
Self-assisting robot-assisted pulmonary lobectomy has favorable outcome compared to VATS lobectomy
Introduction
Lung cancer has been estimated to be the leading cause of cancer-related deaths in the United States in 2021 (1). For early-stage lung cancer, surgical resection remains the gold standard treatment. Although the traditional approach has been to accomplish this resection through an open thoracotomy, over the last few decades, minimally invasive techniques such as video-assisted thoracoscopic surgery (VATS) have been adopted due to fewer complications, less pain, and decreased length of stay (LOS) (2-5). However, the adoption of VATS pulmonary resection has been slow, in part due to the significant learning curve involved in the operation (6,7). Since 2000, robot-assisted pulmonary resection has provided a new approach to surgical treatment of pulmonary tumors. It has improved clinical outcomes compared to open pulmonary resection (8,9). While robot and VATS pulmonary resection have improved clinical outcomes compared to open thoracotomy, VATS and open thoracotomy require a skilled surgical assistant. A surgical assistant performs the important task of retracting tissue and suctioning blood from the surgical field, allowing the surgeon to complete operational tasks. A skilled assistant is necessary to complete thoracotomy or VATS operations (10).
In contrast, a skilled assistant is a great asset (11) but may not be a requirement with robot-assisted surgery. After ports are placed, and the robot is docked, the surgeon sits on the robot console and can control the three robotic instruments and camera. This control provides a surgeon an opportunity to self-assist to complete the surgery. However, there is variability in robot-assisted lobectomy procedures in terms of using an assistant during the case (12-14). Some robot lobectomy techniques require two assistant ports when the assistant performs division of the hilar structure with a hand-held stapler and retraction of the lung (14). We wanted to determine if self-assistance during robot-assisted pulmonary lobectomy has similar outcomes compared to VATS lobectomy that requires active assistant. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-176/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board at the Houston Methodist Hospital Research Institute (Pro00013680, 11/5/2015) and informed consent was taken from all the patients. We obtained data from the Houston Methodist Hospital Society of Thoracic Surgery (HMH-STS) database. We performed a retrospective analysis of the HMH-STS database of patients who underwent consecutive pulmonary lobectomies by a single surgeon from 10/2017 to 5/2020 using a DaVinci Xi Robotic platform (Intuitive Surgical, Sunnyvale, CA). We assessed surgeon experienced in the number of robotic cases and lobectomy surgical procedures before the starting of self-assisting during robot lobectomy.
Self-assisting during robotic lobectomy was performed with a surgical technician at the bedside with a general surgery resident typically on the second console. The initial technique for robot pulmonary resection has been described previously (15,16); however, there were three modifications needed to fully self-assist during the pulmonary lobectomy. The modifications were placing four ports only, precise dissection around hilar structures without blunt dissection, and complete dissection around the pulmonary artery (Video 1). Ultimately, we converted to a four-port technique from the five-port technique, where the assistant port was removed (Figure 1). We placed the first port by the scapula tip, where we placed either the tip up grasper or caudiere forceps to help retract the lung. The camera is placed in the posterior axillary line port, and the ports in the anterior axillary line and posterior inferior ports were used to dissect hilar structures and staple vessels, bronchus, or fissures.
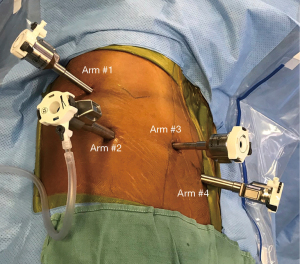
When we first started the four-port technique, to ensure we were prepared for potential conversion, we marked the skin incision for lateral thoracotomy over the hilum. We practiced with the team on the conversion steps. We ensured that we had a rib spreader and Raytec sponge on ringed forceps for the conversion to thoracotomy. We discussed that we would retain the camera arm and one of the other arms to keep the pressure on the bleeding vessel. We used one of the other ports for suction to keep the field dry. We felt that this technique allowed us to perform the thoracotomy in a controlled fashion and to obtain proximal control of the injured vessel (17). We also had a partner available to assist if we needed to emergently convent to open thoracotomy. Furthermore, the surgical technician used the robot port to retrieve lymph nodes with a four-port technique. Second, we stopped performing blunt dissection around hilar structures; instead, we performed precise dissection of pre-vascular space using the bipolar energy device. This led to minimal blood loss during the operation. Finally, we performed a complete dissection of the pulmonary artery branch before stapling. The precise dissection of the pre-vascular space minimized blood loss and allowed for better visualization during the dissection of the pulmonary artery. A more distal dissection of the pulmonary artery branches allowed for little to no tension when a stapler is placed around the pulmonary artery. The precise dissection of the hilar structures and pulmonary artery added additional time during the operation compared to blunt dissection (Video 1).
