Chronic cough—the limitation and advances in assessment techniques
Introduction
Over the last two decades, advances have been made in understanding the underlying mechanisms of the cough reflex and the pathophysiology of excessive coughing. These advances in clinical or preclinical cough research came with the development and application of novel metrics of objective and subjective cough assessment (1).
A range of validated methods to measure the severity of cough have been used, which can be broadly classified into subjective and objective approaches (2). The subjective assessments focus on the patients’ perception of cough itself and the impact it has on cough-related quality of life (QoL) (3). However, subjective patient-reported outcomes (PROs) are only moderate correlated with cough counting (4,5). Twenty-four-hour cough counting is a semi-objective assessment and has been suggested as the best method to quantify cough (6), but due to its labour-intensive and time-consuming nature, it has been largely confined to the research setting. Finally assessing cough reflex sensitivity, usually by inhalational challenge, can be used to dissect the pathophysiologic mechanisms of cough and evaluate the effect of drug intervention in clinical trials (7,8).
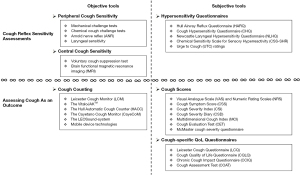
These three metrics of cough, PROs, cough counting, and cough challenge represent different, but linked, facets of coughing. To help understand the assessment of these metrics in clinical trials and clinical practice we have reviewed the current “state of the art” (Figure 1).
Cough reflex sensitivity assessments
Cough sensitivity refers to the response of cough reflex to external noxious stimuli, including mechanical, chemical, and thermal stimuli (9). Patients with chronic cough (CC) often present with allotussia to innocuous stimuli, abnormal sensations or irritability in the throat (laryngeal paresthesia), and increased response to tussive stimuli (hypertussia). These characteristics led to the description of a distinct clinical entity named the cough hypersensitivity syndrome (CHS) (10,11). CHS is due to excessive activation of cough reflex consequent on neuronal dysregulation. This may occur at the peripheral airway sensory level and/or the dysfunction in the higher brain pathways (12-14). The accurate assessment of peripheral/central cough sensitivity is essential for driving forward advances in improving the understanding and management of CC.
Objective tools
Peripheral cough sensitivity
Mechanical challenge tests
Cough can be elicited by the activation of mechanically sensitive vagal afferent nerves projecting to the large airways (some specialised Aδ fibres, commonly named cough receptors) and lungs [mechanoreceptors, including rapidly adapting receptors (RARs) and slowly adapting receptors (SARs)] (15). In preclinical animal studies, the invasive intratracheal mechanical stimulation was found to induce the cough reflex in anaesthetised rabbits/cats/dogs (16-20). For humans in clinical practice, the common area where the mechanical stimulation has been applied are the large airways level and the chest (8,9). In 2004, Lee and Eccles reported the first study to elicit cough in humans by the vibration of the airway at the trachea level with a modified men’s shaver. This research group also subsequently applied 70 Hz percussion stimulation to the chest to induce cough. In both studies, patients with upper respiratory tract infection (URTI) had an increased cough response to the mechanical stimulation compared with the healthy subjects (21,22). An enhanced cough reflex to percussive stimulation was also observed in patients with idiopathic pulmonary fibrosis (IPF), which was the most prominent when low-frequency stimulation (20 Hz) was applied to the most extensive area of fibrosis, the posterior lung base (23). Kamimura et al. demonstrated that mechanical stimulation of the cervical trachea is a feasible cough challenge test by evaluating the following three tests in patients with cough: (I) tracheal compression test (TCT): the cervical trachea was compressed softly with the fingers several times; (II) tracheal stretch test (TST): the trachea was stretched by retroflexion of the neck; (III) tuning fork test (TFT): vibratory stimulation was provided by placing a tuning fork on the cervical trachea for 20 s (24). However, the cough detection rate of the tests was only 27.7%, 39.8%, and 36.9%, respectively and no stimulus response relationship can be detected.
Chemical cough challenge tests
Vagal C fibres are thought to be the primary mediators in regulating pathological cough. They express receptors responsive to various chemical stimuli, including transient receptor potential vanilloid-1 (TRPV1), sensitive to capsaicin and pH (25-27), transient receptor potential ankyrin 1 (TRPA1), sensitive to multiple irritants including allyl isothiocyanate (AITC) and cinnamaldehyde (28,29). TRPV4, sensitive to anandamide, arachidonic acid and osmotic stimuli such as distilled water) (30,31), transient receptor potential melastatin 8 (TRPM8), sensitive to the cooling compounds menthol, icilin, and eucalyptol (32) and purinergic receptors (P2X3 and P2X2/3) sensitive to adenosine triphosphate (ATP) (33). Since the first introduction of citric acid as a challenge agent in the human study (34), the controlled inhalation of tussive chemical stimuli has been used in assessing the cough reflex sensitivity for over 60 years. Thus, challenges and their responses may be used to probe different receptors/pathways subtending the cough reflex (34-38).
The standardized methodologies of cough challenge tests have been described elsewhere (6,9,39). The first administered concentrations of tussive stimuli causing two (C2) and five (C5) coughs are commonly taken as the endpoints of patients being told “cough if you need to”. However, the traditional endpoints may not best reflect clinical relevance due to the extensive overlap between CC patients and healthy subjects and the poor correlation with 24-h cough frequency (C2: r=−0.08 and C5: r=−0.03) (40,41).
Hilton et al. measured the cough responses utilizing two novel endpoints with nonlinear mixed-effects modelling: Emax (the maximum cough numbers evoked by any concentration of capsaicin) and ED50 (the capsaicin dose needed to induce half-maximal response), which could discriminate health subjects from CC and imply the neuronal pathways controlling cough. However, maximal dose challenge test may not be tolerated and compared to C2/C5, it takes a long time (30–45 min) to reach the plateau at the maximum tolerated dose (42,43). Another alternative method can be used for the longitudinal follow-up of patients: comparison of cough numbers elicited by the individualised ED50 dose between every follow-up, which may be more time efficient (44,45). To date, no methodology provides meaningful utility in clinical practice.
In antitussive drug development, challenge tests including capsaicin, ATP, interferon γ (IFN-γ), distilled water have achieved common usage in clinical trials, but there is poor correlation with other cough metrics (41,46-49). The highly specific antagonists of TRPV1, XEN-D0501, and SB-705498, demonstrated efficacy and potency in preclinical and capsaicin cough challenge studies, thus showing target engagement but failed to show any efficacy in patients with refractory chronic cough (RCC) (50-52). This indicates individual receptors may have different roles in the complex pathophysiology of cough.
Arnold nerve reflex (ANR)
ANR, also known as the ear-cough reflex, was first described by Arnold in 1831 and named after the German anatomist (53). ANR is a mechanical challenge test, which is evaluated by stimulating the external auditory canal of each ear with a cotton-tipped applicator. Coughing occurring within 10 s of stimulation is considered induced by the irritation of the external auditory canal innervated by the auricular branch of the vagus nerve, which was present in about 25% of CC adults and 3% of CC children and as with CC is more commonly observed in female patients. Most patients showed a unilateral positive reflex. For adults, the prevalence in CC patients demonstrated to be 11–12-fold higher than in those healthy subjects or patients without CC, which meant the positive ANR would reflect the vagal hypersensitivity; but this increased prevalence was not found among children, which suggested CHS as an acquired condition, resulting from irritation of the vagus (54,55). Castro et al. reported three cases of “Oto-tricho-tussia”, which meant “ear-hair-cough” (56). The urge to cough (UTC) in these patients was triggered by the irritation of Arnold’s branch of the vagus nerve caused by the hair located on the tympanic membrane and the external auditory canal, which responded to the removal of the irritant hair. Japanese researchers recently found daily aural stimulation with capsaicin ointment for 2 weeks or 6 months could enhance the cough reflex and reduce the incidence of aspiration pneumonia in elderly dementia patients, which may be explained by the capsaicin-TRPV1-mediated ANR (57,58). These results further support the important role of vagal hypersensitivity in CC. Provided the Arnold nerve is the only cutaneous peripheral branch of the vagus nerve, it has been considered a window that transmits biofeedback from its peripheral receptors to the brain (59). However, in the study conducted by Mai et al. (60) compared with 0% of healthy subjects, 73% of CC patients had positive ANR or UTC, of which 87.5% were negative after one-month treatment. However, the incidence was not related to phenotype, cough duration, cough severity, QoL, or treatment outcomes. Thus, a positive ANR can indicate the common feature, CHS, in CC patients, but is not suitable as a routine test in a clinic.
