
The role of postoperative radiotherapy for stage I/II/III thymic tumor—results of the ChART retrospective database
Introduction
Thymic tumors, including thymoma and thymic carcinoma, are the most common primary malignancies located in anterior mediastinum. They are relatively rare and usually grow indolently. The incidence of thymic tumors in the USA is 0.13 per 100,000 person-years according to the Surveillance, Epidemiology, and End Results (SEER) database (1). More than 30% of thymic tumors may be accompanied with myasthenia gravis (2). Surgical resection is the most important treatment for thymic tumors. Complete resection of the entire tumor has consistently been found to be an independent prognostic factor (3-6). The International Thymic Malignancy Interest Group (ITMIG) recommended that en bloc resection of the entire thymus gland and surrounding areolar tissue for complete resection (7). Local recurrence is the major failure pattern after surgery (3,5,8,9). The recurrence rate was lower for patients received complete resection than those received incomplete resection, resulted in a better survival in the former group (10). Chemotherapy has been used commonly, especially at the induction setting. Preoperative chemotherapy has been reported to increased R0 resection rate (7). Postoperative chemotherapy is not recommended for thymoma after complete resection. Chemotherapy is adopted in inoperable or gross residual disease after local treatment (11,12). Postoperative radiotherapy (PORT) is usually administrated in Masaoka-Koga stage IV thymoma for the purpose of local control (5,8,13). Indications of PORT for complete resected Masaoka-Koga stage I to III thymic tumors are controversial, although it has been used frequently in clinical practice. Most authors suggested that complete resection alone be adequate for Masaoka-Koga stage I to III thymic tumors, although some studies indicated potential survival benefits from PORT (4,5,9,14-21). Most of the knowledge on thymic tumors comes from retrospective, single-institutional studies. No randomized prospective trial has ever been conducted to date to evaluate the effect of PORT on thymic malignancies. The Chinese Alliance for Research in Thymomas (ChART) was founded in 2012, with the purpose of improving treatment for thymic tumors through collaborative studies. A retrospective database was established, gathering data from 18 tertiary referral centers in China. Our objective was to investigate the role of PORT in patients who underwent surgery for stage I to III thymic epithelial tumors using the ChART registry.