We analyzed the intraoperative and postoperative outcomes of patients who underwent the operation with self-assistance when performing robot lobectomy. We compared the outcomes to the propensity matched group of HMH-STS VATS lobectomy patients (2012–2017). We obtained patient demographics, comorbidities, pulmonary function tests, ECOG score, ASA classification, and indications for surgery. We then evaluated the intraoperative outcomes and the postoperative outcomes. The procedure time was calculated from the time of incision to skin closure. It included some patient's additional procedures such as wedge resection before lobectomy and cryoablation of the intercostal nerve. We evaluated the major postoperative complications, including atrial arrhythmias requiring treatment, air leak greater than five days, pneumonia, surgical site infections, pulmonary embolus, bronchopleural fistula, deep vein thrombosis (DVT) requiring treatment, new central neurological events, ventricular arrhythmia requiring treatment, myocardial infarct (MI), empyema, tracheostomy, adult respiratory distress syndrome (ARDS), respiratory failure, renal failure, and sepsis.
Statistical analysis
Demographic and clinical data were reported as frequencies and proportions for categorical variables and as medians and interquartile ranges (IQR) or means (± standard deviation, SD) for continuous variables as appropriate. We also determined the similar variables found in Reddy et al. (8) report on the outcomes of robot-assisted lobectomy and VATS lobectomy and compared the outcomes of our series. Comparisons on common outcomes between studies were conducted by the chi-square or Fisher’s exact tests for the categorical variables and the unpaired one-sided t-test for the continuous variables.
Difference between groups in all patients (pre-matched) was compared using the Chi-square or Fisher’s exact tests for categorical variables and unpaired t-test or Kruskal Wallis test for continuous variables as appropriate. Patients in the self-assisted robot assisted surgery and video-assisted thoracoscopic surgery (VATS) groups were matched using the non-replacement propensity score (PS) matching with a ratio of 1:1 based on the following criteria: age at surgery, gender, race, body mass index, hypertension, congestive heart failure, coronary artery disease, diabetes, preoperative chemotherapy or immuno-therapy, preoperative thoracic radiation therapy, FEV1 predicted, DLCO predicted, smoking, ASA classification, pathologic stage, lung cancer-number of nodes.
Balance of the covariates after matching were determined by the standardized bias percent. Differences in the outcomes between the two groups in the PS matched cohort were compared using the univariable exact logistic regression (for categorical outcomes) or linear regression for continuous outcomes, adjusting for the matched pair using the clustered sandwich variance estimator. Difference in the proportion of select post-operative complications was presented by the bar charts and box plots. Generalized linear modeling (GLM) were performed to determine the characteristics associated with major and minor post-operative complications. Variables included in the multivariable models were selected using the least absolute shrinkage and selection operator (LASSO) method with the cross-validation (CV) selection option [1, 2], and also based on the clinical importance. All the analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, TX, USA). A P value of <0.05 was considered statistically significant. All analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, TX, USA).
Results
Ninety-five robot-assisted consecutive pulmonary resections were performed with full self-assistance. By the time the study surgeon began self-assisting during the pulmonary lobectomy, the surgeon had performed 400 thoracic robot-assisted operations with 51 cases of pulmonary lobectomy. The median age of patients was 70 years old (IQR 65, 74), most patients were females (n=54, 56.8%) and white (n=81, 85.2%), and were undergoing lobectomy for treatment of lung cancer (n=85, 89.5%). The three most common comorbidities were hypertension (n=63, 55.3%), coronary artery disease (n=25, 26.3%) and diabetes (n=19, 20%). Prior to lobectomy, few patients had preoperative chemotherapy (n=10, 10.5%), radiation therapy (n=1, 1.1%) and prior cardiothoracic surgery (n=11, 11.6%). A majority of patients were either current or former smokers (n=64, 67.4%). Most of the patients had an ECOG score of 0 (n=73, 76.8%) and ASA classification of 3 (n=63, 66.3%, Table 1).