Laryngeal sensitivity
RCC and laryngeal hypersensitivity appear to display significant overlap in symptoms and aetiology (61,62). According to the data from the Phase 2 study of the P2X3 receptor antagonist gefapixant (63), 95% to 96% RCC patients reported abnormal laryngeal symptoms, such as an irritation or tickle sensation in the throat, which is consistent with the Chinese RCC population (95.9%, unpublished recent data). Some local direct therapies, such as bilateral thyroarytenoid injections with botulinum toxin type A and superior laryngeal nerve block, have demonstrated significant improvement in RCC (64,65).
Phua et al. measured the laryngopharyngeal sensitivity (LPS) by mechano-stimulation and chemo-stimulation (66). The threshold to mechanical stimulation was defined as the lowest air pressure required to trigger an involuntary transient adduction of the vocal folds, which was known as the laryngeal adductor reflex (LAR). The repeatability, and the coefficient of variation of this test were 0.96, and 9.7%, respectively, according to their priorly reported data (67). The chemo-sensitivity was defined as the threshold volume of chemical solution (0.1 N hydrochloric acid or normal saline) infused into the pyriform sinus to initially trigger a complete adduction of the vocal folds, an irrepressible swallow, or a cough. In healthy subjects, a strongly negative correlation was found between thresholds to mechano-stimulation and acid-stimulation; in patients with gastroesophageal reflux disease (GERD), a heightened chemo-sensitivity and a diminished mechano-sensitivity were presented, by which the authors speculated a compensatory mechanism between them and the presence of noxious acid stimuli might desensitize the mechanoreceptors (66).
Central cough sensitivity
Voluntary cough suppression test (CST)
Cough can be voluntarily initiated and inhibited (68). The CST was established by modifying the capsaicin challenge test and the subjects were told “try not to cough”. The lowest capsaicin concentrations required to induce one cough (CS1), two coughs (CS2), and 5 coughs (CS5) were recorded. Hutchings et al. reported that healthy subjects demonstrated their voluntary ability to suppress the capsaicin-evoked cough (69). In contrast the impaired cough suppression is observed in RCC patients (70), which provides evidence for dysfunction of central inhibitory control in cough hypersensitivity. The CS5 value was moderately correlated with objective cough frequency, more reproducible than CS1 and CS2, and better than C5 in discriminating RCC patients from healthy subjects with a sensitivity (100%) and specificity (91.3%) when 39 µmol/L was adopted as the threshold. Cho et al. also found that both patients with chronic obstructive pulmonary disease (COPD) associated RCC had increased cough reflex sensitivity, but only RCC patients lost the ability to suppress capsaicin-evoked cough (71). This indicated different underlying mechanisms causing CC in COPD from those in RCC. So far, CST is a relatively understudied area. CST seems to be a better discriminator of CHS than the traditional cough challenge test, and is therefore a more promising indicator in clinical research going forward. However, both of them were subject to test-retest variability (72). It is necessary to study the difference in the voluntary ability to suppress cough between various aetiologies induced CC, and ascertain its relationship with treatment outcomes.
Brain functional magnetic resonance imaging (fMRI)
Brain fMRI affords researchers a way to peek under the hood of cough and into the central (brain and brainstem) sensory and motor networks. Upregulated brain region activity causes additional neural firing and thus more locally increased energy requirement. This in turn leads to an increase in haemoglobin-carrying oxygen (Hb) and changes in magnetic properties. Using fMRI images visualized by high T2 signals (73,74), Stuart Mazzone and Michael Farrell have made great progress in our understanding of the neuronal circuitry underlying cough reflex. The activation of the cortical and subcortical neuronal networks was found to be involved in shaping the capsaicin inhalation-induced UTC in 2007 (75,76). Sex-related differences were later found in these regional responses, being significantly larger in the primary somatosensory cortices of females (77). In this context, the predominance of females in CC is understandable. Subsequently, the supramedullary inhibition of cough was demonstrated in 2011 (78). These data suggest that an intact cough reflex requires the involvement of brainstem-mediated response to irritation of the airways and the active facilitation by cortical regions, which is further regulated by distinct higher order inhibitory processes. In healthy humans, the brain response to capsaicin inhalation showed dissociable patterns (79,80). A dose graduated response was found in the majority of brain regions, including the insula and mid-cingulate cortex. In the brainstem, capsaicin produced dose-dependent activations in respiratory-related regions of the dorsal pons and lateral medulla. Subject ratings of UTC correlated with the activation in the somatosensory and mid-cingulate cortices. Prefrontal and parietal regions demonstrated all or nothing responses, which were dose-independent. Inhibitory neural regions, including the inferior frontal gyrus and supplementary motor area, were activated exclusively at the high dose of capsaicin, which indicated the cough (motor) suppression engagement. The existence of an inhibitory circuit can also help to explain the placebo antitussive response (81). In CC patients, the activation of the midbrain during airway irritation was detected by fMRI but not in healthy subjects. In contrast, the activation in the medial prefrontal cortex was shown in healthy subjects, but was absent in CC. This indicates dysfunction of the cough-suppression network in CHS (82). Interestingly, enhanced activity of the central inhibition network was found to coincide with smoking-induced sensitization of these neural circuits, which provided a novel target of upregulation of nicotinic receptors in the treatment of RCC (83). Further work has shown decreased grey matter volume in the left frontal cluster (correlated with a longer cough duration) and enhanced functional connectivity within the left fronto-parietal network (correlated with a greater psychological and social impact of coughing) in RCC. This study provides a basis for the development of interventions targeting cognitive modulation in CC patients (84).
Some limitations such as low signal-to-noise ratios, experimental methods, and statistical challenges, have to be acknowledged, but fMRI data has enormously enhanced our understanding of the neurophysiology of the cough reflex. Thus, fMRI should be combined with multiple other methods to overcome its intrinsic constrain (73).
Subjective tools: questionnaires indicating hypersensitivity (Table 1)
Table 1
| Tools | Items/features | Validity | Reliability | Responsiveness | |
|---|---|---|---|---|---|
| Internal consistency | Repeatability | ||||
| HARQ (10) | A self-administered 14-cough-related-item questionnaire with a maximum score of 70 | Adopt 13 as a cut-off point, the sensitivity (94%) and specificity (95%) of the HARQ was high, with an area under the ROC curve of 0.99 | Cronbach’s α: 0.81 | r=0.78 | MCID: 16 points (P<0.0001) |
| NLHQ (85,86) | A 14-item self-administered questionnaire regarding symptoms of abnormal laryngeal sensation across three domains: obstruction, pain/thermal, and irritation | A cut-off for normal laryngeal function could be considered to be 17.1; moderately correlated with LCQ (r=0.673); moderately correlated with cough frequency in a real-world study (r=−0.430) | Cronbach’s α: around 0.9 | NA | No available data for CC |
| CHQ (87) | A 23-item self-reported questionnaire assessing the presence and severity of cough triggers and laryngeal sensations on a categorical yes/no scale, with scores ranging from 1 to 23 | Moderately correlated with cough counts (r=0.54), VAS (r=0.57) and LCQ (r=−0.68) | Cronbach’s α=0.90 | NA | NA |
| CSS-SHR (88,89) | A short 11-item self-reported questionnaire to quantify the experience of SHR patients regarding odorous/pungent substances | With a cut-off for the normal range of 43, 92% of SHR could be correctly diagnosed, with a sensitivity of 73% and a specificity of 97% | R ranged between 0.76 and 0.84 | r=0.87 | NA |
HARQ, Hull Airway Reflux Questionnaire; ROC, receiver operating characteristic; MCID, minimum clinically important difference; NLHQ, Newcastle Laryngeal Hypersensitivity Questionnaire; LCQ, Leicester Cough Questionnaire; CC, chronic cough; CHQ, Cough Hypersensitivity Questionnaire; VAS, Visual Analogue Scale; CSS-SHR, Chemical Sensitivity Scale for Sensory Hyperreactivity; NA, not applicable.