Materials and methods
Records of surgical patients between 1994 and 2012 in ChART database were retrospectively reviewed. Patients were included in the analysis if there was complete information on tumor stage, surgery and radiation therapy. Patients who received neoadjuvant treatment, who had history of other malignance, and who underwent a biopsy alone were excluded. All cases were restaged according to Masaoka-Koga staging system (22). Histological subtypes were classified according to World Health Organization (WHO) criteria published in 2004 (23).
Statistical analysis was performed with SPSS statistical software package version 19.0 (SPSS Inc, Chicago, IL). Continuous data variables were analyzed using Student’s t-test. Nominal data were analyzed using crosstabs and Pearson’s chi-square test. Kaplan-Meier survival curves were constructed and compared using the log-rank test. Multivariate analysis was performed according to the Cox proportional hazard model. Significance was set at a probability value less than 0.05. Because only de-identified data were used for the study, informed consent was waived by IRB.
Results
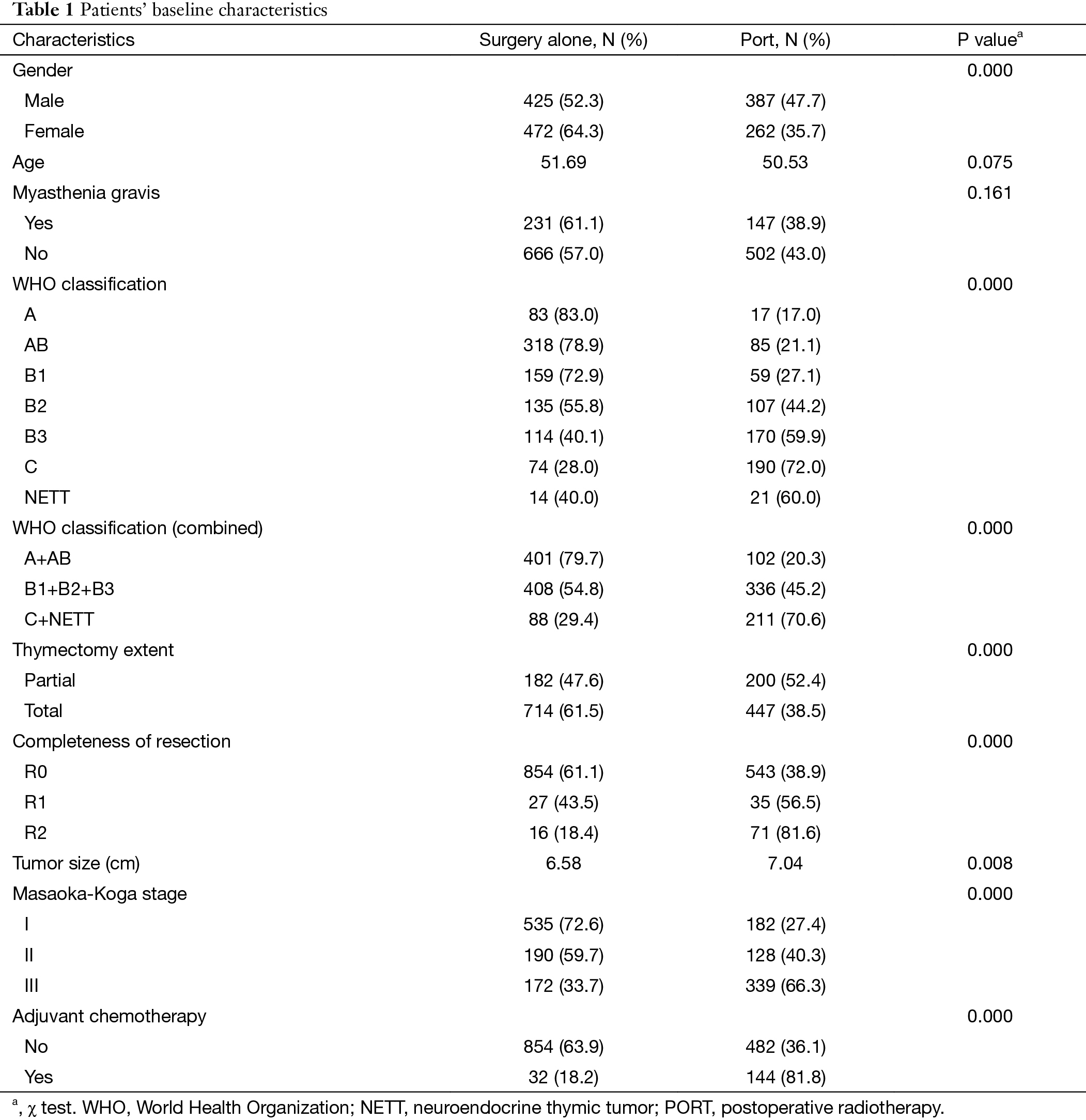
We From the ChART database 2,159 patients with Masaoka-Koga stage I to III thymic epithelial tumors were identified. Among them, 1,546 patients with complete data on staging, radiation therapy, and surgery made the final study cohort. There were 717 patients classified as Masaoka-Koga stage I, 318 patients as stage II, and 511 patients as stage III. Patients’ baseline characteristics were presented in Table 1. These included 649 patients (41.98%) who received PORT and 897 patients (58.02%) who received surgery alone. Significant differences were found in gender, WHO histological subtype, tumor size, thymectomy extent, complete resection, Masaoka-Koga stage and adjuvant chemotherapy between the two groups of patients.
Full table
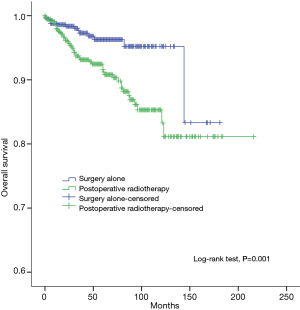
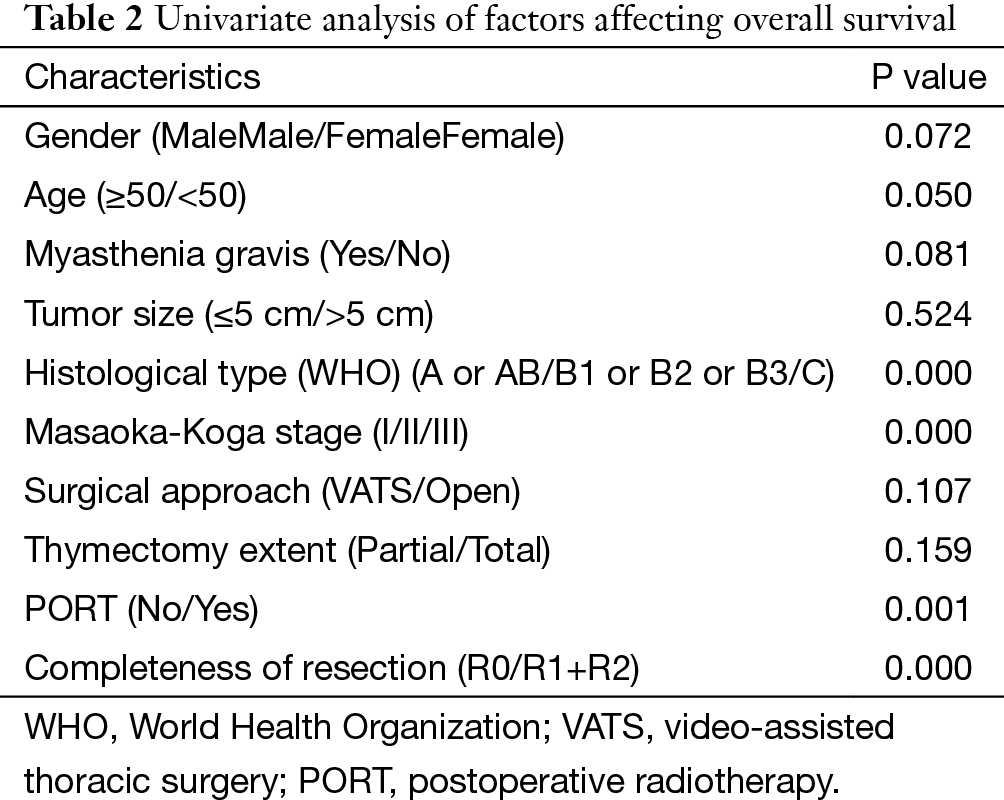
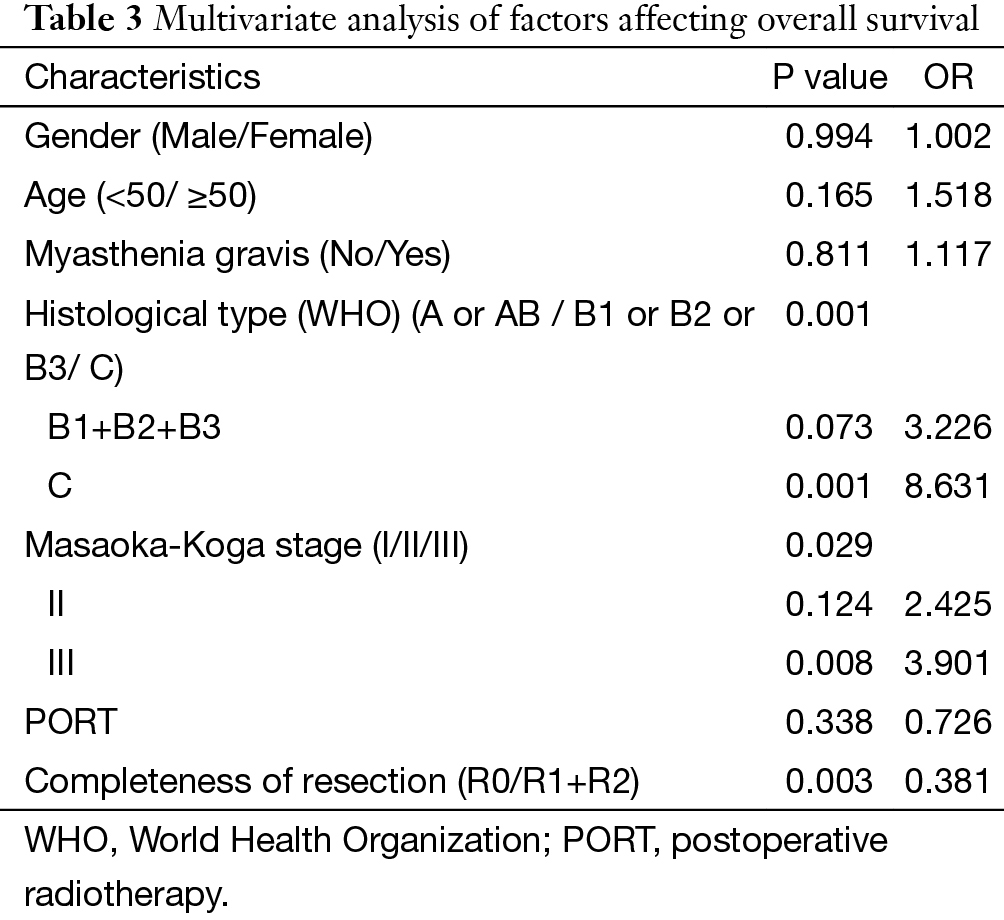
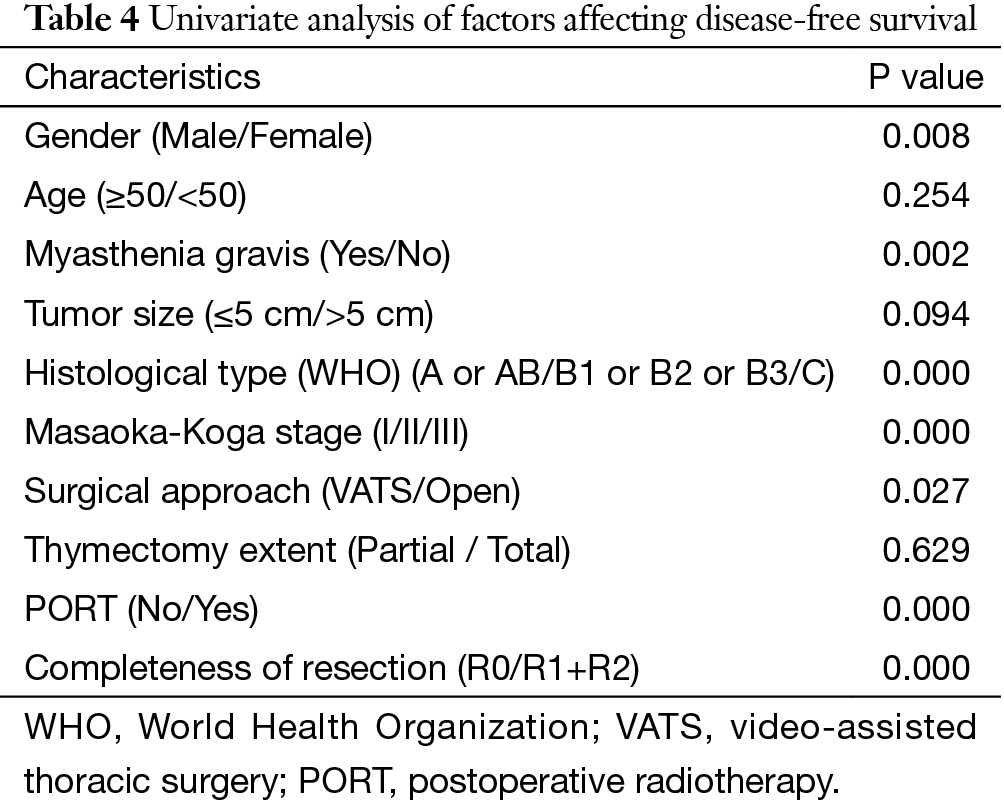
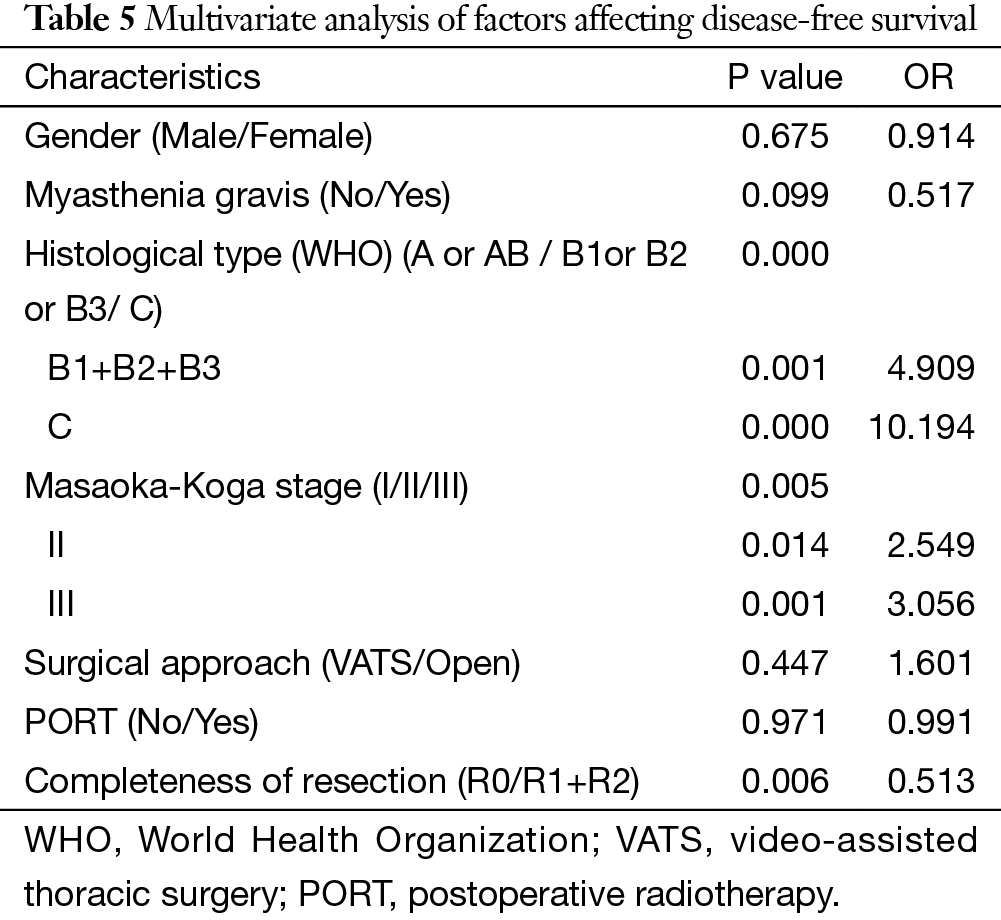
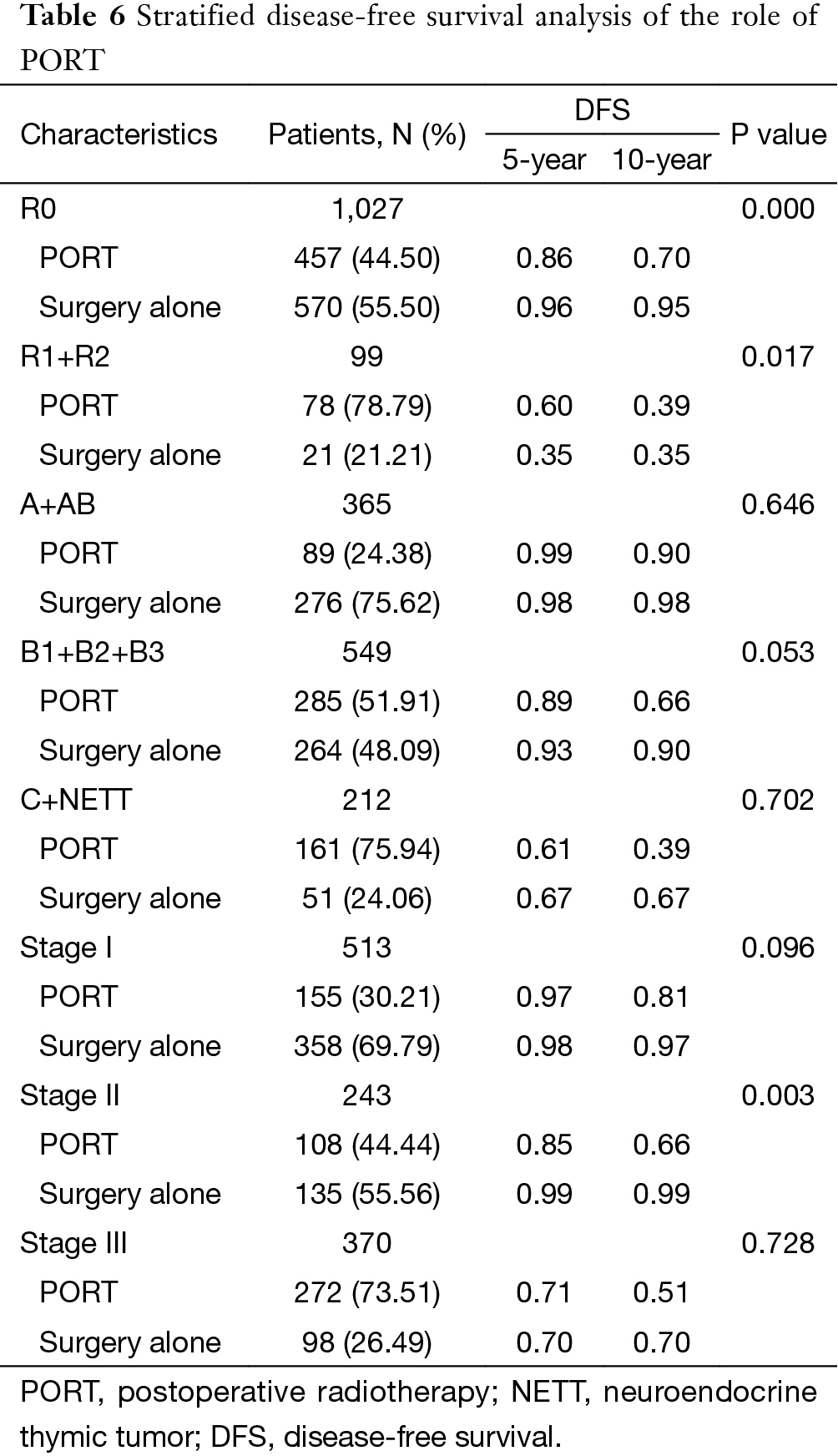
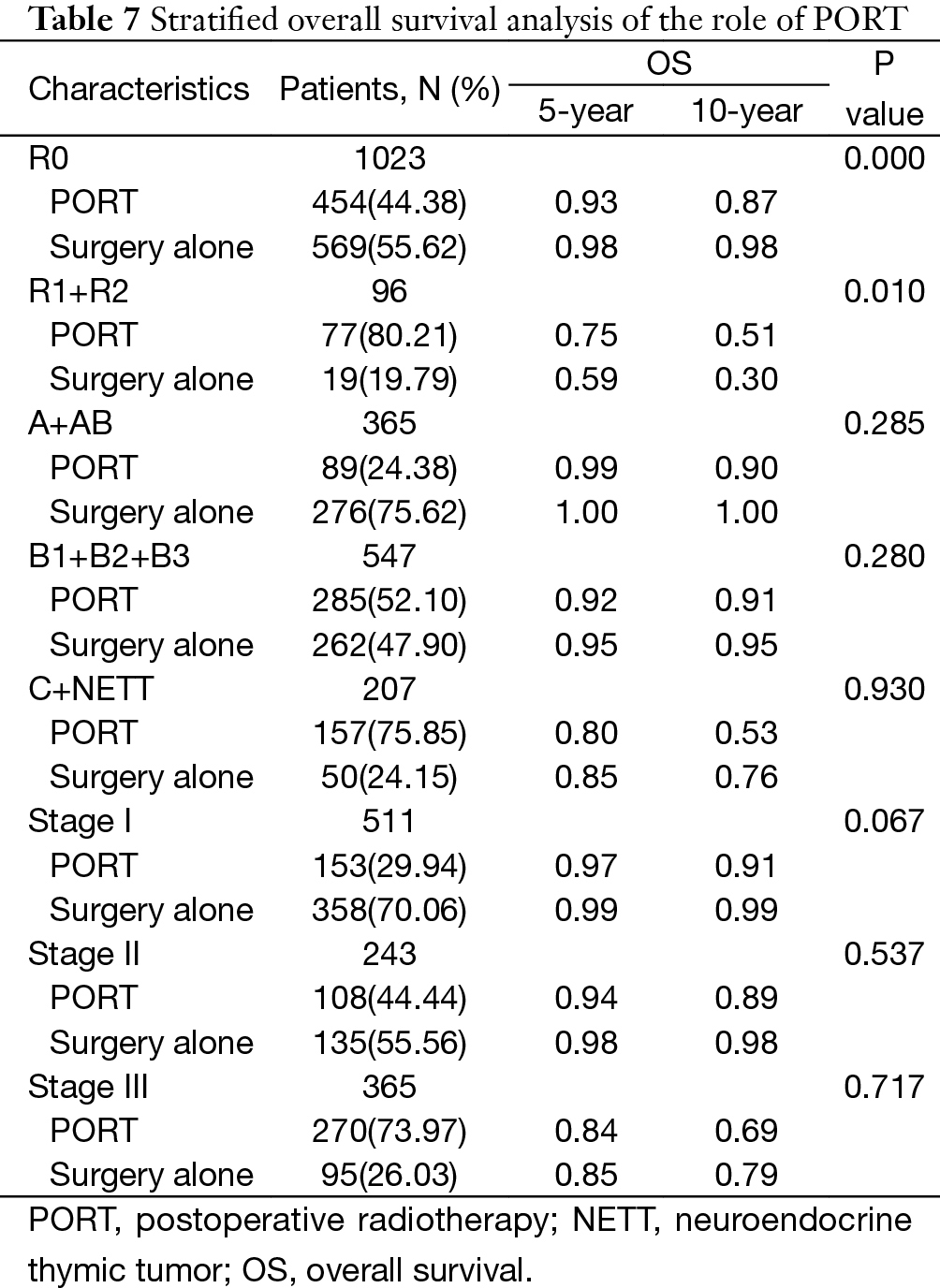
The 5-year and 10-year overall survival (OS) rates and disease-free survival (DFS) rates for patients having surgery followed by PORT were 90% and 80%, 81% and 63%, comparing with 96% and 95%, 92% and 90% for