Table 1
| Variable | Total (n=211) | Robot (n=95) | VATS (n=116) | P value |
|---|---|---|---|---|
| Age at time of surgery (years), median (IQR) | 68.0 (60.0, 74.0) | 70.0 (65.0, 74.0) | 68.0 (57.0, 74.5) | 0.14 |
| Male | 94 (44.5) | 41 (43.2) | 53 (45.7) | 0.71 |
| Non-White | 42 (20.0) | 14 (14.7) | 28 (24.3) | 0.08 |
| Height (cm), median (IQR) | 170.2 (162.6, 177.8) | 170.0 (163.0, 178.0) | 170.2 (162.3, 177.8) | 0.45 |
| Weight (kg), median (IQR) | 78.8 (66.3, 92.3) | 80.6 (69.4, 97.9) | 74.9 (63.8, 89.6) | 0.03 |
| Body mass index (kg/m2), median (IQR) | 27.5 (24.0, 31.3) | 28.8 (24.8, 32.6) | 26.8 (23.3, 30.8) | 0.01 |
| Hypertension | 137 (64.9) | 63 (66.3) | 74 (63.8) | 0.70 |
| Congestive heart failure | 5 (2.4) | 1 (1.1) | 4 (3.4) | 0.38 |
| Coronary artery disease | 52 (24.6) | 25 (26.3) | 27 (23.3) | 0.61 |
| Interstitial fibrosis or interstitial lung disease | 5 (2.4) | 2 (2.1) | 3 (2.6) | 1.00 |
| Cerebrovascular history | 26 (12.3) | 9 (9.5) | 17 (14.7) | 0.30 |
| Diabetes | 42 (19.9) | 19 (20.0) | 23 (19.8) | 0.98 |
| Currently on dialysis | 1 (0.5) | 0 (0.0) | 1 (0.9) | 1.00 |
| Preoperative chemotherapy or immunotherapy | 19 (9.0) | 10 (10.5) | 9 (7.8) | 0.48 |
| Preoperative thoracic radiation therapy | 6 (2.8) | 1 (1.1) | 5 (4.3) | 0.16 |
| Prior cardiothoracic surgery | 28 (13.3) | 11 (11.6) | 17 (14.7) | 0.51 |
| FEV1 predicted, median (IQR) | 87.0 (75.0, 100.0) | 89.0 (75.5, 101.0) | 85.0 (75.0, 98.0) | 0.26 |
| DLCO predicted, median (IQR) | 79.0 (65.0, 95.0) | 80.0 (68.0, 96.0) | 76.0 (60.5, 92.0) | 0.10 |
| Cigarette smoking | 159 (75.4) | 64 (67.4) | 95 (81.9) | 0.02 |
| Reoperation | 23 (10.9) | 9 (9.5) | 14 (12.1) | 0.55 |
| ASA classification | 0.22 | |||
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 2 | 26 (12.3) | 14 (14.7) | 12 (10.3) | |
| 3 | 134 (63.5) | 63 (66.3) | 71 (61.2) | |
| 4 | 51 (24.2) | 18 (18.9) | 33 (28.4) | |
| Lung cancer | 186 (88.2) | 85 (89.5) | 101 (87.1) | 0.67 |
| Pathologic stage | 0.003 | |||
| Stage 0–1 | 121 (60.2) | 54 (63.5) | 67 (57.8) | |
| Stage 2 | 47 (23.4) | 21 (24.7) | 26 (22.4) | |
| Stage 3 | 19 (9.5) | 10 (11.8) | 9 (7.8) | |
| Stage 4 | 14 (7.0) | 0 (0.0) | 14 (12.1) | |
| Lung cancer, number of nodes | 10.0 (7.0, 14.0) | 11.0 (8.0, 14.0) | 9.0 (7.0, 12.0) | 0.02 |
Data is presented as medians and interquartile ranges or numbers (percentages). VATS, video-assisted thoracoscopic surgery; IQR, interquartile range; cm, centimeter; kg, kilogram; m2, meters squared; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity of the lung for carbon monoxide; ASA, American Society of Anesthesiologists.