Hull Airway Reflux Questionnaire (HARQ)
The HARQ is a self-administered 14-item tool to access the likelihood of the presence of airway reflux based on the common sequelae of gaseous, non-acidic reflux from the gastrointestinal tract (90). This airway reflux cannot be visualized on a radiograph, but micro-aspiration existence was later objectively verified by the new technique of reflux scintigraphy (91). Each item of HARQ is scored from 0 (no problem) to 5 (serious problem). Higher scores indicate worse cough-related symptoms. The validated HARQ was recommended as a diagnostic instrument (92,93), with good internal consistency (Cronbach’s α=0.81) and reproducibility (r=0.78). The normal scores for healthy subjects range from 0 to 13, with high sensitivity (94%) and specificity (95%). The minimum clinically important difference (MCID) is 16 (10). Zhang et al. established a logistic regression equation with HARQ items, by which the treatment success rate of gabapentin for RCC could reach 83.72%. When the total score was ≥21.5, HARQ had a moderate ability to predict gabapentin efficacy, with a sensitivity of 84.6% and specificity of 63.6% (94).
In over 2,000 rigorously assessed RCC/unexplained chronic cough (UCC) patients who participated in the COUGH-1 and COUGH-2, the mean baseline HARQ score was 40, nearly three times the upper limit of normal (ULN). 95% of the participants had increased scores (92,95). Thus, HARQ describes the symptom complex in CC with a very high degree of accuracy. Whether it accurately predicts and correlates with response to treatment needs to be further studied. Currently, HARQ is available in nearly forty different languages (https://www.issc.info/HullCoughHypersensitivityQuestionnaire.html).
Cough Hypersensitivity Questionnaire (CHQ)
The first version of CHQ is a 35-item self-reported questionnaire assessing the presence and severity of cough triggers and laryngeal sensations on a Likert scale (96), with scores ranging from 0 to 150. The limited results showed its potential to distinguish CHS from healthy subjects, but lacked the reliability and repeatability data. The new CHQ consists of 23 items (including 16 cough triggers and 7 laryngeal sensations) on a categorical yes/no scale and demonstrated a good internal consistency (Cronbach’s α=0.90) in a pulmonary sarcoidosis study. The total score had a moderate corelation with cough severity (r=0.57) and health status (r=−0.68) (87). The Korean version of CHQ was reported a moderate correlation with total Leicester Cough Questionnaire (LCQ) score (r=−0.50) and Visual Analogue Scale (VAS) score (r=0.40), which indicated the potential clinical relevance of evaluating the cough hypersensitivity in CC patients (97).
Newcastle Laryngeal Hypersensitivity Questionnaire (NLHQ)
The NLHQ is a 14-item self-administered questionnaire in an attempt to quantify the laryngeal paraesthesia in patients with laryngeal conditions (involving three domains: obstruction, pain/thermal, and irritation), developed and validated by Vertigan et al. in 2014, using a seven-point Likert scale (each item ranging from 1 All of the time to 7 None of the time) (85). NLHQ could successfully discriminate patients from healthy humans with high internal consistency (Cronbach’s α=0.9) and moderately correlated with LCQ (r=0.673). The low limit score of a normal laryngeal function was 17.1/98. In another real-world study, NLHQ was reported to have a moderate correlation with cough frequency (r=−0.430) (86). NLHQ currently has been recommended as a useful outcome tool to measure the efficacy of neuromodulators and behavioural intervention in CC study (98).
Chemical Sensitivity Scale for Sensory Hyperreactivity (CSS-SHR)
The CSS-SHR is a short 11-item self-reported questionnaire to quantify the experience of SHR patients regarding odorous/pungent substances (88). With the upper limit score of the normal range of 43, a high correct classification rate (92%) for the diagnosis of SHR was demonstrated, with a sensitivity of 73%, a specificity of 97%, good test-retest reliability (rxy=0.87), satisfying internal consistency (rx=0.76–0.84). Utilizing a combination of positive results of CSS-SHR and capsaicin challenge test (induction of 10 and 35 coughs at capsaicin concentrations of 0.4 and 2.0 µmol/L, respectively) to define SHR, the prevalence of SHR was estimated to be 6.3% in a cohort of 693 subjects, which were randomly selected from a population-based study (89). Later, CSS-SHR was further validated and showed high internal consistency (Cronbach’s α=0.78–0.83) in a large-scale population-based study (99). However, CSS-SHR has not been widely used currently.
UTC ratings
The UTC sensation is characterized by a perception of airway irritation and the resultant cough desire, an important clinical feature in CHS (100). In inhalation cough challenge, the perceptible sensation or motor response may not occur at sufficiently low concentration of tussive stimuli; however, with concentrations increasing, the participant will report an UTC but 0 cough event happens, based on a modified Borg scale ranging from zero (no discernible urge) to 10 (maximal urge) (72,81). The minimum concentration of stimuli for perception is deemed Cu, which was proved to be effective and reproducible without sex difference and had moderate to strong linear correlations with C2/C5 (r=0.74 and 0.57, respectively) (101). Increasing UTC ratings correlated with a worse QoL (r=−0.31 for total LCQ, −0.34 for psychological domain and −0.37 for social domain) in CC patients (102) and a higher cough frequency in healthy subjects (r=0.72) (103). Due to the combination with chemical challenge test, the UTC rating was limited into research setting.
Assessing cough as an outcome
Objective measurement of cough frequency
The measurement of 24-hour cough frequency has become the primary endpoint in most clinical studies comparing the effect of treatment. In reality, it is a semi-objective measure since it is impossible to differentiate spontaneous coughing from voluntary coughs. Longer term cough monitoring has been demonstrated and direct insight for the objective evaluation of cough and can indicate early signs of exacerbations of chronic pulmonary disease (1,104-107). Cough may occur as a single event or in clusters described variously as paroxysms, bouts, peals, attacks, or cough epochs (Figure 2). Much debate surrounded the apparently simple task of deciding what is a cough. Physiologists had defined cough as an inspiration followed by a forcible expiration suggesting that the subsequent peal of sounds were not coughs but expiration reflexes (ERs) (108). It is now generally accepted that an individual sound event constitutes individual cough, which divided into explosive, intermediate and viced phases (109). Different parameters were proposed to count cough, including individual cough sound (5), cough epochs (110), cough seconds (47,109). The typical cough sound waveform and different counting methods were illustrated with Fig. 1 & Fig. 2 in Kelsall’s study (109). In an unexplained CC patients study, all the three units demonstrated strong correlation with each other and had a moderate association with subjective assessments and QoL, whereas cough epochs were a less satisfactory alternative (109). In the tuberculosis (TB) treatment study, total time spent coughing (seconds per hour) was also found to be a better predictor of the disease severity and microbiologic treatment response than cough epochs (111). However, in another prospective observational study, which compared the nocturnal cough in healthy subjects and in patients with cystic fibrosis (CF) and primary ciliary dyskinesia, the repeatability of cough epochs/h was found higher than coughs/h [intraclass correlation coefficient (ICC) =0.75 versus 0.49] (112). Furthermore, in a recent double-blind randomised placebo-controlled trial, lesogaberan [a novel peripheral gamma-aminobutyric acid type B (GABAB) receptors agonist) could decrease the cough response to capsaicin in RCC patients, accompanied by the reduction in cough bouts/h, but not the coughs/h, when compared with placebo. The authors attributed this difference to the particular role that the GABAB receptors played in triggering cough bouts, with little influence on the subsequent bout duration (113). Most of the current studies utilized the individual explosive cough sound as the cough counting unit although simply averaging the number of coughs per hour over a given period of time is not highly correlated with the patient experience (5).