patients having surgery alone (P=0.001, P<0.001) respectively (Figures 1,2). In univariate analysis, age, WHO histological subtype, Masaoka-Koga stage, completeness of resection, and PORT were associated with OS (Table 2). Multivariate analysis showed that WHO histological type (P=0.001), Masaoka-Koga stage (P=0.029) and completeness of resection (P=0.003) were independently prognostic factors for OS, while PORT was not (Table 3). In univariate analysis, gender, myasthenia gravis, WHO histological type, Masaoka-Koga stage, surgical approach, PORT and completeness of resection were associated with DFS (Table 4). Multivariate analysis again showed that only WHO histological type (P<0.001), Masaoka-Koga stage (P=0.005) and completeness of resection (P=0.006) were independently prognostic factors for DFS (Table 5). Subgroup analysis showed that patients with incomplete resection underwent PORT achieved better OS and DFS (P=0.010, 0.017, respectively) than those having surgery alone. However for patients with complete resection, PORT was associated with worse OS and DFS (P<0.001, P<0.001, respectively). And no survival difference was detected between patients with or without PORT in the majority of stage or histology categories, except in stage II disease where PORT was associated with a worse DFS (Tables 6,7).

Full table
Full table
Full table
Full table
Full table
Full table
Discussion
The role of PORT in thymic tumors remains controversial. Local recurrence is the most common pattern of failure in thymic tumors after surgery. It has been suggested that PORT may reduce the recurrence rate from about 30% to below 5% (24,25). Given the rarity of the thymic tumors, their indolent natural history, and the large number of patients died from unrelated causes, no prospective randomized study has ever investigated the true benefit of PORT.
In this large multicenter study, a total of 1,546 patients with Masaoka-Koga stage I to III thymic tumors were elected from the ChART database. Unfortunately, PORT was not found to be associated with improved OS. The 5- and 10-year OS rates for patients who underwent surgery followed by PORT were 90% and 80%, comparing with 96% and 95% for patients who underwent surgery alone (P=0.001), respectively. This may be attributed to the higher proportion of patients with thymic carcinoma, stage III disease, and palliative surgery in the PORT group. However, PORT was significantly associated with improved OS in patients having palliative surgery.
