A single-center analysis of 71 patients with thymic carcinoma: the chronological changes in the surgical procedure and prognosis
Introduction
Thymic carcinoma (TC) is a rare and aggressive mediastinal neoplasm with a poor prognosis. Although several studies have been reported concerning TC (1-8), the clinicopathologic characteristics and prognosis of TC remain unclear, due to its rarity.
Among thoracic malignant tumors, lung cancer, which is the leading cause of death worldwide, has an improved overall survival (OS) compared with several decades ago (9,10). However, there have been few reports concerning the changes in the prognosis over time for thymic epithelial tumors (TETs), especially TC, due to the small number of such cases. Therefore, whether or not the treatment or prognosis of TC has improved over the years remains unclear.
In recent years, small-sized TETs have been treated more frequently than before due to the spread of computed tomography (CT) (11,12). Based on the background of widely spread CT, chest CT has become a tool for us in general physical examinations (13), and small-sized tumors in the lung field have been detected more frequently than before in recent years. In addition to small pulmonary nodules, small-sized anterior mediastinal tumors have also been detected and treated in the early stage. In addition, the application of minimally invasive surgery (MIS) for general thoracic surgery, including that for TET, has spread recently (11,14,15). Several studies have demonstrated that MIS for TET was equal in survival outcomes and reduced perioperative complications, compared to open surgery (16,17). In our institution, MIS for TETs, which includes video-assisted thoracoscopic surgery (VATS) and robot-assisted thoracoscopic surgery (RATS), has been available since January 2009, with the frequency of performance increasing in association with the increase in small-sized anterior mediastinal tumors.
While there are few large-scale reports of TC, some have indicated an improved prognosis due to the development of medical care over time. In the present study, we examined the results of continuous treatment of 71 TC cases, which is considered to be a relatively large number of cases reported by a single institution, and clarified the changes in the clinical characteristics, surgical procedures and prognosis of TC patients over time. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-490/rc).
Methods
Study population
A total of 71 patients underwent treatment for TC from January 1983 to January 2020 at Nagoya City University Hospital. The clinical and pathological data of these patients were retrospectively reviewed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Nagoya City University Graduate School of Medical Sciences (IRB No. 60-20-0136). Because of the retrospective nature of the study, patient informed consent was not required. Regarding the diagnosis of TC, imaging diagnosis was performed by chest CT. The biopsy of TC for pathological diagnosis was performed when it was thought to be difficult to perform an initial surgery due to the tumor progression. To investigate the chronological changes in the clinical characteristics, surgical procedure and prognosis, the patients were divided into two groups at January 2009, when MIS for TET was started at our institution. Regarding the treatment strategy for TC at our institution, the patients were considered inoperable as initial surgery if the following findings were noted on preoperative diagnostic images: (I) massive invasion of two or more great vessels, (II) cervical lymphogenous metastasis, (III) distant metastasis, or (IV) pleural or pericardial dissemination. Regarding the treatment of inoperable patients, chemotherapy was selected in the patients with distant metastasis, while chemo-radiotherapy was selected in the inoperable patients with locally advanced TC. When chemoradiotherapy was considered to be intolerable, radiotherapy was selected. Postoperative additional treatments including chemotherapy, radiotherapy, and chemo-radiotherapy, were considered for incomplete resection cases. As chemotherapy, ADOC regimen (50 mg/m2 of cisplatin and 40 mg/m2 of doxorubicin on day 1, 0.6 mg/m2 of vincristine on day 3, and 700 mg/m2 of cyclophosphamide on day 4, at 3 weeks interval) or CP regimen (AUC6 of carboplatin and 200 mg/m2 of paclitaxel on day 1 at 3 weeks interval) was selected if tolerable (18,19). Adjuvant therapy was considered an option after complete resection, but the criteria for adjuvant therapy have not been stablished. Preoperative treatment was performed in some patients, whose radiotherapy was limited to 40 Gy, whereas radical radiation was fully performed at a dose of 60 Gy. Salvage surgery underwent for patients with good response to chemoradiotherapy and expected complete resection. Regarding the indication of MIS for TET, only small-sized TETs which were 3 cm or less were selected at first, however, MIS has been performed in the cases with the clinical Masaoka stage I–III tumors except for those needed great vessels resection followed by reconstruction nowadays. In the cases with invasion of the left brachiocephalic vein, MIS has been selected. Regarding the lymph node dissection for TET in MIS, lymphadenectomy for N1 lymph nodes were performed in the cases with total thymectomy. For small-sized anterior mediastinal tumors, limited thymectomy is selected in some cases at our institution. There is no clear indication of limited thymectomy, however, patients whose tumor is located in the relatively caudal part of the thymus and with a certain distance from left brachiocephalic vein are tend to be selected. In the present study, TC was diagnosed by the pathologist of the Department of Pathology at Nagoya City University. Clinical and pathological staging was performed in accordance with the Masaoka classification and the Masaoka-Koga classification respectively (20). OS was calculated from the date of having a surgery to that announcing a death or the December 31, 2021.