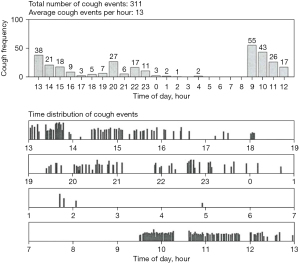
To date, there are no universal standardized approaches for counting cough. Non-automated cough counting (manual counting) can achieve high agreement in different listeners and remains the gold standard method of quantifying coughs (114,115). A wide variety of techniques have attempted at capturing the cough sound information from the patients (7). A meta-analysis reported by Pavesi et al. for the first time showed the feasibility of a fully computerized cough acquisition and analysis system combined with the following manual counting of audio and video displays methodology in evaluating the antitussive efficacy of dextromethorphan (116). However, the huge labour costs led to the development of modern automated cough frequency monitors. An ideal cough monitor should be robust, compact, portable, and capable of recording for at least 24 hours, while accurately, consistently, and automatically identifying all coughs and distinguishing them from all speech, sneezing, laughter, and background noise (117). Although several modern automated or semi-automated cough monitors have been developed, as yet, they have been rarely introduced into routine clinical practice and are confined to experimental centres. Among them, two systems, the Leicester Cough Monitor (LCM) and VitaloJAKTM, have demonstrated good validity and are being widely used in research work.
LCM
The LCM is an automated sound-based ambulatory cough monitor, consisting of a portable battery-powered digital recorder and a flip-collar microphone, and was developed and initially validated by Birring et al. in 2008 (118). The Leicester Cough Algorithm was used to analyse the 6–24 hours of sampling sounds data, which was divided into contiguous 10 s segments and classified as possible cough sound or background noise. This would be further manually calibrated to eliminate those wrongly classified cough events, which took 5 min for a 24-h recording. Cough was defined as an individual explosive sound no matter whether it occurred as a single event or in a cluster. The reported sensitivity and specificity of LCM were 91% and 99%, respectively, for the classification of cough events, with a false positive rate of 2.5 events/hour. The accuracy of manual and automated cough counts appeared similar. The LCM also demonstrated highly repeatable cough counts (ICC =0.9) over 3 months. Further validation also showed a highly significant correlation (r=0.97) and consistency (ICC =0.98) between manual and automated coughs/per patient/hour (119). Another validation work conducted on 20 subjects (8 healthy subjects and 12 patients with respiratory disease) reported the sensitivity and specificity of LCM were 82.3% and 99.9% in healthy volunteers, and 83.8% and 99.9% in patients (41). Automated cough counting appeared more repeatable than aurally counting in patients compared with healthy subjects (ICC =0.98 versus 0.85).
Currently, the LCM system can record consecutive data for more than 24 hours (up to 4 days) (2) and has been applied to measure coughs in a wide range of clinical studies, including the investigation of healthcare use and costs in CC (120), post-exercise cough in asthma and cough patterns in asthma and non-asthma (121,122), CC in vocal cord dysfunction (VCD) (123), inhaled sodium cromoglicate in IPF (124), cough frequency in acute cough (125), COPD exacerbation (126), bronchiectasis (127) and TB (128,129), Physiotherapy and Speech And Language Intervention (PSALTI) for CC (130-132), as well as the evaluation of the antitussive efficacy of various neuromodulators, including gabapentin, pregabalin (133,134). A recent real-world retrospective study provided the prospects of the feasibility and clinical utility of LCM in the outpatient clinical setting, in which cough monitor was responsive to intervention and claimed to identify different diseases by the cough frequency and pattern (86).
The VitaloJAKTM
The VitaloJAKTM is a semi-automated cough monitoring system, developed by the collaboration between Vitalogragh and clinical academics, and is currently the only system with the regulatory approvals necessary for use in clinical trials of investigational medicinal products (135). This system is comprised of a digital sound recording device (with a lapel microphone and a contact microphone attached to the upper sternum), a web-based portal for data transfer, tracking, and storage, and a digital signal processing algorithm to filter from a 24-hour recording and only retain possible cough sounds. An approx. 1.5-hour compressed audio is produced before a trained analyst aurally counts coughs (1,2). The preliminary validation study with a small sample size (n=20) reported the ability of VitaloJAKTM filtering algorithm to reduce up to 98% irrelevant noise or silence while preserving close to 100% of recorded cough sounds (136,137). A recent larger-scale evaluation, which is the currently most extensive testing of cough monitoring system further supported the sensitivity and efficiency of VitaloJAKTM to measure cough frequency across a range of diagnoses and age groups without being influenced by cough numbers (135).
The VitaloJAKTM has been used for recording and measuring coughs in a range of clinical research, including the antitussive efficacy evaluation of GSK2339345 (a novel sodium channel inhibitor) (138), GSK2798745 (he selective TRPV4 channel blocker) (139), lesogaberan (a novel peripherally acting GABAB agonist) (113), Eliapixant (also called BAY 1817080, a P2X3 receptor antagonist) (140) and Gefapixant (a P2X2/3 receptor antagonist) (33,141) in RCC, a phase 2a trial of Navafenterol (also called AZD8871, a novel bronchodilator) in COPD (142), as well as cough frequency measurement in IPF (143) and asthma (144).
The Hull Automatic Cough Counter (HACC)
The HACC was developed for automatically recognising and analysing cough and non-cough sounds (the silence period was omitted) (145). It was preliminarily validated by calculating coughs in one hour after rising in 33 smoking subjects, which turned out to save 97.5% of counting time with a sensitivity of 80%, specificity of 96%, and reproducibility of 100%. The 20% of false positive rate indicated its deficiency in classifying cough sounds and the ambient coughs. In the later validation study, although HACC was strongly correlated with the aural cough counting (r=0.87), it consistently missed actual cough by around 15% (5). Thus, in the subsequent studies, a hybrid HACC/LCM system was used to replace HACC. The mean sensitivity of the HACC/LCM system in acute exacerbations of COPD (AE-COPD) was lower than that of a single LCM in CC and only moderate correlations were reported between QoL and objective cough counting. However, daytime coughs were strongly correlated with nighttime coughs on every visit day, which demonstrated the validity of HACC/LCM as any conscious modification of cough would be more likely during the day than at night (126,146). Recently, the hybrid HACC/LCM system was also used to access the effect of modulator therapy in CF (147).
The Cayetano Cough Monitor (CayeCoM)
The CayeCoM is a semi-automated cough detection system developed for tracking cough in TB patients (148). The automatically detected cough epochs in the pre-screening stage would be manually reviewed to eliminate false positives. CayeCoM has been validated for 24 h recordings, with an accuracy of 75.6% (4 false positives per hour) and specificity of 99.6% in reporting epochs (the sensitivity was only 51.4% when reporting individual coughs), reducing mutually reviewing time by 95% (149). However, in the following investigation of evaluating cough frequency in pulmonary TB in the real-life setting, using CayeCoM technique, 30–42% of recording files were excluded because of the high levels of ambient noise (150,151). Recently, this research group replaced the previous audio-based cough recording with a vibration-based method, by which the spoken word was intelligible and only one of 363 recordings was unusable (111).