The majority of the patients had stage I lung cancer (n=54, 56.8%) with a median of 11 lymph nodes removed (IQR 8, 14) and a median of 3 lymph node stations sampled (IQR 3, 4). The median estimated blood loss was 25 mL (IQR 25, 50) with no intraoperative blood transfusions (n=0, 0%) and no conversion to open or VATS surgery (n=0, 0%). The median procedure time was 4 hours (IQR 3.4, 4.6). The three most common complications after surgery were atrial arrhythmia requiring treatment (n=7, 7.4%), air leak greater than 5 days (n=6, 6.3%), and pneumonia (n=3, 3.2%). The median length of stay was 2 days (IQR 1, 4) with 30-day readmission rate of 6.3 % (n=6) and 0% (n=0) 30-day mortality.
The propensity-matched analysis of self-assisting robot lobectomy and VATS lobectomy showed that self-assisting robot lobectomy group had significantly less conversion to open surgery (n=0, 0% vs. n=10, 12.2%, P=0.002), less intraoperative blood transfusions (n=0, 0% vs. n=6, 7.3%, P=0.03), less any post-operative event (n=20, 24.4% vs. n=41, 50%, P=0.003), and less median length of stay (2 days, IQR 2, 5 vs. 4 day, IQR 3, 6 days , P<0.001) compared to VATS lobectomy group. There was no significant difference with procedure time (P=0.40), readmission within 30 days (P=0.77) and mortality (Table 2).
Table 2
| Outcome | Total (n=164) | Robot (n=82) | VATS (n=82) | P value |
|---|---|---|---|---|
| Conversion to open surgery or VATS | 10 (6.1) | 0 (0.0) | 10 (12.2) | 0.002 |
| Procedure time (min), median (IQR) | 249.5 (201.5, 291.5) | 242.5 (206.0, 276.0) | 260.5 (197.0, 304.0) | 0.40 |
| Intraoperative blood transfusion | 6 (3.7) | 0 (0.0) | 6 (7.3) | 0.03 |
| Any post-operative events occurred (major or minor) | 61 (37.2) | 20 (24.4) | 41 (50.0) | 0.003 |
| Major post-operative complications* | 38 (23.2) | 14 (17.1) | 24 (29.3) | 0.10 |
| Air leak greater than five days | 10 (6.1) | 5 (6.1) | 5 (6.1) | 1.00 |
| Surgical site infection | 1 (0.6) | 1 (1.2) | 0 (0.0) | 1.00 |
| Pulmonary embolus | 1 (0.6) | 1 (1.2) | 0 (0.0) | 1.00 |
| Atrial arrhythmia requiring treatment | 19 (11.6) | 6 (7.3) | 13 (15.9) | 0.12 |
| Ventricular arrhythmia requiring treatment | 1 (0.6) | 0 (0.0) | 1 (1.2) | 1.00 |
| Pneumonia | 11 (6.7) | 3 (3.7) | 8 (9.8) | 0.21 |
| Myocardial infarct | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Empyema | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Bronchopleural fistula | 1 (0.6) | 1 (1.2) | 0 (0.0) | 1.00 |
| Tracheostomy | 1 (0.6) | 0 (0.0) | 1 (1.2) | 1.00 |
| Adult respiratory distress syndrome | 1 (0.6) | 0 (0.0) | 1 (1.2) | 1.00 |
| Respiratory failure | 4 (2.4) | 0 (0.0) | 4 (4.9) | 0.13 |
| Renal failure | 1 (0.6) | 0 (0.0) | 1 (1.2) | 1.00 |
| Sepsis | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| DVT requiring treatment | 2 (1.2) | 2 (2.4) | 0 (0.0) | 0.50 |
| New central neurological events | 2 (1.2) | 0 (0.0) | 2 (2.4) | 0.50 |
| Minor post-operative complications* | 40 (24.4) | 11 (13.4) | 29 (35.4) | 0.004 |
| Ileus | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Urinary retention | 17 (10.4) | 3 (3.7) | 14 (17.1) | 0.003 |
| Delirium | 1 (0.6) | 0 (0.0) | 1 (1.2) | 1.00 |
| Discharge with foley | 5 (3.0) | 1 (1.2) | 4 (4.9) | 0.38 |
| Other pulmonary events | 11 (6.7) | 2 (2.4) | 9 (11.0) | 0.07 |
| Urinary tract infection | 9 (5.5) | 3 (3.7) | 6 (7.3) | 0.45 |
| Post-operative packed red blood cell | 5 (3.0) | 0 (0.0) | 5 (6.1) | 0.06 |
| Other neurological events | 1 (0.6) | 0 (0.0) | 1 (1.2) | 1.00 |
| Other infection requiring IV antibiotics | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Other cardiovascular events | 4 (2.4) | 1 (1.2) | 3 (3.7) | 0.62 |
