
Clinical characteristics and prognostic factors of pulmonary sarcomatoid carcinoma
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a type of rare malignant carcinoma of the lung. The incidence rate of PSC is about 0.1–0.4% among all types of pulmonary malignant carcinomas (1,2). In 1908, the first case of pulmonary carcinosarcoma was reported by Kika (3). In the 2004 World Health Organization (WHO) classification of lung tumors, PSC was divided into the following five subtypes: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma. Pleomorphic carcinoma is the most common subtype of PSC. The classification of PSC in 2021 is same as the classification of PSC in 2004 (4-6). Most patients do not have any specific symptoms. These symptoms are mainly associated with location of the tumor or invasion of the tumor (4).
In this article, we collected the long-term follow-up information of patients with PSC. The purpose of this study is to analyze the clinical characteristics and the prognostic factors of PSC. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-393/rc).
Methods
Patients
All of these patients who received treatment in the Cancer Hospital Chinese Academy of Medical Sciences from January 1, 2006, to December 31, 2015, were enrolled in this study, and all of them were diagnosed with pathologically confirmed PSC. Gender, age, body mass index (BMI), smoking history, family history, clinical symptoms, pathological type, tumor diameter, tumor-node-metastasis (TNM) stage (all of these patients were restaged based on the 8th edition of the TNM classification for lung cancer), tumor status, N staging, M staging, tumor location, surgery or no surgery, lung resection, types of treatment, and survival were retrospectively reviewed. All of these clinical characteristics were collected by retrieving patients’ medical records.
This was a retrospective study and the information of patients was anonymized. Most patients in our study died because of PSC, so it was impossible to sign informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The study was approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 22/304-3506). This research did not influence the treatment and prognosis, and the requirement for obtaining written patient consent was waived.
Survival
All follow-up results were collected through patients’ medical records or telephone interviews. Until the last follow-up, 33 (27.7%) patients were still alive, and the median follow-up time was 82.8 months (Q1–Q3: 63.6–118.7 months); 86 (72.3%) patients died, and the median survival was 11.87 months (Q1–Q3: 6.38–21.48 months). All of these patients in our study were followed up for more than 5 years.
Statistical analysis
The frequency and percentages of categorical variables and the median and range of continuous variables were calculated using SPSS 26.0 (IBM Co., Armonk, NY, USA). R version 4.1.0 (R Foundation, Vienna, Austria) was used to draw the survival curve and forest plot. One-year survival rate, three-year survival rate, five-year survival rate, and overall survival (OS) were calculated using the Kaplan-Meier method. The comparison of each group was assessed using the log-rank test. When the P value was less than 0.05, the difference in each group was considered significant.
Results
Patients’ characteristics
All basic clinical characteristics of these 119 patients who were treated in our hospital from 2006 to 2015 are listed in Table 1. Male patients (n=94, 79.0%) easily developed PSC, and the gender ratio was 3.76:1. The mean patient age was 60.67±10.50 years (range, 26–89 years). The mean BMI was 23.39±3.24 kg/m2 (range, 17.1–31.88 kg/m2), and the BMI levels in 8 patients were lower than 18.5 kg/m2. Because of respiratory symptoms, such as cough and blood-stained sputum, 82 (68.9%) patients visited the hospital and were diagnosed with PSC; 14 (11.8%) patients had chest pain, back pain, or shoulder pain; 3 (2.5%) patients were diagnosed with PSC because of weight loss; 2 (1.7%) patients had supraclavicular lymph node enlargement; 18 (15.1%) patients were asymptomatic. A total of 27 (22.7%) patients had a family history of cancer. A total of 79 (66.4%) patients had a smoking history; 76 patients were male. Sixty out of the 79 smokers were heavy smokers (smoking index >400).