The LEOSound-system
LEOSound is a commercially available, automated lung sound monitor, working like a “long-term stethoscope”. It can continuously record the lung sounds by an ambient microphone and three small bio-acoustical sensors, one of which was attached to the patient’s trachea and two to the back (152). The respiratory sounds, such as cough and wheezing, will be recorded, classified, and stored in the database automatically. The trained observers can listen to all the three channels and further verify the automated recognition. In children, the sensitivity of cough detection by LEOSound was 89% and the specificity was 99%, with a strong correlation with the subjective measure (r=0.85) (110). As yet, there is no available validation data in adults, but currently, LEOSound has been widely used in overnight cough recording to evaluate the relationship between CC and other disease entities, such as COPD (153-155), CF (112), asthma (156) and aspiration pneumonia in dysphagic stroke survivors (157).
Mobile device technologies
Mobile cough devices are portable, low-cost, real-time, and patient-friendly. Thus, in recent years, there has been an increased focus on the advanced techniques based on mobile technologies and wearable devices in the acquisition and automatic processing of cough sounds, especially after the outbreak of coronavirus disease 2019 (COVID-19), more studies have attempted to rapidly identify COVID-19 by this technique (158-166). Hoyos-Barceló et al. proposed an efficient and power-saving smartphone-based cough detection system, which could classify cough in a noisy environment with 88.94% sensitivity and 98.64% specificity (167). Kvapilova et al. collected continuous sound with smartphones and measured cough with machine learning. This has been preliminarily validated and the final goal is to achieve a 92% sensitivity at 99% specificity in the real world (168). This technique was also tested to identify dry cough and wet cough with reported sensitivity and specificity values of 88% and 86% (169). Claxton et al. developed a smartphone-based algorithm, which could rapidly and accurately diagnose AE-COPD with a sensitivity of 82.6% and specificity of 91.0% (170). This technique is promising to accurately identify COPD, with or without the presence of infection (171). In another study, a smartphone-based system, TussisWatch, for the first time, proved its feasibility in self-detect cough episodes for early identification of COPD or congestive heart failure (CHF) (105). Using smartphone-based monitoring, Gabaldón-Figueira et al. found that longitudinal monitoring was more accurate than 24-h monitoring and the optimal monitoring period would depend on the baseline cough frequency (172). This technology was also used to collect the nocturnal cough of patients with physician-diagnosed asthma (173-175) and helped with detecting the presence of an asthma exacerbation as well (176).
Subjective tools: cough-related PROs
Cough scores (Table 2)
Table 2
| Tools | Items/features | Validity | Reliability | Responsiveness | |
|---|---|---|---|---|---|
| Internal consistency | Repeatability | ||||
| VAS (5,87,125,127,177-179) | A 100-mm cough scale | Moderately correlated with LCQ (r=−0.41) and CSD (r=0.53); variedly correlated with cough frequency (r=0.03–0.75); poorly correlated with C5 (r=−0.32) | NA | ICC: for CC, 0.45–0.51; for COPD, 0.87 |
MCID for acute cough: ≥17 mm; for CC: ≥30 mm; invalid for assessing cough change in ILD |
| K-CSS (180) | Korean version of CSS. Two questions about the subjective recognition of cough frequency during day- and night-time; simple, short, and practical | Well correlated with LCQ (r ranged between −0.66 and −0.60) and VAS scores (r=0.52) | A weak correlation between the day and night scores (r=0.24) | High repeatability (ICC: 0.75) | Medium of improved score after treatment: 2 |
| sCSS (181) | A Chinese version of simplified CSS | Highly correlated with CSS (day: r=0.82; night: r=0.92) and poorly correlated with C2/C5 (r=−0.20 to −0.030) | NA | Day: ICC =0.90; night: ICC =0.89 | ES of 1–2 weeks post-treatment: 0.52–1.16 for day and 0.71–1.09 for night |
| CSI (182) | 10 simplified questions developed from a 49-item cough specific questionnaire | Well correlated with CLCQ (r=0.60) | r=0.928 | Strong reliability (r=0.83) | NA |
| CSD (183) | Seven self-administered items to rate the frequency (three items), intensity (two items), and disruptiveness (two items) of cough | Moderately correlated with LCQ (r=−0.59 to −0.64) and VAS (r=0.42–0.57) | Cronbach’s α=0.771–0.923 | ICC ranged between 0.87 and 0.92 | MCID: a change threshold of ≥1.3-point reduction on the total and subscale scores |
| MCI (184) | A Practical Scale to Measure Cough intensity, frequency, physical impact, psychosocial impacts and sputum characteristics | Well correlated with VASfrequency (r=0.651), VASintensity (r=0.543), and LCQ (r=−0.824) | Cronbach’s α=0.819 | ICC =0.779 | A Cough Index ≥4 distinguished respiratory patients from healthy subjects, with a sensitivity of 80% and a specificity of 85% |
| CET (185) | A short, simple patient-completed 5-item test involves the dimensions of cough severity, social impact and psychological effect | Strongly correlated with LCQ (r=−0.74), CSS (r=0.71) and VAS (r=0.70) | Cronbach’s α=0.80 | ICC: 0.84 | MCID: 2 of 25 |
Other tools: McMaster Cough Severity Questionnaire (a current conceptual framework for measuring cough severity in RCC/UCC patients) (186). C2, the first administered concentration of tussive stimuli causing two coughs; C5, the first administered concentration of tussive stimuli causing five coughs; VAS, Visual Analogue Scale; LCQ, Leicester Cough Questionnaire; CSD, Cough Severity Diary; NA, not applicable; ICC, intraclass correlation coefficient; ES, effect size; CC, chronic cough; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; K-CSS, Korean Cough Symptom Score; VAS, Visual Analogue Scale; sCSS, simplified cough symptom score; CLCQ, Cough Quality of Life Questionnaire; CSI, Cough Severity Index; MCI, Multidimensional Cough Index; CET, Cough Evaluation Test; MCID, minimum clinically important difference; RCC, refractory chronic cough; UCC, unexplained chronic cough.
VAS and Numeric Rating Scales (NRS)
The anchors on a linear scale make the evaluation of cough severity in a standardized manner. A standardized VAS is a 0 to 100 mm linear scale. Higher scores suggest higher severity (187). Due to its useability and practicality, VAS is one of the most widely used tools in both research and clinics as an endpoint for assessing cough severity in a broad range of disease (104,125,177,188). A good repeatability was reported in CC (ICC =0.604) (5) and COPD (ICC =0.87) (178). In a recent validation study in CC, VAS demonstrated moderate test-retest reliability (ICC =0.45 to 0.51) and moderately correlated with other subjective tools. However, its correlation with cough frequency varied in different timepoints and different studies (125,127). As a responsive outcome, VAS reduction ≥30 mm was estimated as a clinically meaningful change threshold (179). In acute cough, the MCID for the VAS has been reported to be 17 mm (125). The NRS (0–10 point) was also recommended as a cough severity assessment (93,187). A standardized consensus must be made when using VAS or NRS, such a simple outcome measure.
Cough Symptom Score (CSS)
The CSS was proposed by Hsu et al. (189), and consists of two questions about the subjective recognition of cough frequency during the day- and night-time, which is a simple, short, and practical tool. Each question score ranges from 0 to 5, and the total score ranges from 0 (no cough at all) to 10 (most severe cough) (23). It has been adapted and widely used in research area in many countries after translation and/or amendment, such as Korea and China (180,181). The Korean CSS (K-CSS) correlated well with other subjective approaches with high repeatability (ICC =0.75) and significantly responded to treatment. But the internal consistency was low (0.0006), given the weak correlation between day and night time scores, which also reflected its effectiveness as most patients present mild nocturnal coughs (180). In China, a simplified Cough Symptom Score (sCSS) is adopted, of which each question score ranges from 0 to 3, and the total scores are 0 to 6. The sCSS was highly correlated with CSS (day: r=0.82; night: r=0.92), with high repeatability over 3 days (day: ICC =0.90; night: ICC =0.89), and could be a good responsive outcome [effect size (ES): 0.52–1.16 for day and 0.71–1.09 for night, 1–2 weeks post-treatment] (181).
