
The association between thoracic sarcopenia and survival is gender specific in early-stage lung cancer
Introduction
Lung cancer is the leading cause of cancer related deaths worldwide, with more than 131,000 deaths projected for 2021 in the United States (1). Surgical resection remains the gold standard therapy for patients presenting with early-stage non-small cell lung cancer (NSCLC), with 5-year survival rates reported to range from 25–73% (2-5). Unfortunately, early detection alone does not ensure long-term survival, as 1 in 5 patients with stage IA NSCLC will die from disease recurrence within 5 years of surgery and adjuvant chemotherapy offers no survival advantage in unselected populations (6,7). Methods capable of selecting subsets of patients with aggressive disease at the time of resection could aid clinicians in determining if adjuvant chemotherapy or closer surveillance is likely to provide a benefit.
Sarcopenia, or the physiologic state of reduced muscle mass and function, has recently emerged as a prognostic factor in the outcomes of patients with malignancy (8,9). It has been shown to be highly prevalent in lung cancer patients, with rates as high as 56%, and been associated with major perioperative complications and reduced overall survival (OS) (10-13). Similarly, thoracic sarcopenia, or reduced thoracic muscle mass, has been associated with impaired respiratory status and has been found to be a prognostic factor in the outcomes of cardiothoracic surgeries (14,15). Although there have been multiple investigations into the association between sarcopenia and outcomes of patients with lung cancer, few have examined the implications of measuring sarcopenia at various vertebral levels (10,12,16-20). Since a majority of patients with early-stage lung cancer do not receive abdominal computed tomography (CT) imaging, it is important to understand the relationship between thoracic sarcopenia and outcomes in this population. Therefore, we investigated the association between sarcopenia, measured at three separate vertebral levels, and survival in patients with early-stage lung cancer who underwent surgical resection. Our hypothesis was those with sarcopenia would have worse outcomes including OS and disease-free survival (DFS). We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-273/rc).
Methods
After Institutional Review Board approval was obtained, a retrospective review of an institutional database was conducted. Inclusion criteria included patients who underwent anatomic lung resection for pathologic T1–T2, N0, M0 NSCLC (AJCC 8th edition) between 2010–2019. All patients had histologic confirmed adenocarcinoma, R0 resection, thoracic lymphadenectomy and no history of induction therapy. Exclusion criteria included less than 90 days of follow-up or technically inadequate preoperative CT chest or abdominal imaging. Because arm position (raised vs. by the side) has been shown to alter the total muscle area obtained from a single-slice CT scan, patients unable to raise their arms over their head were classified as technically inadequate.
Demographic, treatment, and outcome data were collected. Sarcopenia was assessed by measuring skeletal muscle cross-sectional area (SMA) at the 5th thoracic level (T5), 12th thoracic level (T12), and 3rd lumbar level (L3) utilizing staging CT scans performed within 90 days prior to surgery (Figure 1). Chest CT scans were utilized for obtaining body composition analysis at T5 while CT abdomen/pelvis scans were utilized for obtaining body composition analysis at the L3 level. Both CT chest and CT abdomen/pelvis scans were utilized for obtaining body composition analysis at the T12 level. SliceOMatic v5.0 revision 8 (TomoVision, Magog, Canada) was utilized for sarcopenia analysis. All CT scans were assessed by two operators trained on the software. Sarcopenia at L3 was defined using skeletal muscle index (SMI) cut-off values of <41 cm2/m2 for females, <43 cm2/m2 for males with a body mass index (BMI) <25 kg/m2, and <53 cm2/m2 for males with a BMI ≥25 kg/m2, based on previous reports (1). Sarcopenia at T5 and T12 were defined as gender-specific lowest quartile values.