Table 1
| Characteristics | Case | P value |
|---|---|---|
| Gender | ||
| Female | 25 (21.0%) | 0.923 |
| Male | 94 (79.0%) | |
| Age, years | 60.67±10.50 | |
| BMI, kg/m2 | 23.39±3.24 | |
| <18.5 | 8 (6.7%) | 0.324 |
| ≥18.5 | 111 (93.3%) | |
| Symptoms | ||
| None | 18 (15.1%) | 0.835 |
| Respiratory symptoms | 82 (68.9%) | |
| Pain | 14 (11.8%) | |
| Emaciation | 3 (2.5%) | |
| Cervical mass | 2 (1.7%) | |
| Family history | ||
| No | 92 (77.3%) | 0.382 |
| Yes | 27 (22.7%) | |
| Smoking | ||
| No | 40 (33.6%) | 0.085 |
| Yes | 79 (66.4%) | |
| Tumor diameter, cm | ||
| ≤5 | 74 (62.2%) | 0.160 |
| >5 | 45 (37.8%) | |
| TNM | ||
| I | 13 (10.9%) | 0.000039 |
| II | 38 (31.9%) | |
| III | 55 (46.2%) | |
| IV | 13 (10.9%) | |
| T | ||
| 1&2 | 61 (51.3%) | 0.031 |
| 3&4 | 58 (48.7%) | |
| N | ||
| 0 | 56 (47.1%) | 0.005 |
| 1&2&3 | 63 (52.9%) | |
| M | ||
| 0 | 105 (88.2%) | 0.001 |
| 1 | 14 (11.8%) | |
| Pathological type | ||
| PSC NOS† | 48 (40.3%) | 0.633 |
| Pleomorphic carcinoma | 53 (44.5%) | |
| Spindle cell carcinoma | 11 (9.2%) | |
| Giant cell carcinoma | 3 (2.5%) | |
| Carcino-sarcoma | 2 (1.7%) | |
| Pulmonary blastoma | 2 (1.7%) | |
| Tumor location | ||
| NOS‡ | 8 (6.7%) | 0.521 |
| Left upper lobe | 37 (31.1%) | |
| Left lower lobe | 12 (10.1%) | |
| Right upper lobe | 52 (43.7%) | |
| Right middle lobe | 1 (0.8%) | |
| Right lower lobe | 9 (7.6%) | |
| Surgery | ||
| No | 8 (6.7%) | 0.261 |
| Yes | 111 (93.3%) | |
| Lung resection | ||
| No | 8 (6.7%) | 0.072 |
| Wedge resection | 10 (8.4%) | |
| Lobectomy | 70 (58.8%) | |
| Sleeve lobectomy | 8 (6.7%) | |
| Pneumonectomy | 17 (14.3%) | |
| Combined lung resection§ | 6 (5.0%) | |
| Types of treatment | ||
| Without surgery | 8 (6.7%) | 0.236 |
| Only surgery | 26 (21.8%) | |
| Surgery + adjuvant | 82 (68.9%) | |
| Neoadjuvant + surgery + adjuvant | 3 (2.5%) | |
| Status | ||
| Died | 86 (72.3%) | – |
| Alive | 33 (27.7%) | |
Data are presented as n (%) or mean ± SD. P value: the P value in each group was calculated separately through the Kaplan-Meier method. Tumor diameter: the maximum diameter of tumor. NOS†, the subtypes in these 48 patients (sarcomatoid carcinoma) were not determined at that time; NOS‡, the size of the tumor in these patients were relatively more significant, and the location of tumors in these cases could not be classified into other groups combined lung resection; §, lobectomy combined with lobectomy or wedge resection. OS, overall survival; BMI, body mass index; TNM, tumor-node-metastasis; PSC, pulmonary sarcomatoid carcinoma; NOS, not otherwise specified; SD, standard deviation.
Tumor characteristics
The median maximum tumor diameter was 5.0 cm (range, 1.0–22.0 cm), and in 74 (62.2%) patients, the maximum tumor diameter was no more than 5.00 cm; 51 (42.9%) patients were in the T1 + T2 group. A total of 56 (47.1%) patients did not develop lymph node metastasis; 105 (88.2%) patients did not develop distant metastasis. Pleomorphic carcinoma was PSC’s most common pathological subtype; 53 (44.5%) patients were diagnosed with pleomorphic carcinoma; 48 (40.3%) patients did not determine the pathological subtype then. PSC was usually located in the upper lobe, especially the right upper lobe; 52 (43.7%) tumors were located in the right upper lobe. A total of 111 (93.3%) patients underwent surgery; lobectomy was performed in 70 (58.8%) patients. Eight (6.7%) patients were diagnosed with PSC by biopsy and received only non-surgical treatment. Most patients (n=82, 68.9%) who underwent surgery received adjuvant therapy. A total of 26 (21.8%) patients received only surgical treatment, and 20 patients of these 26 patients were in stage I or stage II. Three (2.5%) patients were diagnosed with PSC before surgery and received neoadjuvant therapy before surgery and adjuvant therapy after surgery (Table 1).
