Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery
Introduction
Approximately 27% of “at-risk” patients who undergo chest computed tomography (CT) screening are found to have pulmonary nodules larger than 4 mm in size according to a recent national lung screening trial (1). Despite the fact that the majority of these nodules may not actually be malignant, biopsy and/or surgical intervention along with follow-up CT to confirm or support their indolent characteristics is recommended for nodules greater than 1 cm in diameter (2). Single-port video-assisted thoracoscopic surgery (VATS) has become an increasingly popular modality and provides a minimally invasive approach to the removal of pulmonary nodules (3,4). However, this technique also raises additional issues compared with conventional three-port VATS. During single-port VATS, the surgical instruments and camera need to be located within a single incision, thus making intraoperative localization of these lesions perhaps even more challenging (5). Furthermore, manual palpation may well be impossible for single-incisional subxiphoid VATS approach (6). Small pulmonary lesions of 1 cm, for instance, or those located at a distance from the periphery of the lung, particularly when they are at a depth greater than 2.5 times the lesion diameter, can be difficult to locate intraoperatively using palpation alone. Localization becomes more problematic when dealing with part-solid lesions with high ground-glass opacity (GGO), which are hard to palpate even if they are substantial or near the periphery.
Classically, the use of preoperative CT-guided marking by dye, contrast medium, radio nucleotide labelling, or hookwire/microcoil localization has been useful in aiding the intraoperative localization of small lesions. Occasionally, however, such methods lead to complications. Recent studies have demonstrated the efficacy of identifying pulmonary lesions immediately before or during surgery using a hybrid operating room (OR) with intraoperative CT to implant a hookwire or metallic fiducial, intraoperative localization using ultrasound, or electromagnetic navigational bronchoscopy (ENB)-guided localization. In this paper, we review the development of techniques for localizing small pulmonary nodules and discuss some of their advantages and limitations, with a particular emphasis on approaches that can be used in single-port VATS.
Common techniques to localize pulmonary nodules without the need for palpation
Multiple methods, mostly performed under the guidance of preoperative CT, have been developed to assist the identification of pulmonary nodules. In general, they can be classified into three categories: implantation of metallic substances, injection of vital dye visualized by colour or intraoperative fluoroscopy, and radionuclide labelling detected by an endoscopic probe.
Metallic implantation
Although lacking an official standard, preoperative CT-guided hookwire placement is currently the most common method used to localize small pulmonary lesions and has a relatively high success rate. The wire is often placed several hours before the commencement of surgery. Any displacement of the wire can lead to a failure of localization in approximately 9.5% of patients (7), though this may be partly resolved by the use of a spiral wire (8) or addition of a suture system (9). The problem of displacement mainly occurs during the transportation of the patient to the OR or during the surgical procedure itself, either when the lung is deflated after general anaesthesia or through the gentle retraction of the wire by the surgeon. Likewise, migration of the microcoil, which is normally implanted preoperatively via percutaneous route and detected through intraoperative fluoroscopy in the lung parenchyma, during the period between coil insertion and operative resection can occur in 3% to 10% of cases (10,11). Other complications, such as pleuritic pain, pneumothorax, hemothorax, or air embolus, can arise due to the invasive puncture of the visceral pleura.
Dye or contrast medium injection
The injection of methylene blue tattooing (12) or another water-soluble contrast medium with lipiodol or barium sulphate (13) under CT guidance close to the tumour site assists the surgeon in locating the lesion during surgery either by direct sight or through intraoperative fluoroscopy. Apart from pleural-related complications, the diffusion of the dye within the parenchyma and at time spillage into the pleural space can lead to a failure in localizing the lesion. Thus, the time between labelling and thoracoscopy must be as short as possible; otherwise, an excessive amount of dye will diffuse beyond the target region. Moreover, the possibility of anaphylaxis and the potential risk of embolism may be non-negligible if this material accidently reaches the systemic circulation (14). In addition, there are physical limitations to the use of intraoperative fluoroscopy in the deflated lung when the patient is in the decubitus position.