Cough Severity Index (CSI)
The CSI comprises 10 simplified questions statistically developed from a 49-item cough-specific questionnaire. It has been validated and turned out to be a responsive outcome measure with high internal consistency (0.928) and test-retest repeatability (r=0.83). A moderate correlation was found between CSI and the cough quality of life questionnaire (CQLQ) (r=0.60) (182). CSI has been used as an outcome to evaluate the treatment efficacy in several cough studies (65,190).
Cough Severity Diary (CSD)
The CSD is a seven-item daily diary, self-rated by patients along three dimensions: frequency (three items), intensity (two items), and disruptiveness (two items). CSD is measured on an 11-point scale and higher scores indicate greater severity (191,192). The performance properties of CSD were preliminarily assessed in a small sample size of CC or subacute patients, which demonstrated a high internal consistency (α=0.89 to 0.96), good reproducibility (ICC =0.68 to 0.94) and moderate-to-strong correlation with LCQ (r=−0.62) and VAS (r=0.84). CSD scores in subacute patients also showed significant responsiveness (192). A larger validation study included 253 RCC patients (183), which reported a high internal consistency (α=0.89), moderate test–retest reliability (ICC =0.68) and meaningful responsiveness of CSD scores. The relationships between CSD with LCQ and VAS were the same as that in the preliminary validation study. A threshold of ≥1.3-point reduction on the total and subscale scores was appropriate to define MCID. CSD was later evaluated and utilised in investigating the antitussive efficacy of gefapixant, and was proven to be a meaningful endpoint (63,193,194).
Multidimensional Cough Index (MCI)
The MCI is an easy-to-use scale composed of nine items covering cough intensity, frequency, physical impact, psychosocial impacts, and sputum characteristics. The first four components were scaled with a range of 0 to 20. The performance of MCI has been validated and showed strong enough psychometric and validating properties: Cronbach’s α=0.819, ICC =0.779, significantly correlated with VASfrequency (r=0.651), VASintensity (r=0.543), and LCQ (r=−0.824). A Cough Index ≥4 could classify patients from healthy subjects, with a sensitivity of 80% and a specificity of 85% (184).
Cough Evaluation Test (CET)
The CET is a newly developed, short, simple, patient-completed 5-item test that involves the dimensions of the physical, social and psychological effect. Each item is scaled using a 1–5 points Likert scale. CET has been verified as a reliable, valid, and responsive tool to evaluate cough with a high internal consistency (Cronbach’s α=0.80) and repeatability (ICC =0.84). The MCID was 2. CET also highly correlated with VAS (r=0.70) (185).
McMaster cough severity questionnaire
The McMaster cough severity questionnaire is a newly developed cough symptom severity instrument for RCC patients and provided 43 items addressing the following domains: urge-to-cough sensations (subdomains: frequency and intensity) and cough symptom (subdomains: frequency, control, bout duration, intensity, and associated features/sequelae) (186,195). This currently is a conceptual framework. Further studies are needed to simplify this questionnaire and address items and domains that are most important to RCC patients.
Cough-specific QoL questionnaires (Table 3)
Table 3
| Tools | Items/features | Validity | Reliability | Responsiveness | |
|---|---|---|---|---|---|
| Internal consistency | Repeatability | ||||
| LCQ (86,196,197) | A 19-item QoL questionnaire involving the physical, psychological and social domains. Higher scores indicate better QoL | Strongly correlated with cough VAS (r=−0.72); moderately negatively correlated with cough frequency in a real-world study (r=−0.430); poorly correlated with C2 (r=0.32) | Cronbach’s α: varied within domains from 0.79 to 0.89 | ICC: 0.88 to 0.96 | ES: 0.84 to 1.75; MCID: 1.3 |
| CQLQ (198-200) | A 28-items QoL mentioning 6 domains (physical complaints, extreme physical complaints, psychosocial issues, emotional well-being, personal safety fears, and functional abilities). Lower scores indicate better QoL | Significantly correlated with cough VAS (r=0.63) and LCQ (r=−0.42 to −0.60) | Cronbach’s α =0.92 for the total CQLQ; and 0.62 to 0.86 for the six subscales | ICC: 0.89 for total CQLQ; and 0.75 to 0.93 for the subscales | MCID: 21.89±15.38 of a total 112 points |
| CCIQ (201) | A 16-item questionnaire with 4-dimensional structure (sleep/concentration, social relationship, mood and daily life impact) | Poorly correlated with SF-36 | Highly satisfactory levels of internal consistency for sleep/concentration and relationship, and acceptable levels for daily life impact and mood | R ranged between 0.67 and 0.88 | After treatment, statistically significant differences were recorded in 16 of 21 items |
| COAT (202) | A short, patient-completed questionnaire comprises five items regarding cough frequency, daily activity, sleep disturbance, fatigue and cough hypersensitivity | Well correlated with LCQ (r=−0.71 to −0.81) and NRS (r=0.62–0.82) | Item-to-total correlations =0.71 to 0.84; item-to-item correlations =0.32 to 0.67 | ICC: 0.88 | MCID: 2.0 of 20 |
PROs, patients reported outcomes; QoL, quality of life; LCQ, Leicester Cough Questionnaire; VAS, Visual Analogue Scale; C2, the first administered concentration of tussive stimuli causing two coughs; ICC, intraclass correlation coefficient; ES, effect size; MCID, minimum clinically important difference; CQLQ, Cough Quality of Life Questionnaire; CCIQ, Chronic Cough Impact Questionnaire; SF-36, 36-Item Short Form Health Survey; COAT, Cough Assessment Test; NRS, numeric rating scale.
LCQ
The LCQ comprises 19 items divided into three domains (physical, psychological, and social). Each item score ranges from 1 to 7. Higher scores indicate better QoL. In CC, LCQ had good internal reliability (Cronbach’s α=0.79 to 0.89) and high repeatability (ICC for domains varying between 0.88 and 0.96). It was also responsive to successful treatment of cough (ES for domains =0.84 to 1.75) and strongly correlated with VAS (r=−0.72) (196). Cough frequency in CC patients correlated significantly with LCQ scores (4,5,47,109). The total LCQ score has an established MCID of 1.3 points, which for domains were 0.2 for physical, 0.2 for social 0.2, and 0.8 for psychological (197).
LCQ has been the most widely used of all cough-related QoL questionnaires and was recommended by ERS cough guidelines [2020] (93). It was translated into a wide range of languages (https://www.physio-pedia.com/Leicester_Cough_Questionnaire). It is now routinely used in assessing the overall efficacy of interventions in cough, including PSALT (131), behavioural cough suppression therapy (BCST) (203,204), proton pump inhibitors (PPIs) (205), and neuromodulators (206-209). It also experienced robust validation and has been introduced to studies in other diseases, such as acute cough (125), asthma (122), COPD (210,211), CF (212), IPF (23), and bronchiectasis (127).
CQLQ
The CQLQ was also recommended by ERS cough guidelines [2020] (93), consisting of 28 items with six domains including physical complaints, psychosocial issues, functional abilities, emotional well-being, extreme physical complaints, and personal safety fears. Each item is scored on a 4-point Likert-type response scale. Lower scores indicate better QoL (198). It’s a valid and reliable tool to assess the QoL in CC and acute cough (Cronbach’s α=0.92, item-to-total correlations =−0.06 to 0.72, ICC =0.89) and responsive to successful treatment (199). In CC, the correlation between CQLQ and LCQ was moderate (r=−0.42) before treatment and high after treatment (r=−0.60). The concurrent validity of LCQ was higher but the ES was slightly lower than CQLQ (213,214), the mean MCID of the CQLQ was 21.89±15.38 out of 112 points (198). CQLQ has also been validated in COPD and IPF (46,200,215).