Gene mutations
Most of the patients in our study were diagnosed with PSC at an early period. Therefore, the use of whole-genome sequencing and the examination of checkpoint antibodies were rarely seen in our study. Only 18 patients received whole genome sequencing. Five patients had Kirsten rat sarcoma viral oncogene homolog (KRAS) exon 12 mutations, two patients had epidermal growth factor receptor (EGFR) mutations, and one patient had anaplastic lymphoma kinase (ALK) rearrangement. The other ten patients did not have any gene mutations.
Survival analysis
From the date of diagnosis to the date of death or the date of the last follow-up time represented the OS. The median OS in this study was 17.47 months (Q1–Q3: 7.93–62.53 months). All patients’ 1-, 3-, and 5-year survival rates were 61.3%, 34.5%, and 31.9%, respectively (Table 2).
Table 2
| Characteristic | n (%) or number | 1-year survival | 3-year survival | 5-year survival | |||||
|---|---|---|---|---|---|---|---|---|---|
| Rate (%) | P value | Rate (%) | P value | Rate (%) | P value | ||||
| Tumor diameter | |||||||||
| ≤5 cm | 74 (62.2) | 64.9 | 0.283 | 36.5 | 0.324 | 35.1 | 0.230 | ||
| >5 cm | 45 (37.8) | 55.6 | 31.1 | 26.7 | |||||
| TNM | |||||||||
| I | 13 (10.9) | 84.6 | 0.058 | 76.9 | 0.000151 | 76.9 | 0.000072 | ||
| II | 38 (31.9) | 71.1 | 47.4 | 44.7 | |||||
| III | 55 (46.2) | 54.5 | 23.6 | 20.0 | |||||
| IV | 13 (10.9) | 38.5 | 0.0 | 0.0 | |||||
| T | |||||||||
| I&II | 61 (51.3) | 65.6 | 0.302 | 41.0 | 0.094 | 39.3 | 0.066 | ||
| III&IV | 58 (48.7) | 56.9 | 27.6 | 24.1 | |||||
| N | |||||||||
| 0 | 56 (47.1) | 71.4 | 0.081 | 50.0 | 0.004 | 46.4 | 0.004 | ||
| 1&2&3 | 63 (52.9) | 52.4 | 20.6 | 19.0 | |||||
| M | |||||||||
| 0 | 105 (88.2) | 64.8 | 0.021 | 39.0 | 0.001 | 36.2 | 0.001 | ||
| 1 | 14 (11.8) | 35.7 | 0.0 | 0.0 | |||||
| Overall | 119 | 61.3 | 34.5 | 31.9 | |||||
P value: the P value in each group was obtained separately through the Kaplan-Meier method. TNM, tumor-node-metastasis.
In all patients included in this study, OS was not associated with gender, symptoms, family history of cancer, smoking history, pathological type, tumor location, surgery or no surgery, lung resection, and treatment modes (P>0.05, Table 1).