Statistical analysis
Statistical analyses of the survival were performed using the Kaplan-Meier and univariable log-rank tests. Statistical significance was defined as P<0.05. All statistical analyses were performed with the JMP® 14 software program (SAS Institute Inc., Cary, NC, USA).
Results
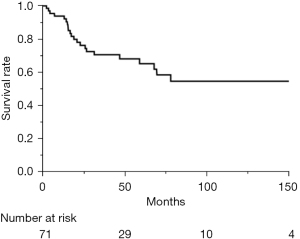
The clinical characteristics of the 71 patients are shown in Table 1. There were 44 men and 27 women, with a mean age of 60.7 (range, 27–86) years old. Thirty-four of the 71 patients visited a hospital with subjective symptom and underwent treatment. The remaining 37 patients were found to have an abnormal shadow without any symptoms. The clinical Masaoka stage of the 71 patients was as follows: stage I in 4, stage II in 8, stage III in 13, stage IVa in 6, stage IVb in 20, and the stages of the remaining 20 patients were unknown. Among the 45 patients who underwent surgery, R0 resection was performed in 31. Regarding non-surgical treatment, 14 patients received chemotherapy, 2 radiotherapy, 7 chemo-radiotherapy, and 1 only best supportive care. As a pathological diagnosis, 48 patients were diagnosed with squamous cell carcinoma, 4 with adenocarcinoma, 3 with lymphoepithelioma-like carcinoma, and 12 with other types of carcinoma; the subtypes of 4 patients were not identified. Regarding the prognosis, the 5-year OS of the 71 patients was 65.1% (Figure 1).
Table 1
| Factors | Value |
|---|---|
| Gender | |
| Male | 44 |
| Female | 27 |
| Mean age (years) | 60.7 (range, 27–86) |
| Subjective symptom | |
| Yes | 34 |
| No | 37 |
| Modality of identification | |
| X-ray | 22 |
| CT | 15 |
| Clinical Masaoka stage | |
| I | 4 |
| II | 8 |
| III | 13 |
| IVa | 6 |
| IVb | 20 |
| Unknown | 20 |
| Treatment | |
| Surgery | 45 |
| In-operation | 26 |
| Resection | |
| R0 | 31 |
| R1–2 | 14 |
| Non-surgical treatment | |
| Chemotherapy | 14 |
| Radiotherapy | 2 |
| Chemo-radiotherapy | 7 |
| Best supportive care | 1 |
| Unknown | 2 |
| Histology | |
| Squamous cell carcinoma | 48 |
| Adenocarcinoma | 4 |
| Lymphoepithelioma-like carcinoma | 3 |
| Others | 12 |
| Unclassifiable | 4 |
TC, thymic carcinoma; CT, computed tomography.