Statistical analysis
Baseline demographic and treatment data were compared between patients with and without sarcopenia at each of the three vertebral levels. Patients with missing outcome data were excluded from analysis. Differences in baseline demographic and treatment data were assessed with Student’s t-test for parametric continuous variables and Wilcoxon-Ranked Sum for non-parametric continuous variables and Chi-square tests and Fisher’s Exact test for dichotomous and categorical variables. Primary outcomes included OS and DFS. Secondary outcomes included postoperative complications, hospital length-of-stay (LOS), and 30-day readmission rate. OS and DFS were examined using Kaplan-Meier survival analysis curves. Cox Proportional Hazard regression was utilized to perform adjusted survival analysis and to model which variables were independently associated with OS and DFS. Variables found to either be significant on univariate analysis or those previously reported in the literature were included in the Cox proportional hazard regression model. A proportional hazards assumption test was conducted for all Cox proportional hazard regression models. Results with alpha ≤0.05 was considered statistically significant for all analysis. All statistical analyses were performed using STATA/IC software (version 16.1, StatCorp, College Station, TX, USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Rush University Medical Center institutional review board (IRB number 19121401), and individual consent for this retrospective analysis was waived.
Results
Overall, 296 patients that underwent anatomic lung resection for early-stage NSCLC met inclusion criteria during the study period. Patients were excluded from analysis for having less than 90 days of follow-up (n=30) and incomplete or missing CT imaging (n=45). Overall, 221 patients met inclusion criteria with 70% (154/221) being female, a median BMI of 26.5 kg/m2 [interquartile range (IQR), 23.3–29.9 kg/m2], age of 69 years (IQR, 62.4–74.9 years), and follow-up of 46.9 months (IQR, 25.0–70.7 months) (Table 1).
Table 1
| Characteristics | Upper thoracic level | Lower thoracic level | Lumbar level | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| T5 sarcopenic (n=51) |
T5 non-sarcopenic (n=142) |
T12 sarcopenia (n=53) |
T12 non-sarcopenic (n=168) |
L3 sarcopenia (n=108) |
L3 non-sarcopenia (n=70) |
||||
| Age, years, median (IQR) | 71.3 (64.5–75.2) | 68.4 (61.0–73.8) | 69.7 (65.2–75.4) | 68.4 (61.9–74.3) | 72.2 (65.0–77.3) | 67.0 (60.3–72.9) | 0.001 | ||
| Sex | |||||||||
| Female | 34 [67] | 97 [68] | 38 [72] | 116 [69] | 74 [69] | 42 [60] | 0.075 | ||
| Height, cm, median (IQR) | 161.3 (149.9–168.5) | 160.0 (134.6–165.1) | 160.0 (142.2–165.1) | 160.0 (134.6–165.1) | 160.0 (147.3–166.4) | 160.0 (144.8–165.1) | 0.566 | ||
| BMI, kg/m2, median (IQR) | 23.9 (21.2–26.7) | 27.5 (24.0–31.9) | 22.2 (20.3–25.1) | 27.7 (24.6–31.6) | 25.7 (22.1–28.1) | 27.2 (24.2–32.3) | 0.001 | ||
| SMI, cm2/m2, median (IQR) | 42.2 (39.5–46.6) | 54.3 (49.6–61.7) | 23.9 (22.3–24.9) | 33.2 (29.4–38.4) | 38.6 (35.4–41.9) | 47.1 (44.0–53.6) | 0.001 | ||