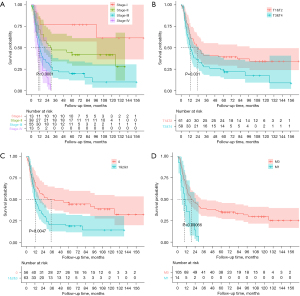
The OS of patients in different TNM stages was different. A higher TNM stage had a lower OS (P<0.05, Table 1 and Figure 1A). The median OS values of stage-I, stage-II, stage-III, and stage-IV patients were 77.3, 29.4, 12.8, and 10.4 months, respectively. The TNM stage influenced the 3- and 5-year survival significantly (P<0.05), but not the 1-year survival (P>0.05, Table 2). The maximum tumor diameter did not influence the 1-, 3-, or 5-year survival and OS (P>0.05, Tables 1,2). Patients in the T1&T2 group had a significantly longer OS than patients in T3&T4 group (P<0.05, Table 1 and Figure 1B). The 1-, 3-, and 5-year survival rates were not significantly different in the two groups, but the 1-, 3-, and 5-year survival rates in the T3&T4 group were lower than those in the T1&T2 group (P>0.05, Table 2). Patients without lymph node metastasis had better OS than those with metastasis (P<0.05, Table 1 and Figure 1C). We could also see the difference in the 1-, 3-, and 5-year survival rates between the two lymph node statuses (P<0.05, Table 2). Patients with distant metastasis had worse OS, 1-, 3-, and 5-year survival rates than those without distant metastasis (P<0.05, Tables 1,2, and Figure 1D). In the COX multivariate regression analysis, the N and M stages were independent prognostic factors (P<0.05, Figure 2).

Discussion
PSC is a rare type of non-small cell lung cancer with a poor prognosis. Most past research studies about PSC were small-scale case reports or database analyses (7-9). Tumor diameter did not influence the OS, but the T stage influenced the OS. It was inevitable that other tumor characteristics, such as invasion of the pleura and spread through air spaces, also influenced the OS (10,11). T-stage, lymph node status, and distant metastasis can influence the OS, 1-, 3-, and 5-year survival rates. Patients with lower TNM stage had a better prognosis. Just like the study of Gu et al., TNM was the main prognostic factor for PSC (7). Male patients and smokers, especially heavy smokers can easily suffer from PSC. Pleomorphic carcinoma is the primary subtype of PSC (1,3,8). Respiratory symptoms, such as cough and hemoptysis, are the most common symptoms of PSC (9). The nutritional status of patients may influence surgical or non-surgical treatment (12,13). BMI is one of the factors in assessing patients’ nutritional status. When BMI is <18.5 kg/m2, we think it indicates malnutrition. Irrespective of whether the patients receive surgery, target therapy, or any other treatment, they may have a poor prognosis. In our study, out of eight patients whose BMI was <18.5 kg/m2, six died until the last follow-up. The median OS of these eight patients was 10.00 months, which was lower than the OS of all patients in our study, but poor nutritional status did not influence the OS significantly (P=0.324). Metastatic locations of PSC are similar to those of the other types of non-small cell lung cancer, including lung, pleura, bone, brain, and adrenal glands (14-17). The metastatic locations of patients with stage-IV in our study were similar to the above. PSC can also metastasize to the upper lip and skin. However, the OS was generally low when PSC metastasized to these unusual organs (16,18,19).
Surgery is the main therapeutic method for PSC, especially for early-stage diseases; most patients in our study underwent surgery (8,20,21). PSC is rarely diagnosed by biopsy; thus, only a few patients receive neoadjuvant therapy. In our study, only three patients received neoadjuvant therapy. It is still unclear whether the patients should receive adjuvant chemotherapy and whether adjuvant chemotherapy can improve the prognosis. In the researches by Cen et al. and Abdallah et al., patients in stage-II and stage-III who received adjuvant chemotherapy achieved a better result in terms of OS, whereas the OS of patients in stage-I was not improved (21,22). Moreover, patients with PSC can easily develop resistance to traditional chemotherapy (17). In our study, we could not determine any difference in OS between surgery and surgery combined with adjuvant chemotherapy (P>0.05).