| Atelectasis requiring bronchoscopy | 3 (1.8) | 0 (0.0) | 3 (3.7) | 0.25 |
| Post-op pleural effusion requiring drainage | 5 (3.0) | 2 (2.4) | 3 (3.7) | 1.00 |
| Pneumothorax | 3 (1.8) | 1 (1.2) | 2 (2.4) | 1.00 |
| Total number of ICU days, mean (± SD) | 0.9 (±3.4) | 1.5 (±0.7) | 0.9 (±3.5) | 0.80 |
| Total length of stay (days), median (IQR) | 3.0 (2.0, 5.0) | 2.0 (1.0, 4.0) | 4.0 (3.0, 6.0) | <0.001 |
| Readmission within 30 days of discharge | 14 (8.5) | 6 (7.3) | 8 (9.8) | 0.77 |
| 30-day mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
Values are in frequency (%) for categorical variables and median (IQR) for continuous variables. *, patients may have more than one complication. VATS, video-assisted thoracic surgery; min, minutes; IQR, interquartile range; DVT, deep vein thrombosis; IV, intravenous; ICU, intensive care unit; SD, standard deviation.
We then compared our data to the propensity-matched analysis of lobectomy performed by surgeons who performed 20 or more annual robotic lobectomies published by Reddy et al. (8) (Table 3). We found that patients who underwent self-assisting robot-assisted lobectomy in our series had significantly less conversion to open or VATS from robot compared to robot-assisted series from Reddy et al. (0% vs. 4.8%, P=0.03) with similar procedure time (4±1 vs. 4.12±1.3 hours, P=0.38) and similar complication rate (23.2% vs. 30.7%, P=0.16). However, patients in our series had significantly shorter mean length of stay (3.1±2.8 vs. 6.3±4.5, P<0.001) and more patients going home after surgery (100% vs. 93.7%, P=0.004) without difference in a 30-day mortality (0% vs. 1.3%, P=0.62). Moreover, patients in our series had significantly better outcomes than patients who underwent VATS lobectomy in the Reddy et al. series. Patients had significantly less conversion to open (P=0.001), fewer complications (P=0.01), less length of stay (P<0.001), and more patients going home (P=0.01) without significant difference in mortality (P=0.24).
Table 3
| Outcomes | Kim et al. Robotic self-assisting (n=95) |
Reddy et al. Robotic-assisted (n=838) | P value | Reddy et al. VATS lobectomy (n=838) | P value |
|---|---|---|---|---|---|
| Conversion to open surgery or VATS | 0 (0.0) | 40 (4.8) | 0.03 | 67 (8.0) | 0.001 |
| Procedure time (hours), mean (± SD) | 4.0 (±1.0) | 4.12 (±1.3) | 0.38 | 3.71 (±1.2) | 0.02 |
| All complications | 22 (23.2) | 257 (30.7) | 0.16 | 307 (36.7) | 0.01 |
| Total length of stay (days), mean (± SD) | 3.1 (±2.8) | 6.3 (±4.5) | <0.001 | 6.6 (±5.0) | <0.001 |
| Discharge status | 0.004 | 0.01 | |||
| Health facility | 0 (0.0) | 53 (6.3) | 62 (7.4) | ||
| Home | 95 (100) | 785 (93.7) | 776 (92.7) | ||
| 30-day mortality | 0 (0.0) | 11 (1.3) | 0.62 | 12 (1.4) | 0.24 |
Values are in frequency (%) for categorical variables and mean (± SD) for continuous variables. VATS, video assisted thoracoscopic surgery; SD, standardized deviation.
Discussion
We discovered that full self-assisting during pulmonary lobectomy is safe. This study shows that pulmonary lobectomy can be performed by the surgeon with self-assistance during the operation. The outcomes are better compared to VATS lobectomy that requires an assistant. This is a significant advancement over requiring a skilled surgical assistant during open lobectomy and VATS lobectomy. When performing open lobectomy and VATS lobectomy, the surgical assistant is necessary to complete the case. Open lobectomy requires a surgical assistant to retract the lung(s) and suction the blood from the field. A VATS lobectomy requires a surgical assistant providing retraction, keeping the field dry, and allowing for visualization of the field by holding the camera.