| Tobacco use | 0.070 | ||||||||
| Current | 8 [16] | 25 [18] | 9 [17] | 29 [17] | 20 [19] | 14 [20] | |||
| Former | 33 [65] | 97 [68] | 33 [62] | 116 [69] | 72 [67] | 48 [69] | |||
| Never | 10 [20] | 20 [14] | 11 [21] | 23 [14] | 16 [15] | 8 [11] | |||
| DM | 10 [20] | 24 [17] | 6 [11] | 32 [19] | 22 [20] | 16 [23] | 0.225 | ||
| CAD | 6 [12] | 20 [14] | 9 [17] | 20 [12] | 18 [17] | 10 [14] | 0.748 | ||
| Race/ethnicity | 0.397 | ||||||||
| Caucasian | 39 [76] | 113 [80] | 42 [79] | 129 [77] | 90 [83] | 51 [73] | |||
| African American | 9 [18] | 17 [12] | 6 [11] | 28 [17] | 12 [11] | 12 [17] | |||
| Hispanic | 1 [2] | 1 [1] | 1 [2] | 1 [1] | 0 [0] | 0 [0] | |||
| Asian | 1 [2] | 4 [3] | 3 [6] | 3 [2] | 1 [1] | 2 [3] | |||
| Other | 1 [2] | 7 [5] | 1 [2] | 7 [4] | 5 [5] | 5 [7] | |||
| Pathologic stage | 0.795 | ||||||||
| IA1 | 21 [41] | 65 [46] | 22 [42] | 70 [42] | 51 [47] | 35 [50] | |||
| IA2 | 10 [20] | 34 [24] | 9 [17] | 43 [26] | 22 [20] | 16 [23] | |||
| IA3 | 2 [4] | 6 [4] | 1 [2] | 7 [4] | 3 [3] | 2 [3] | |||
| IB | 16 [31] | 30 [21] | 17 [32] | 41 [24] | 28 [26] | 12 [17] | |||
| IIA | 2 [4] | 7 [5] | 3 [6] | 8 [5] | 4 [4] | 5 [7] | |||
All data presented as n [%], unless otherwise indicated. T5, 5th thoracic vertebra; T12, 12th thoracic vertebra; L3, 3rd lumbar vertebra; IQR, interquartile range; BMI, body mass index; SMI, skeletal muscle index; DM, diabetes mellitus; CAD, coronary artery disease.
Upper thoracic skeletal muscle analysis
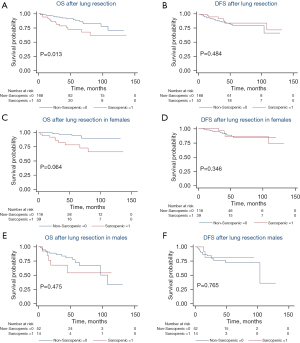
A total of 193 patients were analyzed at T5 with 68% (131/193) being female, a median BMI of 26.4 kg/m2 (IQR, 22.7–29.9 kg/m2), age of 68.8 years (IQR, 62.3–74.7), and follow-up of 47.4 months (IQR, 25.8–68.6 months). At T5, 26% (51/193) patients were determined to have sarcopenia with a median upper thoracic SMI of 42.2 cm2/m2 (IQR, 39.5–46.6 cm2/m2) compared to 54.3 cm2/m2 (IQR, 49.6–61.7 cm2/m2) in the non-sarcopenic group (P<0.001). BMI was found to be lower in the sarcopenic group, with a median value of 23.9 kg/m2 (IQR, 21.2–26.7 kg/m2) compared to 27.5 kg/m2 (IQR, 24.0–31.9 kg/m2) in the non-sarcopenic group (P<0.001). There were no differences in height, age, race/ethnicity, smoking status, medical comorbidities, or pathologic stage between groups. With a median follow-up of 47.4 months, patients with upper thoracic sarcopenia at time of lung resection had similar OS [median 45.5 (IQR, 26.3–75.6) vs. 47.7 (IQR, 25.7–66.8) months, P=0.257) and DFS (median 31.3 (IQR, 15.9–72.2) vs. 36.6 (IQR, 20.1–58.3) months, P=0.296] compared to patients without sarcopenia (Figure 1A,1B). However, sarcopenic males had worse OS [median 41.0 (IQR, 13.8–53.7) vs. 42.0 (IQR, 23.1–55.1) months, P=0.023] and DFS [median 15.8 (IQR, 8.4–30.8) vs. 34.8 (IQR, 20.1–50.5) months, P=0.007] when compared to non-sarcopenic males (Figure 1C,1D). No difference in OS or DFS was identified between sarcopenic and non-sarcopenic females when assessed at T5 (Figure 1E,1F).