Chronic Cough Impact Questionnaire (CCIQ)
The CCIQ comprises 16 items addressing four dimensions: sleep/concentration, social relationship, mood, and daily life impact. Each item is self-evaluated on a 5-point Likert scale. Numeric values for responses to the CCIQ items are converted to 0–100 points, with 100 reflecting the worse health-related QoL (216). CCIQ was a valid tool for CC, with satisfactory internal consistency (Cronbach’s α for sleep/concentration was 79.98, 86.98 for relationship, 65.41 for mood, and 69.04 for daily life impact), good reliability (r=0.67 to 0.88) and responsiveness to treatment improvement (201).
COugh Assessment Test (COAT)
The COAT is a short, patient-completed questionnaire comprising five items regarding cough frequency, daily activity, sleep disturbance, fatigue, and cough hypersensitivity. Each item scores on a 0–4 scale and higher scores reflect worse QoL. COAT has been validated in Korean population and showed good repeatability, reliability and validity, with clear discriminative properties (ICC: 0.88, item-to-total correlations =0.71 to 0.84, item-to-item correlations =0.32 to 0.67, correlations with Korean LCQ =−0.71 to −0.81, MCID =2.0) (202,217).
Conclusions and prospects
Each of the available assessment tools have strengths and weaknesses. Cough frequency monitors are suitable as the primary outcome of clinical trials and currently appear to be effective, however, beyond objective frequency reduction, subjective improvement in patient perception is also key to assessing treatment effect. Therefore, cough counts should always be complemented by the subjective assessment of QoL. Further exploration is needed to select the best combination of cough assessments for a specific clinical scenario. Indeed, a composite endpoint of objective and subjective measures may be the ideal metric to give a global assessment of cough.
With the recent breakthroughs in objective cough detection technology, cough counting will be more accurate in the future and promises to be less obtrusive, labour intensive, and costly. These developments also open up the potential for diagnosis and monitoring of disease progression. The ongoing development of affordable mobile smartphone apps and wearable devices offers the possibility of transferring the detection technology into routine clinical practice.
Acknowledgments
Funding: This study was supported by the China Scholarship Council (No. 202006260287) and University of Hull PhD scholarship.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Woo-Jung Song and Kian Fan Chung) for the series “Novel Insights into Chronic Cough” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-874/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-874/coif). The series “Novel Insights into Chronic Cough” was commissioned by the editorial office without any funding or sponsorship. AHM serves as an unpaid editorial board member of Journal of Thoracic Disease. MZ was funded by the China Scholarship Council (No. 202006260287) and University of Hull PhD scholarship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hall JI, Lozano M, Estrada-Petrocelli L, et al. The present and future of cough counting tools. J Thorac Dis 2020;12:5207-23. [Crossref] [PubMed]
- Spinou A, Birring SS. An update on measurement and monitoring of cough: what are the important study endpoints? J Thorac Dis 2014;6:S728-34. [Crossref] [PubMed]
- Wang Z, Wang M, Wen S, et al. Types and applications of cough-related questionnaires. J Thorac Dis 2019;11:4379-88. [Crossref] [PubMed]
- Birring SS, Matos S, Patel RB, et al. Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir Med 2006;100:1105-9. [Crossref] [PubMed]
- Faruqi S, Thompson R, Wright C, et al. Quantifying chronic cough: objective versus subjective measurements. Respirology 2011;16:314-20. [Crossref] [PubMed]
- Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007;29:1256-76. [Crossref] [PubMed]
- Cho PSP, Birring SS, Fletcher HV, et al. Methods of Cough Assessment. J Allergy Clin Immunol Pract 2019;7:1715-23. [Crossref] [PubMed]
- Mai Y, Fang L, Zhong S, et al. Methods for assessing cough sensitivity. J Thorac Dis 2020;12:5224-37. [Crossref] [PubMed]
- Mei H, Gu W, Ran L, et al. Evaluation methods and influencing factors of cough sensitivity. Ther Adv Respir Dis 2022;16:17534666211070134. [Crossref] [PubMed]
- Morice AH, Faruqi S, Wright CE, et al. Cough hypersensitivity syndrome: a distinct clinical entity. Lung 2011;189:73-9. [Crossref] [PubMed]
- Mazzone SB, McGarvey L. Mechanisms and Rationale for Targeted Therapies in Refractory and Unexplained Chronic Cough. Clin Pharmacol Ther 2021;109:619-36. [Crossref] [PubMed]
- Sykes DL, Zhang M, Morice AH. Treatment of chronic cough: P2X3 receptor antagonists and beyond. Pharmacol Ther 2022;237:108166. [Crossref] [PubMed]
- Mazzone SB, Farrell MJ. Heterogeneity of cough neurobiology: Clinical implications. Pulm Pharmacol Ther 2019;55:62-6. [Crossref] [PubMed]
- Mazzone SB, McGovern AE, Farrell MJ. Endogenous central suppressive mechanisms regulating cough as potential targets for novel antitussive therapies. Curr Opin Pharmacol 2015;22:1-8. [Crossref] [PubMed]
- Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 2006;152:223-42. [Crossref] [PubMed]
- Demoulin B, Coutier-Marie L, Ioan I, et al. In Vivo Documentation of Stimulus Velocity Tuning of Mechanically Induced Reflex Cough. Physiol Res 2020;69:S139-45. [Crossref] [PubMed]
- Basin S, Valentin S, Demoulin-Alexikova S, et al. Impact of Inhaled Corticosteroids on the Modulation of Respiratory Defensive Reflexes During Artificial Limb Exercise in Ovalbumin-Sensitized Rabbits. Front Physiol 2021;12:804577. [Crossref] [PubMed]
- Shen TY, Pertzborn MC, Rose MJ, et al. Influence of intrathoracic vagotomy on the cough reflex in the anesthetized cat. Respir Physiol Neurobiol 2022;296:103805. [Crossref] [PubMed]
- Widdicombe JG. Receptors in the trachea and bronchi of the cat. J Physiol 1954;123:71-104. [Crossref] [PubMed]
- Tedeschi RE, Tedeschi DH, Hitchens JT, et al. A new antitussive method involving mechanical stimulation in unanesthetized dogs. J Pharmacol Exp Ther 1959;126:338-44.
- Lee P, Eccles R. Cough induced by mechanical stimulation of the upper airway in humans. Acta Otolaryngol 2004;124:720-5. [Crossref] [PubMed]
- Lee PC, Eccles R. Cough induction by high-frequency chest percussion in healthy volunteers and patients with common cold. Respir Med 2004;98:771-6. [Crossref] [PubMed]
- Jones RM, Hilldrup S, Hope-Gill BD, et al. Mechanical induction of cough in Idiopathic Pulmonary Fibrosis. Cough 2011;7:2. [Crossref] [PubMed]
- Kamimura M, Mouri A, Takayama K, et al. Cough challenge tests involving mechanical stimulation of the cervical trachea in patients with cough as a leading symptom. Respirology 2010;15:1244-51. [Crossref] [PubMed]
- Satia I, Tsamandouras N, Holt K, et al. Capsaicin-evoked cough responses in asthmatic patients: Evidence for airway neuronal dysfunction. J Allergy Clin Immunol 2017;139:771-779.e10. [Crossref] [PubMed]
- Xu X, Chen Q, Qiu Z, et al. Association of cough hypersensitivity with tracheal TRPV1 activation and neurogenic inflammation in a novel guinea pig model of citric acid-induced chronic cough. J Int Med Res 2018;46:2913-24. [Crossref] [PubMed]
- Liviero F, Scarpa MC, De Stefani D, et al. Modulation of TRPV-1 by prostaglandin-E2 and bradykinin changes cough sensitivity and autonomic regulation of cardiac rhythm in healthy subjects. Sci Rep 2020;10:15163. [Crossref] [PubMed]
- Long L, Yao H, Tian J, et al. Heterogeneity of cough hypersensitivity mediated by TRPV1 and TRPA1 in patients with chronic refractory cough. Respir Res 2019;20:112. [Crossref] [PubMed]
- Liu C, Chen M, Dong P. Establishment and evaluation of a new measurement of cough reflex sensitivity: Cough provocation by agonist of TRPA1 (cinnamaldehyde). Eur Respir J 2014;44:579.