A skilled surgical assistant is a great asset during open or VATS lobectomy. The surgical assistant who can predict the next surgical move and expose the correct anatomy so the surgeon can complete the task will allow the case to proceed smoothly and with great success. Even though skilled surgical assistants are necessary during the open or VATS procedures, not all practices have the availability of such resources. When I started my practice, I had a novice surgical assistant who needed considerable prompting for the surgeon to complete the case. During a VATS lobectomy, I would need to move the novice assistant’s hand holding the instrument and the camera to help expose and show the necessary anatomy. This “hand-holding” adds significant variability to the operation, and this was performed every month since I had a new resident every month. The variability of the assistant leads to variability in surgical outcomes. For example, one assistant would pull too hard on the parenchyma of the lung, which would lead to tearing of the lung and oozing throughout the case. In my experience, there are differences in how tissue is handled and how different surgical areas are exposed between having an intern assist with a lobectomy compared to having an attending assisting a fellow through the surgery. This variability is usually not present with a skilled surgical assistant, but the variability is completely removed with the ability to self-assist during pulmonary lobectomy. With robot-assisted lobectomy being performed by a fully self-assisting surgeon, the surgeon controls the third arm that is used to retract the lung and the camera to view the desired area. Once the optimal port placement for the robot is determined, the operation with self-assistance is performed. This action has led to optimal outcomes since there is no variability due to the skill level of the assistant. This improvement in outcomes has been seen with both intraoperative and postoperative outcomes.
Ultimately, the ability to have minimal blood loss and no conversions to VATS or open lobectomies has allowed the successful completion of a case fully autonomously. We have previously shown that robot-assisted pulmonary resections were associated with fewer conversions compared to VATS (18,19) in our practice. This is different from the meta-analysis of the conversion rates of VATS and robot-assisted pulmonary resections, which have shown no differences in the conversion rates between VATS and robot-assisted pulmonary resections (20). The reasons for fewer conversions in our experience have been our change in technique and technology improvement. Utilizing self-assistance allows for precise dissection of the vessels in the pre-vascular plane, mobilization of the vessels, and advancement of stapler technology, which significantly contributes to success. When we first adopted robot lobectomy, we used the procedural techniques from VATS lobectomy, where blunt dissection of the vessels was utilized to complete the operation. This led to significant oozing that required a bedside assistant to provide suction to the surgical field during the operation. We used an Airseal port (ConMED, Largo, FL) to ensure the suction could occur with insufflation of the chest cavity. Once we changed the technique from blunt dissection to precise dissection of the pre-vascular space using the bipolar energy device, we minimized bleeding, making it possible to avoid using the assistant. Additional techniques to avoid conversion have been dissecting the pulmonary artery branch until there is no tension on the pulmonary artery branch when the stapler is placed around it. Less tension on the pulmonary artery has led to less misadventure with the vessel. In addition, the advancement of stapler technology has allowed for better articulation, leading to less tension on the pulmonary artery branch during division. These changes are likely part of the decrease in the amount of bleeding and the decreased probability of needing to convert to VATS or open lobectomy procedures.
Lastly, we started performing autonomous pulmonary lobectomies when the surgeon felt comfortable with the procedure. This phenomenon occurred not due to experience with lobectomy alone, but with continual experience with the use of the robot in all thoracic surgical cases. By the end of the study, the surgeon in this study performed 773 total operations. By the time the surgeon started self-assistance pulmonary lobectomies, the surgeon had completed 400 robot-assisted thoracic surgical cases. The surgeon already had performed other autonomous thoracic operations such as foregut surgery, mediastinal mass resection, and pulmonary wedge resections. Thus, fifty-one pulmonary lobectomies were not the mark where the transition happened, but 400 overall robot cases made the surgeon (and team) feel comfortable performing more complex operations and fully self-assisting during pulmonary lobectomy.