Lower thoracic skeletal muscle analysis
Overall, 221 patients were analyzed at T12 with 70% (154/221) female, a median BMI of 26.6 kg/m2 (IQR, 23.4–30.0 kg/m2), age of 68.9 years (IQR, 62.3–74.7 years), and follow-up of 47.4 months (IQR, 25.4–72.1 months). Fifty-three patients (24%) were determined to have sarcopenia at this vertebral level with a median lower thoracic SMI of 23.9 cm2/m2 (IQR, 22.3–24.9 cm2/m2) compared to 33.2 cm2/m2 (IQR, 29.4–38.4 cm2/m2) in the non-sarcopenic group (P<0.001). BMI was found to be significantly lower in the sarcopenic group, with a median value of 22.2 kg/m2 (IQR, 20.3–25.1 kg/m2) compared to 27.7 kg/m2 (IQR, 24.6–31.6 kg/m2) in the non-sarcopenic group (P=0.002). There were no differences in height, age, race/ethnicity, smoking status, medical comorbidities, or pathologic stage between groups. With a median follow-up of 47.4 months, patients with lower thoracic sarcopenia at time of lung resection had worse OS [median 38.8 (IQR, 23.8–72.6) vs. 49.1 (IQR, 25.7–69.9) months, P=0.013] (Figure 2A). There was no difference in DFS between those with and without sarcopenia at T12 (Figure 2B). When stratifying based on gender, sarcopenic females were similar to non-sarcopenic females in regards to OS and DFS (Figure 2C,2D) and sarcopenic males were similar to non-sarcopenic males in regards to OS and DFS (Figure 2E,2F).
Lumbar skeletal muscle analysis
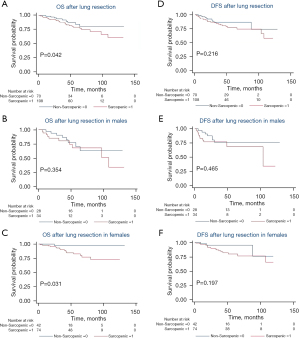
Overall, 178 patients were analyzed at L3 with 65% (116/178) female, a median BMI of 26.2 kg/m2 (IQR, 22.9–29.9 kg/m2), age of 69.2 years (IQR, 62.1–75.0 years), and follow-up of 46.2 months (IQR, 26.5–66.5 months). One hundred and eight patients (61%) were determined to have sarcopenia at the third vertebral level with a median SMI of 38.6 cm2/m2 (IQR, 35.4–41.9 cm2/m2) compared to 47.1 cm2/m2 (IQR, 44.0–53.6 cm2/m2) in the non-sarcopenic group (P<0.001). BMI was found to be significantly lower in the sarcopenic group, with a median value of 25.7 kg/m2 (IQR, 22.1–28.1 kg/m2) compared to 27.2 kg/m2 (IQR, 24.2–32.3 kg/m2) in the non-sarcopenic group (P=0.003). Increased age was also significantly associated with sarcopenia with a median value of 72.2 (IQR, 65.0–77.3) vs. 67.0 (IQR, 60.3–72.9) years in the nonsarcopenic group (P=0.003). There was no difference in height, race/ethnicity, smoking status, medial comorbidities, or pathologic stage between groups. With a median follow-up of 46.2 months, patients with L3 sarcopenia had worse OS [median 43.3 (IQR, 25.9–63.9) vs. 48.8 (IQR, 26.9–75.0) months, P=0.042] (Figure 3A). There was no significant difference in DFS between those with and without sarcopenia at L3 and when comparing genders for OS or DFS. (Figure 3B-3F).
Discussion
Gender-specific differences in the association between sarcopenia at various vertebral levels and survival in patients with resected early-stage NSCLC patients is not well understood. The current data suggest that sarcopenia is associated with OS and DFS at upper vertebral levels in males, but not females. To our knowledge, this is the first report of a gender-specific difference in the ability to prognosticate survival based on skeletal muscle mass at the upper vertebral levels. The ability for physicians to preoperatively risk-stratify patients with lung cancer for OS and DFS is of paramount importance as this can identify patients that could potentially benefit from closer surveillance or adjuvant cancer therapies. As early-stage lung cancer is often identified with low dose CT chest screening programs it will be important to understand how thoracic sarcopenia in particular should be interpreted in this patient populations.