Radionuclide labelling
The technetium-99 (99TC) radiotracer, after injection into the tissue surrounding the lesions, can remain stable for up to 24 hours, thereby increasing the time frame between labelling and surgery (15). Perhaps more importantly, the technique enables the continual localization of nodules during surgery via a gamma probe, thus allowing for their accurate excision. However, centres must have the correct equipment and radiation protection procedures in place to offer this method. In addition, this technique may not be suitable for deep and posterior nodules because of the structure of the probe, which is not able to be moved freely within the chest (7,16). Other complications relating to percutaneous injection are similar to those given for the methods listed above.
Although these preoperative methods facilitate thoracoscopic sublobar resection, most of them require the patient to be transferred to two different locations or departments to complete the entire resection: one to localize the lesion and one for surgery. This may be time-consuming and uncomfortable for patients.
Simultaneous localization and resection of pulmonary nodules
The simultaneous localization of pulmonary nodules and surgical resection, especially after anaesthesia in the OR, could help to minimize complications such as pneumothorax and wire displacement resulting from patient transfer and procedural delays, as well as reduce patients’ discomfort. Recent studies to investigate the use of hybrid OR, which combines localization techniques with immediate single-port VATS, have shed new light on minimally invasive thoracic surgery.
Image-guided video-assisted thoracoscopic surgery (VATS) in hybrid operating room (OR)
The integration of real-time on-table image guidance technology into clinical practice is well established in other specialties, including cardiology and vascular surgery. However, the use of hybrid OR, termed image-guided VATS (iVATS), for the intraoperative assessment and localization of lung nodules was first reported by the Brigham and Women’s Hospital group in 2013. In their prospective clinical trial (17), 23 patients with a mean pulmonary lesion size of 1.30±0.38 cm underwent placement of two T-shaped fiducials using intraoperative C-arm CT followed by standard thoracoscopic resection. Briefly, after general anaesthesia and placement of the patient into a lateral decubitus position, a C-arm CT scan was conducted during an end-inspiratory-hold manoeuvre according to the pre-determined field. Two T-bar fiducials were then implanted for localization under the guidance of fluoroscopy. Notably, the T-bars were successfully implanted in 20 (87.0%) cases and normally required only one CT scan. The average and total procedure radiation doses were low (median: 1,501 mGy × m2; range, 665–16,326). The total amount of time needed for the procedure was also found to be acceptable because the median length of time required for placing the two T-bars was 39 min. Narayanam et al. (18) similarly demonstrated that the combination of lung tattooing and immediate VATS was safe for children in an interventional radiology suite and avoided the need for open thoracotomy to provide a definite diagnosis. Ohtaka et al. (19) introduced an O-arm intraoperative CT scan system integrated with a novel “intraoperative stamping” method (20) for localizing pulmonary lesions. However, the centre of the O-arm in this case may require adjusting more than once due to the lack of a pre-determined scanning field, thus increasing the potential radiation exposure to the patient. Moreover, although the “stamping” method avoids puncturing of the visceral pleura, an additional incision is required to place the dye-containing gauze ball, making its application difficult in single-port VATS.
We subsequently expanded the iVATS method to include single-port VATS in major lung resections of small or GGO lesions, known as image guided single-port VATS (iSPVATS) (21). In our method, the hookwire is inserted within 30 min under the guidance of on-table cone-beam CT (DynaCT) in the OR, thus guaranteeing the accuracy of the localization process (Figure 1). A single-port VATS procedure can then be performed immediately. If the hookwire is dislodged during lung deflation, a salvage CT scan can be performed to re-hookwire or re-localize the lesions although that was never necessary in our experience. Moreover, DynaCT provides essential information for resection margins even in cases unsuitable for hookwire insertion.