Because of the limited effectiveness of chemotherapy in PSC and better effectiveness of targeted therapy and immunotherapy in the other types of lung cancer, an increasing number of research studies about the use of targeted therapy and immunotherapy in PSC have been conducted in recent years. The common mutations of PSC are TP53 mutations, KRAS mutations, EGFR mutations, MET mutations or amplification, ALK rearrangement, and fusion of ROS1 (23,24). About 3–31.8% of PSC patients may have MET exon 14 mutations, but the rate of EGFR mutations in PSC patients is uncertain (4,25). In the research by Chen et al. (2), only 5 (3.5%) patients had ALK rearrangement. In the case report by He et al., during the treatment process of afatinib and crizotinib for patients with EGFR mutations and MET amplification, kidney and adrenal gland metastases occurred in that patient. The patient died after stopping the use of medicine (26). Chen et al. (27) reported that a patient with KRAS mutation and EML4-ALK fusion showed a worse reaction to chemotherapy and crizotinib. The expression of MET mutations may become a sign of using MET tyrosine kinase inhibitors (TKIs), such as crizotinib and capmatinib (23,28). But patients without MET mutations have a better OS than patients with MET mutations. In other words, PSC with MET mutations is also a factor for poor prognosis (28,29). The same as KRAS (24,30). Five patients had KRAS mutations in our study, and all five died; the median survival time was 13.5 months (range, 5.13–60.97 months). The patient with ALK rearrangement in this study was still alive and had been using crizotinib/alectinib until the last follow-up. We can also see a better result in the research by Chen et al. (2). The other two patients with EGFR mutations died. Because of the worse therapeutic effect of chemotherapy, analysis of the whole genome sequencing of PSC patients may help us select suitable targeted drugs and predict the prognosis (31).
Immunotherapy is a novel therapeutic method for non-small cell lung cancer. Because of the lower morbidity of PSC, a large-scale prospective study of immune checkpoint inhibitors (ICIs) in PSC has hardly been conducted. In some small-scale studies, we can note that using ICIs can improve OS and progression-free survival. With an increase in the expression of PD-L1, the patients may achieve a better complete or partial response. Immunotherapy is an emerging therapy, and none of the patients received it in our study.
Conclusions
In summary, T-stage, lymph node, and distant metastases are the main factors that influence the OS of PSC. Complete resection of the tumor is one of the main treatments for PSC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-393/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-393/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-393/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-393/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 22/304-3506). This research did not influence the treatment and prognosis, and the requirement for obtaining written patient consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery 2012;152:397-402. [Crossref] [PubMed]
- Chen X, Zhang Y, Lu J, et al. Pulmonary Sarcomatoid Carcinoma with ALK Rearrangement: Frequency, Clinical-Pathologic Characteristics, and Response to ALK Inhibitor. Transl Oncol 2017;10:115-20. [Crossref] [PubMed]
- Kika G. A rare case of malignant mixed tumor in the respiratory tract. Jpn J Cancer Res 1908;2:574-622.
- Baldovini C, Rossi G, Ciarrocchi A. Approaches to Tumor Classification in Pulmonary Sarcomatoid Carcinoma. Lung Cancer (Auckl) 2019;10:131-49. [Crossref] [PubMed]
- Ung M, Rouquette I, Filleron T, et al. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin Lung Cancer 2016;17:391-7. [Crossref] [PubMed]
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Gu L, Xu Y, Chen Z, et al. Clinical analysis of 95 cases of pulmonary sarcomatoid carcinoma. Biomed Pharmacother 2015;76:134-40. [Crossref] [PubMed]
- Lin Y, Yang H, Cai Q, et al. Characteristics and Prognostic Analysis of 69 Patients With Pulmonary Sarcomatoid Carcinoma. Am J Clin Oncol 2016;39:215-22. [Crossref] [PubMed]