Upper thoracic vertebral levels are increasingly being used to assess for sarcopenia in lung cancer patients (14,15). However, differences have been noted in the ability of thoracic vs. lumbar vertebral levels to assess for generalized sarcopenia. Some authors have reported L3 to be superior given the large percentage of skeletal muscle and absence of ribs at this level (21). However, early-stage lung cancer patients do not always undergo CT abdominal imaging, making it difficult to assess L3 in these patients. Thus, it is necessary to evaluate how measurements at different vertebral levels will impact survival to help clinicians prognosticate outcomes for their patients.
There has been growing consensus that sarcopenia negatively impacts patients with lung cancer. A recent meta-analysis including 13 studies reported sarcopenia in 50% in lung cancer patients and found it was a predictor of reduced OS but not DFS in patients with NSCLC (12). Although this study demonstrated the association between sarcopenia and outcomes in patients with lung cancer, the studies reviewed used different methods to assess for sarcopenia. For example, one studied included in the review assessed sarcopenia via dual-energy X-ray absorptiometry (DXA) (22). It remains unclear how evaluating sarcopenia with DXA compares to CT scans, as there have been no direct comparisons in lung cancer patients. Furthermore, of the studies reviewed that assessed sarcopenia at L3, two computed psoas musculature cross-sectional area (PMA) to assess for presence of generalized sarcopenia (10-19). Utilizing PMA to assess for generalized sarcopenia has been recently reported to not be as accurate as assessing total SMA at L3 (23,24).
Another issue complicating the comparison of different lung cancer sarcopenia studies from the review by Yang et al. was the various definitions adopted by authors in determining which patients met criteria for sarcopenia (12). The most utilized cut-off values used to define sarcopenia were referenced by Prado et al. and Martin et al.; both very large retrospective reviews of patients with gastrointestinal and lung malignancies (8,21). However, 2 studies utilized a cut-off value in which the patient population studied was those with colorectal liver metastasis and another study utilized cut-off values which assessed SMI in health adults (25,26). We utilized L3 cut-off values from Martin et al. in our study, which accounts for the impact obesity may have on sarcopenia values. There are currently no generally accepted cut-off values for upper thoracic vertebral levels and thus we utilized gender specific lowest-quartile cut-off values at T5 and T12 to account for this. Further research is needed to determine uniform cut-off values at these levels for cancer patients who routinely undergo CT chest imaging.
Another recent meta-analysis was performed that included studies of patients that had surgically treated NSCLC (13). It demonstrated that sarcopenia was an independent prognosticator of worse OS in patients with resected NSCLC and only those with early-stage disease had a reduction in DFS. However, no studies included in this meta-analysis assessed sarcopenia at the upper thoracic levels. With the emergence of data associating reduced upper thoracic musculature with short and long-term outcomes in cardiothoracic surgery, there is a need to investigate differences between vertebral levels when assess for sarcopenia.
This study has limitations. First, although our sample size was adequate for a robust survival analysis, we had a larger proportion of females than males, perhaps confounding the results. In addition, the retrospective design of the study allows the potential for unrecognized bias. Future investigations into the role sarcopenia, as measured at various vertebral levels, and outcomes of cancer patients should be performed in prospective manner. Furthermore, utilizing gender-specific lowest quartiles to define sarcopenia at T5 and T12 may underreport the prevalence of sarcopenia at these levels. Unlike the widely used definitions for sarcopenia at the L3 vertebral level there is no consensus on the definition of sarcopenia at thoracic vertebral levels in patients with early-stage lung cancer. This study attempts to provide some guidance for clinicians to understand how to compare thoracic sarcopenia in lung cancer patients that may not have CT abdominal imaging to use the gold standard L3 level for sarcopenia analyses. Finally, almost half of patients reviewed at L3 met definition of sarcopenia whereas only a quarter of those at T5 and T12 met criteria.