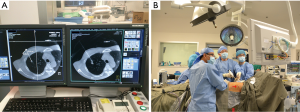
Use of our hookwire iSPVATS approach may reduce the risk of complications associated with the transportation of the patient and delay of the procedure such as prolonged discomfort, hookwire displacement, and pneumothorax. However, a special multidisciplinary team comprising surgeons, radiologists, and anaesthetists requires special training. The DynaCT system also needs experience in positioning for the decubitus lie when conducting thoracic surgery. The procedure would work well for lesions adjacent to the chest wall or near the periphery even if of subcentimetre size, but may not be suitable when the wire has to cross extensive lung tissue to localize a very deep lesion. Furthermore, the lesion may not be accessible percutaneously in areas around the apical, diaphragmatic, or mediastinal regions of the lung because it is shielded or too dangerous to approach (22). In addition, it may be more challenging to “mark” multiple lesions, particularly during pulmonary metastasectomy, using this technique. Currently, there are no large studies providing data on indications for iSPVATS in intra-operative localization of small pulmonary nodules. For experienced surgeons, lesions 1cm or more in size can usually be palpated in SPVATS.
Intraoperative ultrasound localization
In completely deflated lungs, the structures of the pulmonary arterioles and venules are identified as homogeneous hypoechoic areas and the bronchioles as hyperechoic spots. In contrast, pulmonary nodules can be visualized by intraoperative ultrasonography as a hypo-/hyperechoic nodule or a hyperechoic shadow beneath the nodule. It has been reported that pulmonary nodules that underwent wedge resection and that can be successfully located by ultrasound were located at a depth of around 30 mm on CT scan, which corresponds to approximately less than half that distant when the lung is deflated (23). Therefore, ultrasonography would be of use if it were able to detect a lesion at a depth of 15 mm from the surface of an entirely deflated lung. In previous studies, ultrasonography showed a sensitivity of more than 90% in localizing pulmonary lesions (24,25), with a procedural time in VATS ranging from less than 1 to 13 (mean, 4.07±3.99) minutes (26). Perhaps more importantly, the echo-Doppler function was able to reveal details regarding the vascularisation of the nodule and could thus help to predict the nature of the lesion (27). This technique offers a time-saving and less invasive method of localization with almost no reported complications.
The careful placement of the port for thoracoscopic ultrasound is reportedly a crucial factor for successful localization (28), mainly because the linear-type probe without optics has been adopted during VATS in previous reports (16,27). A design such as this may decrease the ability of ultrasound to access lesions in the posterior segment or lateral to the spine (26) and hence hamper its potential use in single-port surgery. In 2011, Rocco et al. (29) described their initial experience using a 10-mm ultrasound probe along with a 5-mm, 0-degree video-thoracoscope and an articulating grasper to identify two peripheral nodules via single-port VATS. Although it has been noted in the past that a high frequency of 12 MHz will allow the near visualisation of targets at a greater depth (for instance, 15 mm in deflated lungs) (23), a frequency of 5 to 10 MHz was adopted with success by Rocco’s team.
Apart from the benefit of a non-invasive approach, ultrasonography in VATS may be simpler than other techniques that require a potentially rapid learning curve. More importantly, in a previous study, ultrasonography was reportedly able to identify two occult lesions that may not have been detected by preoperative spiral CT (24). Wada et al. (23) recently introduced a prototype convex ultrasound thoracoscope that was able to visualise targets over a much wider range of lungs in an animal model. Additionally, they added a real-time fine-needle aspiration system to the probe through a single port to enable surgeons to take a diagnostic biopsy of the nodules in advance of diagnostic resection. These features could be particularly important when dealing with multiple pulmonary lesions. Fundamentally, intraoperative ultrasonography requires the complete collapse of the lung for accurate imaging, which makes it difficult and time-consuming in patients with emphysema or pleural adhesions (27). Low-pressure CO2 insufflation (<10 mmHg) during VATS does not have an adverse haemodynamic impact on the clinical setting (30) and may help to expedite the collapse of the lungs, however, further investigation is warranted.
The main criticism of intraoperative ultrasound localization is that the technique is highly operator-dependent and thus cannot be applied without a specialist in ultrasonography with extensive experience. The procedure is extremely difficult to carry out when the intraoperative localization of subcentre lesions with air filled cavity is required. Nevertheless, it should be pointed out that ultrasound localization is possibly the only available “salvage” technique should one be caught out intraoperatively by an unexpectedly difficult to localize pulmonary nodule, since no prior preparations are required for its use.