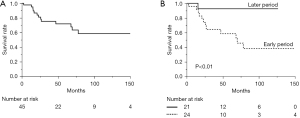
To investigate the chronological changes in the surgical procedure and prognosis, the 45 patients who underwent surgical resection were divided into 2 groups based on the surgical timing: before or after January 2009, when MIS for TC was started at our institution. The clinical characteristics of the two groups are compared in Table 2. There were 24 patients who underwent surgery for TC through December 2008 (earlier group), and 21 patients underwent surgery from January 2009 (later group). The earlier group was more likely to have subjective symptoms than the later group. Regarding the 27 patients without subjective symptoms, those in the later group were more likely to be diagnosed by chest CT, whereas those in the earlier group were more likely to be diagnosed by chest X-ray. The rate of complete resection was significantly higher in the later group (19/21, 90.5%) than in the earlier group (12/24, 50%), and the tumor size was larger in the earlier group rather than in the later group. To investigate the change in the screening modality, the total 71 patients were divided into the two groups and analyzed about the presence of symptom and the modality of identification. The breakdown of them is in Table 3. In summary, there was no significant difference but a tendency that the more patients who had no subjective symptom were diagnosed as TC by CT scan in the later group. There are 37 patients who had no subjective symptom and imaging diagnosis. The 5-year OS rate of these 45 patients who underwent surgical resection was 71.8% (Figure 2A), and the rates in the earlier and later groups were 58.7% and 92.8% respectively (Figure 2B) (P<0.01). Regarding the comparison of postoperative prognosis between the complete resection group and the incomplete resection group, the 5-year OS rate of the patients who underwent complete resection and incomplete resection were 81.3% and 53.6% respectively (P=0.02).
Table 2
| Factors | Early period (n=24) | Later period (n=21) | P value |
|---|---|---|---|
| Mean age (years) | 57.3 | 61.2 | 0.27 |
| Gender | 0.23 | ||
| Male | 13 | 15 | |
| Female | 11 | 6 | |
| Subjective symptom | 0.03 | ||
| Yes | 13 | 5 | |
| No | 11 | 16 | |
| Modality of identification | 0.02 | ||
| X-ray | 9 | 6 | |
| CT | 2 | 10 | |
| Tumor size (cm) | |||
| Mean | 6.8 | 4.4 | <0.01 |
| Median | 6.2 | 4.1 | <0.01 |
| Surgical procedure | <0.01 | ||
| Median sternotomy | 24 | 11 | |
| MIS | 0 | 10 | |
| Complete resection | <0.01 | ||
| Yes | 12 | 19 | |
| No | 12 | 2 | |
| Pathological Masaoka stage | <0.01 | ||
| 0 | 0 | 1 | |
| I | 0 | 3 | |
| II | 5 | 11 | |
| III | 7 | 2 | |
| IVa | 6 | 0 | |
| IVb | 6 | 4 |
CT, computed tomography; MIS, minimally invasive surgery.
Table 3
| Factors | Early period | Later period | P value |
|---|---|---|---|
| Subjective symptom (n=71) | 0.05 | ||
| Yes (n=34) | 22 | 12 | |
| No (n=37) | 14 | 23 | |
| Modality of identification (n=37) | 0.06 | ||
| X-ray (n=22) | 11 | 11 | |
| CT (n=15) | 3 | 12 |
TC, thymic carcinoma; CT, computed tomography.
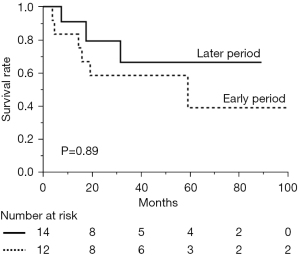
The surgical indication for TC at our institution showed no marked changes throughout the present study. The patients who underwent non-surgical treatment for TC were divided into two groups, similar to the patients who underwent surgical treatment. The data of the two non-surgical groups are compared in Table 4. The clinical Masaoka stage was more likely to be higher in the later group than in the earlier group (P=0.01). The 5-year OS rates of the groups were 66.3% in the later period and 38.9% in the earlier period, respectively, showing no significant difference (Figure 3) (P=0.89).