The available data suggested that Masaoka-Koga stage, completeness of resection and histological classification were the independent prognostic factors (3,4,8). No significant differences in survival were noted among subgroup of thymoma, which was consistent with the result of the meta-analysis conducted by Detterbeck et al. (3). We also demonstrated that complete resection alone was sufficient to achieve a satisfactory outcome in the thymoma, saving the patients from the potential side effects caused by mediastinal radiation. These would include radiation pneumonitis, chronic pulmonary fibrosis, hematopoietic malignancies, esophageal malignancies, restrictive cardiomyopathy and pericardial effusion (26-30). A retrospective study by Mangi et al. found that most patients with stage III disease could undergo complete resection, and the addition of radiation therapy for patients receiving complete resection did not reduce the recurrence rate (21). The use of adjuvant radiation after complete resection of stage III thymoma needs to be re-addressed. For thymoma in WHO types A, AB, B1, as well as those classified as having Masaoka-Koga stage I and II disease, Utsumi et al. insisted that complete resection alone was sufficient treatment strategy (19). Furthermore, there was no significant difference in survival noted with regard to the status of PORT among the patients classified as stage III/IV, and WHO types B2/B3 (19). Kondo et al. reviewed 1,320 patients with stage II and III thymomas, and their finding revealed that local recurrence rates were not significantly decreased by PORT, and the prognosis of invasive thymoma were not improved by PORT (5). Complete resection was the most important factor in the treatment of thymic epithelial tumors. A meta-analysis by Korst et al. reviewed no statistically significant reduction in recurrence after adjuvant radiotherapy for patients with completely resected stage II or III thymic epithelial tumors (20). Weksler et al. reported a retrospective study using SEER database. This large population-based study demonstrated that adding PORT to surgery was associated with improved disease-specific survival. However, in multivariate analysis, postoperative adjuvant radiation therapy was not significantly associated with improved overall survival (18). Based on the current results and existing literature, it seems that future studies on PORT for thymomas after resection should be focused on patients at high risk of developing local recurrence.
Thymic carcinoma consists the most aggressive subtype of thymic neoplasms,. Surgery provides the best chance of cure for resectable thymic carcinoma. Patients who received complete excision had a significantly better prognosis than those who did not received surgical therapy. Due to the rarity of this disease, lack of high level of evidences, the role of chemotherapy and radiation after surgery are not well established. Using SEER database, Weksler et al. studied 290 patients with thymic carcinoma. They found that PORT could not improve the overall survival for patients after complete resection, and complete resection was the preferred primary treatment for thymic carcinoma (31). We also found that PORT did not improve the prognosis for patients with thymic carcinoma in this study. However, Hsu et al. suggested that PORT seemed to improve the prognosis for patients with thymic carcinoma, although the difference was not statistically significant (32). Omasa et al. also reported that PORT did not increase RFS or OS for stage II or III thymoma but increased RFS for stage II and III thymic carcinoma (33). Ahmad et al. reported that radiation therapy was associated with improved OS and longer RFS for patients with thymic carcinoma (34).
Radical resection, WHO histology classification, and Masaoka-Koga stage were revealed as t independent prognostic factors for thymic malignancy in the current study. Our results also showed that PORT could not bring any survival benefit to patients with completely resected stage I, II and III diseases. PORT should be administrated to the patients with palliative surgery, as it did improve the outcomes in these patients. However, because of the retrospective nature of this study and that the radiation field and dosage were highly varied, prospective randomized trials aiming at patients at high risk of developing recurrent disease should be conducted to evaluate the true effect of PORT in thymic epithelial tumors.
Acknowledgements
None.
Footnote
Members of The Chinese Alliance for Research in Thymomas (ChART): Yi Shen, Yucheng Wei, Affiliated Hospital of Qingdao University, Qingdao, China; Yin Li, and Guanghui Liang, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, China; Keneng Chen and Hao Fu, Beijing Cancer Hospital, Beijing, China; Hezhong Chen and Shihua Yao, Changhai Hospital, Shanghai, China; Youbin Cui and Yanzhong Xin, First Affiliated Hospital of Jilin University, Changchun, China; Renquan Zhang and Ningning Kang, First Hospital of Anhui Medical University, Hefei, China; Lijie Tan, Jianyong Ding, Hao Wang, Gang Chen, and Jie Wu, Zhongshan Hospital, Fudan University, Shanghai, China; Chun Chen and Wei Zheng, Fujian Medical University Union Hospital, Fuzhou, China; Liewen Pang and Fangrui Wang, Huashan Hospital, Fudan University, Shanghai, China; Yangchun Liu and Qing Lin, Jiangxi People’s Hospital, Nanchang, China; Yongyu Liu and Yongkai Wu, Liaoning Cancer Hospital, Shenyang, China; Wentao Fang, Jie Zhang, Yan Shen, Changlu Wang, Lei Zhu and Zhitao Gu, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Yongtao Han, Lin Peng, Sichuan Cancer Hospital, Chengdu, China; Jianhua Fu and Qianwen Liu, Department of Thoracic Surgery, Guangdong Esophageal Cancer Institute, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China; Zhentao Yu and Jie Yue, Tianjin Cancer Hospital, Tianjin, China; Peng Zhang and Yuan Chen, Tianjin Medical University General Hospital, Tianjin, China; Yun Wang and Yingcai Geng, West China Hospital, Sichuan University, Chengdu, China; Xinming Zhou and Hongguang Zhao, Zhejiang Cancer Hospital, Hangzhou, China.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Qu YJ, Liu GB, Shi HS, et al. Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad Radiol 2013;20:66-72. [Crossref] [PubMed]
- Detterbeck F, Youssef S, Ruffini E, et al. A review of prognostic factors in thymic malignancies. J Thorac Oncol 2011;6:S1698-704. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg 2004;26:412-8. [Crossref] [PubMed]
- Detterbeck FC, Parsons A. Thymic tumors: a review of current diagnosis, classification, and treatment. In: Patterson GA, Cooper JD, Deslauriers J, et al, (editors). Thoracic and esophageal surgery. Philadelphia: Elsevier, 2008:1589-1614.