- Sun L, Dai J, Chen Y, et al. Pulmonary Sarcomatoid Carcinoma: Experience From SEER Database and Shanghai Pulmonary Hospital. Ann Thorac Surg 2020;110:406-13. [Crossref] [PubMed]
- Kadota K, Nitadori JI, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Wang F, Li P, Li F. Nomogram for Predicting the Relationship between the Extent of Visceral Pleural Invasion and Survival in Non-Small-Cell Lung Cancer. Can Respir J 2021;2021:8816860. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Park S, Park S, Lee SH, et al. Nutritional status in the era of target therapy: poor nutrition is a prognostic factor in non-small cell lung cancer with activating epidermal growth factor receptor mutations. Korean J Intern Med 2016;31:1140-9. [Crossref] [PubMed]
- Gendarme S, Matton L, Antoine M, et al. Strong ALK and PD-L1 positive IHC expression related ALK amplification in an advanced lung sarcomatoid carcinoma: A therapeutic trap? Lung Cancer 2021;152:94-7. [Crossref] [PubMed]
- Maneenil K, Xue Z, Liu M, et al. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin Lung Cancer 2018;19:e323-33. [Crossref] [PubMed]
- Li X, Wu D, Liu H, et al. Pulmonary sarcomatoid carcinoma: progress, treatment and expectations. Ther Adv Med Oncol 2020;12:1758835920950207. [Crossref] [PubMed]
- Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013;8:1574-7. [Crossref] [PubMed]
- Le T, Mayer M, Sailors J, et al. Upper lip metastasis of sarcomatoid carcinoma of the lung - an unusual site of disease: a case report. J Med Case Rep 2017;11:18. [Crossref] [PubMed]
- Xu X, Lin M, Wang S, et al. Lung Sarcomatoid Carcinoma Metastasis to Skin: A Case Report and Review of the Literature. Cancer Invest 2016;34:286-92. [Crossref] [PubMed]
- Özkan B, Erdoğdu E, Duman S, et al. Prognostic factors in patients undergoing pulmonary resection for sarcomatoid carcinomas of the lung. Balkan Med J 2021;38:104-10. [PubMed]
- Cen Y, Yang C, Ren J, et al. Additional chemotherapy improves survival in stage II-III pulmonary sarcomatoid carcinoma patients undergoing surgery: a propensity scoring matching analysis. Ann Transl Med 2021;9:24. [Crossref] [PubMed]
- Abdallah HM, Martinez-Meehan D, Lutfi W, et al. Adjuvant chemotherapy for pulmonary sarcomatoid carcinoma: A retrospective analysis of the National Cancer Database. J Thorac Cardiovasc Surg 2022;163:1669-81.e3. [Crossref] [PubMed]
- Mehrad M, Roy S, LaFramboise WA, et al. KRAS mutation is predictive of outcome in patients with pulmonary sarcomatoid carcinoma. Histopathology 2018;73:207-14. [Crossref] [PubMed]
- Ding Y, Shao Y, Na C, et al. Genetic characterisation of sarcomatoid carcinomas reveals multiple novel actionable mutations and identifies KRAS mutation as a biomarker of poor prognosis. J Med Genet 2022;59:10-7. [Crossref] [PubMed]
- Leone A, Graziano P, Gasbarra R, et al. Identification of EGFR mutations in lung sarcomatoid carcinoma. Int J Cancer 2011;128:732-5; author reply 736. [Crossref] [PubMed]
- He Q, Shi X, Zhu H, et al. A case treated with Crizotinib after secondary MET amplification of A double Rare L747S and G719S EGFR mutation Pulmonary Sarcomatoid Carcinoma. Ann Oncol 2020;31:544-6. [Crossref] [PubMed]
- Chen F, Gu Q, Hu C, et al. Poor prognosis of pulmonary sarcomatoid carcinoma with KRAS mutation and ALK fusion. Onco Targets Ther 2019;12:3321-5. [Crossref] [PubMed]
- Mignard X, Ruppert AM, Antoine M, et al. c-MET Overexpression as a Poor Predictor of MET Amplifications or Exon 14 Mutations in Lung Sarcomatoid Carcinomas. J Thorac Oncol 2018;13:1962-7. [Crossref] [PubMed]
- Liu XW, Chen XR, Rong YM, et al. MET exon 14 skipping mutation, amplification and overexpression in pulmonary sarcomatoid carcinoma: A multi-center study. Transl Oncol 2020;13:100868. [Crossref] [PubMed]
- Lococo F, Gandolfi G, Rossi G, et al. Deep Sequencing Analysis Reveals That KRAS Mutation Is a Marker of Poor Prognosis in Patients with Pulmonary Sarcomatoid Carcinoma. J Thorac Oncol 2016;11:1282-92. [Crossref] [PubMed]
- Alì G, Bruno R, Poma AM, et al. Whole transcriptome targeted gene quantification provides new insights on pulmonary sarcomatoid carcinomas. Sci Rep 2019;9:3536. [Crossref] [PubMed]