Table 4
| Factors | Early period (n=12) | Later period (n=14) | P value |
|---|---|---|---|
| Gender | 0.26 | ||
| Male | 6 | 10 | |
| Female | 6 | 4 | |
| Mean age (years) | 65.7 | 61.5 | 0.47 |
| Subjective symptom | 0.25 | ||
| Yes | 9 | 7 | |
| No | 3 | 7 | |
| Clinical Masaoka stage | 0.01 | ||
| III | 4 | 0 | |
| IVa | 4 | 2 | |
| IVb | 4 | 12 | |
| Treatment | 0.57 | ||
| Chemo-radiotherapy | 4 | 3 | |
| Chemotherapy | 5 | 9 | |
| Radiotherapy | 1 | 1 | |
| Best supportive care | 2 | 1 |
Among the 31 patients who underwent complete resection, 9 underwent sub-total thymectomy without lymph node dissection. The details of these nine patients are summarized in Table 5. All nine patients were in the later group and lacked subjective symptoms. The mean tumor size on CT was 2.1 (range, 1.3–3.2) cm. None of them had been diagnosed with TC preoperatively and all underwent MIS. Regarding the pathological Masaoka stage, 2 patients had stage I disease, 6 stage II, and 1 stage III. Although additional resection was considered and proposed in all cases, only 1 patient underwent a second operation, followed by radiation therapy. The remaining 8 cases received follow-up due to be performed R0 operation and patients’ desire. The mean observation period of these 9 patients was 859 days, and all 9 were alive at the latest observation. Postoperative recurrence was seen in one patient with distant metastasis.
Table 5
| Factors | Value |
|---|---|
| Gender | |
| Male | 8 |
| Female | 1 |
| Mean age (years) | 67.3 |
| Subjective symptom | |
| No | 9 |
| Mean CT size (cm) | 2.1 (range, 1.3–3.2) |
| Preoperative pathological diagnosis | |
| No | 9 |
| Surgical procedure | |
| MIS | 9 |
| Adjuvant therapy | |
| Radiation | 1 |
| Surgery + radiation | 1 |
| Pathological Masaoka stage | |
| I | 2 |
| II | 6 |
| III | 1 |
CT, computed tomography; MIS, minimally invasive surgery.
Discussion
The present study was a retrospective analysis of TC in 71 patients, which was a relatively large number for a single-center analysis. To investigate the chronological changes in the surgical procedure and prognosis for TC, two groups divided based on whether or not surgery was performed before January 2009, when MIS for TETs was started at our institution, were established in the present study. The results of a comparison between the two groups suggest that the ease of conducting a survey using CT, which has spread widely over the past few decades, leads to the early detection of TC before patients complain of subjective symptoms. Regarding surgical treatment, complete resection was reportedly associated with a better prognosis (4,21). In the present study, R0 resection surgery was performed in 31 patients (68.9%) among the 45 who underwent surgical treatment, which was almost the same as in a previous report (1). In the comparison analysis of the present study, R0 resection surgery was performed significantly more frequently in the later group than in the earlier group, with the later group also showing a better 5-year OS (92.8% vs. 58.7%, P<0.01). Detection of TCs at the earlier stage was thought to lead to the greater number of R0 resection in the later group compared to the earlier group. In summary, the prognosis of TC has improved over time thanks to early detection by CT screening and complete surgical resection.