Electromagnetic navigation bronchoscopy (ENB) in hybrid operating room (OR)
The clinical use of ENB-guided biopsy to obtain tissue for the diagnosis of peripheral lung lesions dates back almost a decade (31,32), and with its many advantages over percutaneous CT-guided procedures is rapidly gaining acceptance (3). Bolton et al. (33) studied 19 patients who underwent robotic pulmonary surgery immediately after dye marking through ENB under fluoroscopic guidance in the OR. Methylene blue was injected at the site of the lesion and at two other separate locations beneath the pleura, meaning it could be identified more precisely during thoracoscopy. Dye placement by ENB could therefore lower the incidence of marker diffusion from the nodule and the difficulty with visualisation in patients with extensive anthracotic pigmentation. Moreover, the median time for ENB-guided dye placement was 28 min, which provided a limited extension to the total operating time.
ENB can also be a useful adjunct technology for the localization of peripheral lung nodules by the deployment of fiducials to the lesion via the endobronchial route, leading to a much lower production of artefacts than the conventional percutaneous approach. Fiducials are dense markers several millimetres in size and made of either gold or platinum; they are used by stereotactic radiosurgery systems to achieve the real-time tracking of tumours (34). Previously, fiducials are placed under CT guidance via a transthoracic approach, facilitating the subsequent detection and removal of small nodules with intraoperative fluoroscopy (35). The fiducials are associated with negligible risk of allergic reaction and are often placed hours to days before surgery. However, migration of the fiducials is not uncommon with prolonged delay. Fiducials can also be placed using navigation bronchoscopy. Anantham et al. (36) placed 39 fiducials via ENB into nine patients for radiotherapy. However, they found a 10% migration rate after placement, most likely due to coughing. Implantation of the fiducials under ENB after general anaesthesia immediately prior to surgical resection may solve this problem.
Novel ENB-associated technologies that allow for homing-in on lung lesions are currently under development and will, in the future, provide further options for thoracic surgeons. Anayama et al. (37) recently reported their initial experience in a porcine model using ENB-guided transbronchial indocyanine green (ICG) injection. They were able to detect an artificial nodule at a depth of less than 24 mm in the inflated lung using a near-infrared (NIR) fluorescence thoracoscope. The ICG spot was shown to remain at the injection site for more than 6 hours in vivo, and the specific wavelength of ICG fluorescence could be detected by NIR regardless of the colour or texture change of the visceral pleura by anthracosis or other diseases. In addition, ICG was found to be safe with the added advantage that it does not adversely affect the pathological examination. The limitations of this technique were similar to other dye-marking techniques, particularly when treating patients with severe pulmonary emphysema because ICG may diffuse among the alveolar space.
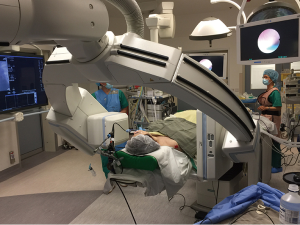
The combination of endobronchial ultrasound (EBUS) and ENB would improve the lesion localization rate to 93% (38), but these techniques may not sufficiently identify subcentimetre lesions with a high GGO content because of the common navigational error range of 4 to 6 mm. Navigational errors of the system and movement of the biopsy tool during its deployment can adversely affect the success of the biopsy or marking. To further increase the accuracy and applicability of this technology, we recently reported the successful use of integrated DynaCT and ENB in a hybrid OR, providing unparalleled real-time images to guide and confirm the successful navigation and biopsy to an 8-mm lesion located in the right middle lobe (39) (Figure 2). The image from the DynaCT revealed its superior accuracy over fluoroscopy or EBUS in determining the direction of the catheter tip. Normally, two intraoperative scans are required: one to identify any minor misdirection and the second to confirm the biopsy after any necessary adjustment has taken place. Thus, the radiation exposure from this technique is similar to a preoperative percutaneous centesis procedure. Hopefully, this hybrid technique will allow more precise placement of marking dye or fiducials, of which we are currently investigating.