Conclusions
The current study represents one of the largest analyses comparing thoracic and lumbar vertebral levels in the assessment of sarcopenia in lung cancer patients. Our data suggest that sarcopenia is associated with worse OS at T12 and L3, however sarcopenia at T5 was associated with worse OS and PFS in males, but not females. Sarcopenia measured at the L3 vertebral level remains the gold standard for prognosticating survival in patients with early-stage lung cancer. However, in cases when abdominal imaging is not available, it is important to understand how to best interpret sarcopenia as measured at higher thoracic levels. Further prospective research is required to better understand what role sarcopenia, measured at various vertebral levels, has on the outcomes of patients with lung cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-273/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-273/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-273/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-273/coif). CWS serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2022 to March 2024. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011;378:1727-40. [Crossref] [PubMed]
- Shah R, Sabanathan S, Richardson J, et al. Results of surgical treatment of stage I and II lung cancer. J Cardiovasc Surg (Torino) 1996;37:169-72. [PubMed]
- Nesbitt JC, Putnam JB Jr, Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg 1995;60:466-72. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694-705. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629-35. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Nakamura R, Inage Y, Tobita R, et al. Sarcopenia in Resected NSCLC: Effect on Postoperative Outcomes. J Thorac Oncol 2018;13:895-903. [Crossref] [PubMed]
- Kawaguchi Y, Hanaoka J, Ohshio Y, et al. Sarcopenia predicts poor postoperative outcome in elderly patients with lung cancer. Gen Thorac Cardiovasc Surg 2019;67:949-54. [Crossref] [PubMed]
- Yang M, Shen Y, Tan L, et al. Prognostic Value of Sarcopenia in Lung Cancer: A Systematic Review and Meta-analysis. Chest 2019;156:101-11. [Crossref] [PubMed]
- Deng HY, Hou L, Zha P, et al. Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: A comprehensive systematic review and meta-analysis. Eur J Surg Oncol 2019;45:728-35. [Crossref] [PubMed]
- Fintelmann FJ, Troschel FM, Mario J, et al. Thoracic Skeletal Muscle Is Associated With Adverse Outcomes After Lobectomy for Lung Cancer. Ann Thorac Surg 2018;105:1507-15. [Crossref] [PubMed]
- Troschel FM, Kuklinski MW, Knoll SJ, et al. Preoperative thoracic muscle area on computed tomography predicts long-term survival following pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2019;28:542-9. [Crossref] [PubMed]
- Go SI, Park MJ, Song HN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer 2016;24:2075-84. [Crossref] [PubMed]
- Kim EY, Kim YS, Park I, et al. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1795-9. [Crossref] [PubMed]
- Kimura M, Naito T, Kenmotsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer 2015;23:1699-708. [Crossref] [PubMed]
- Matsuo Y, Mitsuyoshi T, Shintani T, et al. Impact of low skeletal muscle mass on non-lung cancer mortality after stereotactic body radiotherapy for patients with stage I non-small cell lung cancer. J Geriatr Oncol 2018;9:589-93. [Crossref] [PubMed]
- Shoji F, Matsubara T, Kozuma Y, et al. Relationship Between Preoperative Sarcopenia Status and Immuno-nutritional Parameters in Patients with Early-stage Non-small Cell Lung Cancer. Anticancer Res 2017;37:6997-7003. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Chambard L, Girard N, Ollier E, et al. Bone, muscle, and metabolic parameters predict survival in patients with synchronous bone metastases from lung cancers. Bone 2018;108:202-9. [Crossref] [PubMed]
- Rutten IJG, Ubachs J, Kruitwagen RFPM, et al. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 2017;8:630-8. [Crossref] [PubMed]
- Ebadi M, Wang CW, Lai JC, et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle 2018;9:1053-62. [Crossref] [PubMed]
- van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550-7. [Crossref] [PubMed]
- Yoshizumi T, Shirabe K, Nakagawara H, et al. Skeletal muscle area correlates with body surface area in healthy adults. Hepatol Res 2014;44:313-8. [Crossref] [PubMed]