Overall outcomes in this surgical series were better compared to the reported series of patients who underwent either robot-assisted or VATS lobectomy (2,8). The VATS lobectomy has an overall complication rate of 26%, with a 4-day median length of stay and a 1% 30-day mortality rate (2). The complication rate was 17% in our series, with a 2-day median length of stay and 0% 30-day mortality. Moreover, these results were better than the Premier database outcomes for patients who underwent robotic lobectomy (8). Reddy and coworkers found an overall complication rate of 33%, with a mean length of stay of 5 days and 30-day mortality of 1.3% (8). Thus, self-assisting during robotic lobectomy does not provide poorer outcomes compared to other series of patients who underwent either VATS or robot-assisted lobectomy.
The study’s major limitation is that this is a retrospective, single-surgeon, single-institution study that looks at the outcomes of performing the robot-assisted lobectomy. Thus, this technique might not apply to other surgeons and other facilities. In addition, the surgeon became fully self-assisted when he developed an optimal dissection technique and achieved minimal blood loss during the operation. Thus, the number of pulmonary lobectomies needed prior to full self-assistance during pulmonary lobectomy is hard to quantitate. Despite the limitations, this is the first report of a major advantage of robot-assisted lobectomy compared to VATS or open lobectomy, providing evidence that robot-assisted lobectomy can be performed autonomously.
As the technology and technique have evolved in using the robot platform for pulmonary lobectomy, the ability to safely self-assist and perform this complex operation is a major advancement over open and VATS lobectomy. With the appropriate experience and optimal setting, robot-assisted lobectomy can be performed autonomously.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-176/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-176/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-176/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-176/coif). MPK consults for Veran/Olympus, Medtronic, AstraZeneca, and Intuitive Surgical. He serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2020 to August 2022. EYC consults for Veran/Olympus and Intuitive Surgical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board at the Houston Methodist Hospital Research Institute (Pro00013680, 11/5/2015) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. [Crossref] [PubMed]
- Blasberg JD, Seder CW, Leverson G, et al. Video-Assisted Thoracoscopic Lobectomy for Lung Cancer: Current Practice Patterns and Predictors of Adoption. Ann Thorac Surg 2016;102:1854-62. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Meyerson SL, Balderson SS, D'Amico TA. Training Assistants Improves the Process of Adoption of Video-Assisted Thoracic Surgery Lobectomy. Ann Thorac Surg 2015;100:401-6. [Crossref] [PubMed]
- Yuh B. The bedside assistant in robotic surgery--keys to success. Urol Nurs 2013;33:29-32. [Crossref] [PubMed]
- Sasankan P, Chang S, Cerfolio R. Robotic right upper lobectomy: Twelve steps. JTCVS Tech 2021;7:280-2. [Crossref] [PubMed]
- Veronesi G, Novellis P, Bottoni E, et al. Robotic Lobectomy: Right Upper Lobectomy. Oper Tech Thorac Cardiovasc Surg 2017;21:249-68. [Crossref]
- Usuda J, Inoue T, Sonokawa T, et al. New Technique for Introducing a Surgical Stapler during Robot-Assisted Lobectomy for Lung Cancer. J Nippon Med Sch 2022;89:169-75. [Crossref] [PubMed]
- Kim MP, Chan EY. "Five on a dice" port placement for robot-assisted thoracoscopic right upper lobectomy using robotic stapler. J Thorac Dis 2017;9:5355-62. [Crossref] [PubMed]
- Khan N, Fikfak V, Chan EY, et al. "Five on a Dice" Port Placement Allows for Successful Robot-Assisted Left Pneumonectomy. Thorac Cardiovasc Surg Rep 2017;6:e42-4. [Crossref] [PubMed]
- Gharagozloo F, Meyer M. Technique of robotic lobectomy III: control of major vascular injury, the 5 "P" s. Mini-invasive Surg 2020;4:57. [Crossref]
- Soliman BG, Nguyen DT, Chan EY, et al. Impact of da Vinci Xi robot in pulmonary resection. J Thorac Dis 2020;12:3561-72. [Crossref] [PubMed]
- Kim MP, Nguyen DT, Meisenbach LM, et al. Da Vinci Xi robot decreases the number of thoracotomy cases in pulmonary resection. J Thorac Dis 2019;11:145-53. [Crossref] [PubMed]
- Guo F, Ma D, Li S. Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer: A Meta-analysis. Medicine (Baltimore) 2019;98:e17089. [Crossref] [PubMed]