Conclusions and perspectives
Various techniques have been introduced over the past few decades to help thoracic surgeons identify pulmonary nodules during minimally invasive surgery when detection by manual palpation may be challenging or difficult. Although many small nodules can still be comfortably palpated during VATS or SPVATS, marking of certain lesions, especially subcentimetre or small GGO nodules, may allow the surgeon to more confidently locate the target and define the margin. Most of these techniques require localization to be performed in the radiology suite followed by surgery in the OR. This inevitably takes a considerable amount of time, and patients might experience discomfort due to various complications. In contrast, a hybrid theatre can provide real-time imaging and enhance the possibility of success for hookwire placement or ENB, and also serve as a useful adjunct in the intraoperative ultrasonography technique during uniportal VATS. Other types of physical markers that can be deployed percutaneously in the hybrid OR include the microcoil and standard metal (gold) fiducials, which can enhance haptic feedback and the sensitivity of digital palpation as well as provide a radiopaque nidus for radiological confirmation. DynaCT-guided percutaneous injection of a marking agent (dye, barium, or lipiodol) immediately before surgery can similarly provide a radiopaque beacon to guide resection. A hybrid theatre can also provide relatively simple image guidance by assessing the relationship of the surgical instruments to the lesion. In the future, a combination of ENB-guided metal fiducial placement and hybrid operating theatre image-guided localization may further improve the accuracy and safety of resection, especially for multiple lung lesions in iSPVATS.
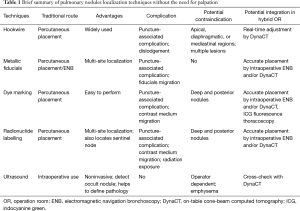
Since no comparative studies have yet been performed, it is difficult to conclude which technique should be applied under what circumstances and which technique would be most effective (Table 1). The evolutionary change in the localization techniques discussed in this review may eventually shift the treatment paradigm toward the patient-centralized caring concept whereby only a single anaesthetic regimen is needed for both localization and resection of the pulmonary nodule(s), iSPVATS would potentially avoid complications, eliminate disruptions from anaesthetic induction, and shorten hospital stay, providing a more cost-effective approach. Finally, we look forward to a brighter future in which greater numbers of pulmonary malignancies are able to be identified at an earlier stage through these advances, thus lowering patient mortality.
Full table
Acknowledgements
Funding: This work was supported by Research Grants Council (RGC) General Research Fund (GRF) [14117715]; and Foundation for Sci & Tech Research Project of Guangdong [2014B020212014].
Footnote
Conflicts of Interest: ZR Zhao and RW Lau have no conflicts of interest to declare. CS Ng has an electromagnetic navigational bronchoscopy system SuperDimension Version 7 on loan from Medtronic.