- Tomaszek S, Wigle DA, Keshavjee S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg 2009;87:1973-80. [Crossref] [PubMed]
- Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009;4:911-9. [Crossref] [PubMed]
- Maggi G, Casadio C, Cavallo A, et al. Thymoma: results of 241 operated cases. Ann Thorac Surg 1991;51:152-6. [Crossref] [PubMed]
- Loehrer PJ Sr, Jiroutek M, Aisner S, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: an intergroup trial. Cancer 2001;91:2010-5. [Crossref] [PubMed]
- Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer 2005;50:59-66. [Crossref] [PubMed]
- Kundel Y, Yellin A, Popovtzer A, et al. Adjuvant radiotherapy for thymic epithelial tumor: treatment results and prognostic factors. Am J Clin Oncol 2007;30:389-94. [Crossref] [PubMed]
- Lucchi M, Ambrogi MC, Duranti L, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg 2005;79:1840-4. [Crossref] [PubMed]
- Nakahara K, Ohno K, Hashimoto J, et al. Thymoma: results with complete resection and adjuvant postoperative irradiation in 141 consecutive patients. J Thorac Cardiovasc Surg 1988;95:1041-7. [PubMed]
- Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9. [Crossref] [PubMed]
- Ogawa K, Uno T, Toita T, et al. Postoperative radiotherapy for patients with completely resected thymoma: a multi-institutional, retrospective review of 103 patients. Cancer 2002;94:1405-13. [Crossref] [PubMed]
- Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg 2012;93:1822-8; discussion 1828-9.
- Utsumi T, Shiono H, Kadota Y, et al. Postoperative radiation therapy after complete resection of thymoma has little impact on survival. Cancer 2009;115:5413-20. [Crossref] [PubMed]
- Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. [Crossref] [PubMed]
- Mangi AA, Wain JC, Donahue DM, et al. Adjuvant radiation of stage III thymoma: is it necessary? Ann Thorac Surg 2005;79:1834-9. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Kuo TT. Tumours of the thymus. In: Travis WD, Brambilla E, Burke AP, et al, editors. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004:146-248.
- Myojin M, Choi NC, Wright CD, et al. Stage III thymoma: pattern of failure after surgery and postoperative radiotherapy and its implication for future study. Int J Radiat Oncol Biol Phys 2000;46:927-33. [Crossref] [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [Crossref] [PubMed]
- Shulimzon T, Apter S, Weitzen R, et al. Radiation pneumonitis complicating mediastinal radiotherapy postpneumonectomy. Eur Respir J 1996;9:2697-9. [Crossref] [PubMed]
- Yeoh E, Holloway RH, Russo A, et al. Effects of mediastinal irradiation on oesophageal function. Gut 1996;38:166-70. [Crossref] [PubMed]
- Velissaris TJ, Tang AT, Millward-Sadler GH, et al. Pericardial mesothelioma following mantle field radiotherapy. J Cardiovasc Surg (Torino) 2001;42:425-7. [PubMed]
- Johansson S, Svensson H, Denekamp J. Timescale of evolution of late radiation injury after postoperative radiotherapy of breast cancer patients. Int J Radiat Oncol Biol Phys 2000;48:745-50. [Crossref] [PubMed]
- Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol 2004;22:3139-48. [Crossref] [PubMed]
- Weksler B, Dhupar R, Parikh V, et al. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299-303. [Crossref] [PubMed]
- Hsu CP, Chen CY, Chen CL, et al. Thymic carcinoma. Ten years' experience in twenty patients. J Thorac Cardiovasc Surg 1994;107:615-20. [PubMed]
- Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. [Crossref] [PubMed]
- Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100, 101.e1-2.


