lesion, preferably by wedge, is not necessarily futile and allows for a new diagnosis or a change in treatment in 85% of cases (24).
In our study, benign lesion resection was relatively safe, with a non-significant difference in terms of postoperative complications between patients who underwent lobectomy versus those who underwent wedge resection for a benign lesion. Still, improvements in techniques are needed to detect indeterminate pulmonary nodules intra-operatively to prevent futile lobectomy or conversion to thoracotomy in these patients. Several methods have already been reported such as preoperative and per operative percutaneous hookwire localization or color dye labeling injection of the nodule under CT scan guidance (25-28). Other teams described electromagnetic navigational bronchoscopic injection of autofluorescent or methylene blue dye (29,30). In these series, about 98% of nodules were successfully localized and no conversion to thoracotomy for lung palpation was reported. So, in case of indeterminate pulmonary nodule difficult to localize, these techniques may help avoid the need for conversion to thoracotomy or for larger resections.
Our study is not without some limitations. It is a retrospective, nonrandomized study, which creates possible biases. Maybe this was circumvented in part by having a multidisciplinary decision for indeterminate pulmonary nodule exploration on each patient in the study. Although 12.7% of our patients had a definitive diagnosis of benign lesion, and reaching 15.1% in the VATS group, the risk of malignancy was felt to be sufficiently high to warrant surgical exploration. However, surgery for a benign lesion is not useless in most cases, as it resulted in a new diagnosis or a treatment change.
Conclusions
With its lower morbidity and its similar oncologic benefit compared to thoracotomy, VATS has become the surgical approach of choice for small, solitary, indeterminate pulmonary nodules. One disadvantage of VATS is the absence of lung palpation, making it sometimes difficult to find small and deep pulmonary nodules. Despite this, VATS was not associated with an increase in lobectomies for benign lesion in our study. Although benign lesion resection was relatively safe, attention must be paid to perform diagnostic lobectomy infrequently and to make benign lesion resections as uncommon as possible without compromising early lung cancer diagnosis and treatment.
Acknowledgments
The authors thank Karine Debbasch for providing language help.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1789/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1789/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1789/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1789/coif). GD reports personal fees from ASTRA ZENECA, outside the submitted work. MD reports personal fees from ROCHE, PFIZER, MSD, Boerhinger Ingelheim, NOvartis, ABBVIE, TAKEDA, BMS and ASTRA ZENECA, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and informed consent was taken from all the patients. In accordance with French legislation, submission to an ethics committee was not required owing to the observational nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al; American College of Chest Physicians. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Azari F, Kennedy G, Singhal S. Intraoperative Detection and Assessment of Lung Nodules. Surg Oncol Clin N Am 2020;29:525-41. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [Crossref] [PubMed]
- Silvestri GA, Bevill BT, Huang J, et al. An Evaluation of Diagnostic Yield From Bronchoscopy: The Impact of Clinical/Radiographic Factors, Procedure Type, and Degree of Suspicion for Cancer. Chest 2020;157:1656-64. [Crossref] [PubMed]
- Maxwell AW, Klein JS, Dantey K, et al. CT-guided transthoracic needle aspiration biopsy of subsolid lung lesions. J Vasc Interv Radiol 2014;25:340-6, 346.e1.
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Yang CJ, Kumar A, Klapper JA, et al. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg 2019;269:163-71. [Crossref] [PubMed]
- Wang BY, Huang JY, Lin CH, et al. Thoracoscopic Lobectomy Produces Long-Term Survival Similar to That with Open Lobectomy in Cases of Non-Small Cell Lung Carcinoma: A Propensity-Matched Analysis Using a Population-Based Cancer Registry. J Thorac Oncol 2016;11:1326-34. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available online: http://www.R-project.org/
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269-74. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Rzyman W, Jelitto-Gorska M, Dziedzic R, et al. Diagnostic work-up and surgery in participants of the Gdansk lung cancer screening programme: the incidence of surgery for non-malignant conditions. Interact Cardiovasc Thorac Surg 2013;17:969-73. [Crossref] [PubMed]
- Sihoe AD, Hiranandani R, Wong H, et al. Operating on a suspicious lung mass without a preoperative tissue diagnosis: pros and cons. Eur J Cardiothorac Surg 2013;44:231-7; discussion 237. [Crossref] [PubMed]
- Smith MA, Battafarano RJ, Meyers BF, et al. Prevalence of benign disease in patients undergoing resection for suspected lung cancer. Ann Thorac Surg 2006;81:1824-8; discussion 1828-9. [Crossref] [PubMed]
- Crestanello JA, Allen MS, Jett JR, et al. Thoracic surgical operations in patients enrolled in a computed tomographic screening trial. J Thorac Cardiovasc Surg 2004;128:254-9. [Crossref] [PubMed]
- Infante M, Chiesa G, Solomon D, et al. Surgical procedures in the DANTE trial, a randomized study of lung cancer early detection with spiral computed tomography: comparative analysis in the screening and control arm. J Thorac Oncol 2011;6:327-35. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Kuo E, Bharat A, Bontumasi N, et al. Impact of video-assisted thoracoscopic surgery on benign resections for solitary pulmonary nodules. Ann Thorac Surg 2012;93:266-72; discussion 272-3. [Crossref] [PubMed]
- Grogan EL, Weinstein JJ, Deppen SA, et al. Thoracic operations for pulmonary nodules are frequently not futile in patients with benign disease. J Thorac Oncol 2011;6:1720-5. [Crossref] [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Chao YK, Pan KT, Wen CT, et al. A comparison of efficacy and safety of preoperative versus intraoperative computed tomography-guided thoracoscopic lung resection. J Thorac Cardiovasc Surg 2018;156:1974-1983.e1. [Crossref] [PubMed]
- Fang HY, Chang KW, Chao YK. Hybrid operating room for the intraoperative CT-guided localization of pulmonary nodules. Ann Transl Med 2019;7:34. [Crossref] [PubMed]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]