In the present study, the chronological changes in the patients who underwent non-surgical treatment for TC were also investigated. The prognosis of these patients was found to not have improved significantly. One reason for this result is that effective systemic therapy, such as chemotherapy or immunotherapy for TC, has not yet been established. The efficacy of immune checkpoint inhibitors in various carcinomas has been demonstrated (22-30). In recent years, there have been several reports on immune checkpoint inhibitors that have been administered to treat TC (31-33), but no promising data on the efficacy or safety of immunotherapy in TC have yet been obtained. Regarding tyrosine kinase inhibitors (TKIs), lenvatinib which is a novel orally administered multi-targeted kinase inhibitor for VEGFR, FGFR, c-Kit, and other kinases, was recently approved for the treatment of advanced TC. In the single-arm phase 2 trial, the objective response rate of the patients with pathologically confirmed unresectable advanced or metastatic TC that progressed following at least 1 session of platinum-based chemotherapy was 38% (16 of 42 patients). This TKI may become a standard option for patients with advanced or metastatic TC (34).
In our institution, indication of MIS for TET had been 3 centimeters or less in maximum tumor diameter at first, which has been extended to clinical Masaoka stage III except for the invasion of great vessels needed reconstruction. To judge whether our indication of MIS for TC is feasible, further studies is needed (35). Regarding the lesion resected in thymectomy for TC in the present study, sub-total thymectomy without lymph node dissection was performed in nine patients. All 9 of these patients were in the later group, and none had a preoperative diagnosis. Regarding the surgical approach, all 9 patients underwent MIS, and 4 of them underwent robotic surgery. The mean observation period of the 9 patients was 859 (81–2,227) days, which was not enough to analyze the long-term prognosis. However, they were all alive at the latest observation and postoperative recurrence was seen in only one patient who was confirmed distant metastasis. Regarding small-sized anterior mediastinal tumors, surgical treatment, including limited thymectomy, is selected without a preoperative diagnosis in most cases (21,36,37). Regarding lymph node dissection for TC, the 8th edition of the TNM classification for TETs defines N1 as the anterior (perithymic) nodes and N2 as the deep intrathoracic or cervical nodes (38,39). However, the optimal extent of lymph node dissection for TC has not been defined. Based on our study, even sub-total thymectomy without lymph node dissection may be acceptable for patients with small-sized TC if it is completely resected with no lymphadenopathy on the preoperative diagnostic images or intraoperative findings. Further large-scale investigations are warranted.
Several limitations associated with the present study warrant mention. First, this was a retrospective study conducted over a long period. Therefore, the clinicopathological data were imperfect in some patients, especially in the early period. Second, length time bias should be taken into consideration in the present study, which may cause an overestimation of the survival outcome in the patients who were diagnosed by imaging modality without subjective symptom. Third, the present study included the 71 patients who underwent treatment for TC from 1983 to 2020 at our institution, which is a relatively long duration. Therefore, the long time period may induce potential bias. Despite these drawbacks, the present study of 71 cases is considered a relatively large-scale study for a single center and may also be quite meaningful as well.
In conclusion, the prognosis of TCs has been improved over time by early detection used CT screening and complete surgical resection. However, the prognosis of advanced patients who cannot undergo surgical treatment has not changed, and the further improvement of systemic therapy is desired. Regarding the extent of resection in thymectomy for TC, even sub-total thymectomy without lymph node dissection may be acceptable for patients with small-sized TC. Further large-scale investigations concerning TC treatment are warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-490/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-490/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-490/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-490/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Nagoya City University Graduate School of Medical Sciences (IRB No. 60-20-0136). Because of the retrospective nature of the study, patient informed consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marx A, Chan JKC, Chalabreysse L, et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J Thorac Oncol 2022;17:200-13. [Crossref] [PubMed]