References
- Aberle DR, Abtin F, Brown K. Computed tomography screening for lung cancer: has it finally arrived? Implications of the national lung screening trial. J Clin Oncol 2013;31:1002-8. [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [PubMed]
- Ng CS, Lau KK, Gonzalez-Rivas D, et al. Evolution in surgical approach and techniques for lung cancer. Thorax 2013;68:681. [PubMed]
- Ng CS. Uniportal VATS in Asia. J Thorac Dis 2013;5 Suppl 3:S221-5. [PubMed]
- Ng CS, Pickens A, Siegel JM, et al. A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devices†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i73-i78. [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [PubMed]
- Sortini D, Feo C, Maravegias K, et al. Intrathoracoscopic localization techniques. Review of literature. Surg Endosc 2006;20:1341-7. [PubMed]
- Eichfeld U, Dietrich A, Ott R, et al. Video-assisted thoracoscopic surgery for pulmonary nodules after computed tomography-guided marking with a spiral wire. Ann Thorac Surg 2005;79:313-6; discussion 316-7. [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography--guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [PubMed]
- Mayo JR, Clifton JC, Powell TI, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250:576-85. [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [PubMed]
- Moon SW, Wang YP, Jo KH, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [PubMed]
- Zaman M, Bilal H, Woo CY, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. [PubMed]
- Sortini D, Feo CV, Carcoforo P, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule and history of malignancy. Ann Thorac Surg 2005;79:258-62; discussion 262. [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol 2015;112:18-25. [PubMed]
- Narayanam S, Gerstle T, Amaral J, et al. Lung tattooing combined with immediate video-assisted thoracoscopic resection (IVATR) as a single procedure in a hybrid room: our institutional experience in a pediatric population. Pediatr Radiol 2013;43:1144-51. [PubMed]
- Ohtaka K, Takahashi Y, Kaga K, et al. Video-assisted thoracoscopic surgery using mobile computed tomography: new method for locating of small lung nodules. J Cardiothorac Surg 2014;9:110. [PubMed]
- Kawada M, Okubo T, Poudel S, et al. A new marking technique for peripheral lung nodules avoiding pleural puncture: the intrathoracic stamping method. Interact Cardiovasc Thorac Surg 2013;16:381-3. [PubMed]
- Ng CS, Man Chu C, Kwok MW, et al. Hybrid DynaCT scan-guided localization single-port lobectomy. Chest 2015;147:e76-8. [corrected]. [PubMed]
- Galetta D, Bellomi M, Grana C, et al. Radio-Guided Localization and Resection of Small or Ill-Defined Pulmonary Lesions. Ann Thorac Surg 2015;100:1175-80. [PubMed]
- Wada H, Anayama T, Hirohashi K, et al. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules†. Eur J Cardiothorac Surg 2016;49:690-7. [PubMed]
- Piolanti M, Coppola F, Papa S, et al. Ultrasonographic localization of occult pulmonary nodules during video-assisted thoracic surgery. Eur Radiol 2003;13:2358-64. [PubMed]
- Matsumoto S, Hirata T, Ogawa E, et al. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS). Eur J Cardiothorac Surg 2004;26:469-73. [PubMed]
- Khereba M, Ferraro P, Duranceau A, et al. Thoracoscopic localization of intraparenchymal pulmonary nodules using direct intracavitary thoracoscopic ultrasonography prevents conversion of VATS procedures to thoracotomy in selected patients. J Thorac Cardiovasc Surg 2012;144:1160-5. [PubMed]
- Kondo R, Yoshida K, Hamanaka K, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg 2009;138:837-42. [PubMed]
- Gow KW, Saad DF, Koontz C, et al. Minimally invasive thoracoscopic ultrasound for localization of pulmonary nodules in children. J Pediatr Surg 2008;43:2315-22. [PubMed]
- Rocco G, Cicalese M, La Manna C, et al. Ultrasonographic identification of peripheral pulmonary nodules through uniportal video-assisted thoracic surgery. Ann Thorac Surg 2011;92:1099-101. [PubMed]
- Wolfer RS, Krasna MJ, Hasnain JU, et al. Hemodynamic effects of carbon dioxide insufflation during thoracoscopy. Ann Thorac Surg 1994;58:404-7; discussion 407-8. [PubMed]
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982-9. [PubMed]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5. [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [PubMed]
- Weiser TS, Hyman K, Yun J, et al. Electromagnetic navigational bronchoscopy: a surgeon's perspective. Ann Thorac Surg 2008;85:S797-801. [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919.
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930-5. [PubMed]
- Anayama T, Qiu J, Chan H, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg 2015;99:224-30. [PubMed]
- Chee A, Stather DR, Maceachern P, et al. Diagnostic utility of peripheral endobronchial ultrasound with electromagnetic navigation bronchoscopy in peripheral lung nodules. Respirology 2013;18:784-9. [PubMed]
- Ng CS, Yu SC, Lau RW, et al. Hybrid DynaCT-guided electromagnetic navigational bronchoscopic biopsy†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i87-i88. [PubMed]