- Hishida T, Nomura S, Yano M, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016;49:835-41. [Crossref] [PubMed]
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol 2014;9:541-8. [Crossref] [PubMed]
- Yano M, Sasaki H, Yokoyama T, et al. Thymic carcinoma: 30 cases at a single institution. J Thorac Oncol 2008;3:265-9. [Crossref] [PubMed]
- Hsu CH, Chan JK, Yin CH, et al. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PloS One 2019;14:e0227197. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Rendina AE, et al. Outcome of surgically resected thymic carcinoma: a multicenter experience. Lung Cancer 2014;83:205-10. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg 2004;26:412-8. [Crossref] [PubMed]
- Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Altorki N, Kent M, Pasmantier M. Detection of early-stage lung cancer: computed tomographic scan or chest radiograph? J Thorac Cardiovasc Surg 2001;121:1053-7. [Crossref] [PubMed]
- Yoon SH, Choi SH, Kang CH, et al. Incidental Anterior Mediastinal Nodular Lesions on Chest CT in Asymptomatic Subjects. J Thorac Oncol 2018;13:359-66. [Crossref] [PubMed]
- Li X, Han X, Sun W, et al. Preoperative misdiagnosis analysis and accurate distinguish intrathymic cyst from small thymoma on computed tomography. J Thorac Dis 2016;8:2086-92. [Crossref] [PubMed]
- Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20:911-20. [Crossref] [PubMed]
- Araki T, Nishino M, Gao W, et al. Anterior Mediastinal Masses in the Framingham Heart Study: Prevalence and CT Image Characteristics. Eur J Radiol Open 2015;2:26-31. [Crossref] [PubMed]
- Yano M, Sasaki H, Moriyama S, et al. Clinicopathological analysis of small-sized anterior mediastinal tumors. Surg Today 2014;44:1817-22. [Crossref] [PubMed]
- Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg 2015;4:495-508. [PubMed]
- Burt BM, Nguyen D, Groth SS, et al. Utilization of Minimally Invasive Thymectomy and Margin-Negative Resection for Early-Stage Thymoma. Ann Thorac Surg 2019;108:405-11. [Crossref] [PubMed]
- Agatsuma T, Koizumi T, Kanda S, et al. Combination chemotherapy with doxorubicin, vincristine, cyclophosphamide, and platinum compounds for advanced thymic carcinoma. J Thorac Oncol 2011;6:2130-4. [Crossref] [PubMed]
- Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015;26:363-8. [Crossref] [PubMed]
- Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S73-80. [Crossref] [PubMed]
- Nakagawa K, Yokoi K, Nakajima J, et al. Is Thymomectomy Alone Appropriate for Stage I (T1N0M0) Thymoma? Results of a Propensity-Score Analysis. Ann Thorac Surg 2016;101:520-6. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017;377:1345-56. Erratum in: N Engl J Med 2018;379:2185. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, andomized, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. [Crossref] [PubMed]
- Quispel-Janssen J, van der Noort V, de Vries JF, et al. Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13:1569-76. [Crossref] [PubMed]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
- Remon J, Passiglia F, Ahn MJ, et al. Immune Checkpoint Inhibitors in Thoracic Malignancies: Review of the Existing Evidence by an IASLC Expert Panel and Recommendations. J Thorac Oncol 2020;15:914-47. [Crossref] [PubMed]
- Yang Y, Ding L, Wang P. Dramatic response to anti-PD-1 therapy in a patient of squamous cell carcinoma of thymus with multiple lung metastases. J Thorac Dis 2016;8:E535-7. [Crossref] [PubMed]
- Yokoyama S, Miyoshi H. Thymic tumors and immune checkpoint inhibitors. J Thorac Dis 2018;10:S1509-15. [PubMed]
- Sato J, Satouchi M, Itoh S, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol 2020;21:843-50. [Crossref] [PubMed]
- Roden AC, Ahmad U, Cardillo GThymic Carcinomas-A Concise Multidisciplinary Update on Recent Developments From the Thymic Carcinoma Working Group of the International Thymic Malignancy Interest Group, et al. J Thorac Oncol 2022;17:637-50. [Crossref] [PubMed]
- Gu Z, Fu J, Shen Y, et al. Thymectomy versus tumor resection for early-stage thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. J Thorac Dis 2016;8:680-6. [Crossref] [PubMed]
- Narm KS, Lee CY, Do YW, et al. Limited thymectomy as a potential alternative treatment option for early-stage thymoma: A multi-institutional propensity-matched study. Lung Cancer 2016;101:22-7. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S81-7. [Crossref] [PubMed]