- Gu QD, Moss CR 2nd, Kettelhut KL, et al. Activation of TRPV4 Regulates Respiration through Indirect Activation of Bronchopulmonary Sensory Neurons. Front Physiol 2016;7:65. [Crossref] [PubMed]
- Morice AH, Kitt MM, Ford AP, et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J 2019;54:1900439. [Crossref] [PubMed]
- Bonvini SJ, Belvisi MG. Cough and airway disease: The role of ion channels. Pulm Pharmacol Ther 2017;47:21-8. [Crossref] [PubMed]
- McGarvey LP, Birring SS, Morice AH, et al. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022;399:909-23. [Crossref] [PubMed]
- Bickerman HA, Barach AL. The experimental production of cough in human subjects induced by citric acid aerosols; preliminary studies on the evaluation of antitussive agents. Am J Med Sci 1954;228:156-63. [Crossref] [PubMed]
- Dicpinigaitis PV, Alva RV. Safety of capsaicin cough challenge testing. Chest 2005;128:196-202. [Crossref] [PubMed]
- Dicpinigaitis PV. Short- and long-term reproducibility of capsaicin cough challenge testing. Pulm Pharmacol Ther 2003;16:61-5. [Crossref] [PubMed]
- Wright CE, Jackson J, Thompson RL, et al. Validation of the ERS standard citric acid cough challenge in healthy adult volunteers. Cough 2010;6:8. [Crossref] [PubMed]
- Wang R, Fowler SJ, Niven R, et al. Investigating the safety of capsaicin cough challenge in severe asthma. Clin Exp Allergy 2019;49:932-4. [Crossref] [PubMed]
- Morice AH, Kastelik JA, Thompson R. Cough challenge in the assessment of cough reflex. Br J Clin Pharmacol 2001;52:365-75. [Crossref] [PubMed]
- Prudon B, Birring SS, Vara DD, et al. Cough and glottic-stop reflex sensitivity in health and disease. Chest 2005;127:550-7. [Crossref] [PubMed]
- Yousaf N, Monteiro W, Matos S, et al. Cough frequency in health and disease. Eur Respir J 2013;41:241-3. [Crossref] [PubMed]
- Hilton EC, Baverel PG, Woodcock A, et al. Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J Allergy Clin Immunol 2013;132:847-55.e1-5.
- King J, Wingfield Digby J, Satia I. Is there clinical value in performing capsaicin cough challenges in patients with severe asthma? Breathe (Sheff) 2021;17:210034. [Crossref] [PubMed]
- Satia I, Watson R, Scime T, et al. Allergen challenge increases capsaicin-evoked cough responses in patients with allergic asthma. J Allergy Clin Immunol 2019;144:788-795.e1. [Crossref] [PubMed]
- Satia I, Badri H, Woodhead M, et al. The interaction between bronchoconstriction and cough in asthma. Thorax 2017;72:1144-6. [Crossref] [PubMed]
- Smith J, Owen E, Earis J, et al. Cough in COPD: correlation of objective monitoring with cough challenge and subjective assessments. Chest 2006;130:379-85. [Crossref] [PubMed]
- Decalmer SC, Webster D, Kelsall AA, et al. Chronic cough: how do cough reflex sensitivity and subjective assessments correlate with objective cough counts during ambulatory monitoring? Thorax 2007;62:329-34. [Crossref] [PubMed]
- Sun J, Zhan C, Deng Z, et al. Expression of interferon-γ and its effect on cough hypersensitivity in chronic refractory cough patients. Thorax 2022;77:621-4. [Crossref] [PubMed]
- Morice AH, Kitt M, Ford A, et al. The effect of MK-7264, a P2X3 antagonist, on cough reflex sensitivity in a randomized crossover trial of healthy and chronic cough subjects. Eur Respir J 2017;50:OA2931.
- Belvisi MG, Birrell MA, Wortley MA, et al. XEN-D0501, a Novel Transient Receptor Potential Vanilloid 1 Antagonist, Does Not Reduce Cough in Patients with Refractory Cough. Am J Respir Crit Care Med 2017;196:1255-63. [Crossref] [PubMed]
- Khalid S, Murdoch R, Newlands A, et al. Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol 2014;134:56-62. [Crossref] [PubMed]
- Zhang L, Sun T, Liu L, et al. The research of the possible mechanism and the treatment for capsaicin-induced cough. Pulm Pharmacol Ther 2018;49:1-9. [Crossref] [PubMed]
- Butt MF, Albusoda A, Farmer AD, et al. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat 2020;236:588-611. [Crossref] [PubMed]
- Dicpinigaitis PV, Kantar A, Enilari O, et al. Prevalence of Arnold Nerve Reflex in Adults and Children With Chronic Cough. Chest 2018;153:675-9. [Crossref] [PubMed]
- Dicpinigaitis PV, Enilari O, Cleven KL. Prevalence of Arnold nerve reflex in subjects with and without chronic cough: Relevance to Cough Hypersensitivity Syndrome. Pulm Pharmacol Ther 2019;54:22-4. [Crossref] [PubMed]
- Castro RA, Zalvan CH, Berzofsky C. Oto-tricho-tussia: An Unexpected Cause of Cough. Case Rep Otolaryngol 2020;2020:3527481. [Crossref] [PubMed]
- Jinnouchi O, Ohnishi H, Kondo E, et al. Aural stimulation with capsaicin prevented pneumonia in dementia patients. Auris Nasus Larynx 2020;47:154-7. [Crossref] [PubMed]
- Ohnishi H, Jinnouchi O, Agawa S, et al. Daily auricular stimulation with capsaicin ointment improved cough reflex sensitivity in elderly patients with dysphagia: a pilot study. Acta Otolaryngol 2020;140:249-53. [Crossref] [PubMed]
- Irwin RS. When Evaluating Patients With Chronic Cough, Should Clinicians Routinely Test the Arnold Nerve Reflex, Look in the Ears, or Do Both? Chest 2020;158:19-20. [Crossref] [PubMed]
- Mai Y, Zhan C, Zhang S, et al. Arnold Nerve Reflex: Vagal Hypersensitivity in Chronic Cough With Various Causes. Chest 2020;158:264-71. [Crossref] [PubMed]
- Ahmad SR, Iyer VN. The Evolving Clinical Practice of Chronic Cough. Mayo Clin Proc 2022;97:1164-75. [Crossref] [PubMed]
- Famokunwa B, Walsted ES, Hull JH. Assessing laryngeal function and hypersensitivity. Pulm Pharmacol Ther 2019;56:108-15. [Crossref] [PubMed]
- Morice AH, Birring SS, Smith JA, et al. Characterization of Patients With Refractory or Unexplained Chronic Cough Participating in a Phase 2 Clinical Trial of the P2X3-Receptor Antagonist Gefapixant. Lung 2021;199:121-9. [Crossref] [PubMed]
- Sasieta HC, Iyer VN, Orbelo DM, et al. Bilateral Thyroarytenoid Botulinum Toxin Type A Injection for the Treatment of Refractory Chronic Cough. JAMA Otolaryngol Head Neck Surg 2016;142:881-8. [Crossref] [PubMed]
- Simpson CB, Tibbetts KM, Loochtan MJ, et al. Treatment of chronic neurogenic cough with in-office superior laryngeal nerve block. Laryngoscope 2018;128:1898-903. [Crossref] [PubMed]
- Phua SY, McGarvey L, Ngu M, et al. The differential effect of gastroesophageal reflux disease on mechanostimulation and chemostimulation of the laryngopharynx. Chest 2010;138:1180-5. [Crossref] [PubMed]
- Phua SY, McGarvey LP, Ngu MC, et al. Patients with gastro-oesophageal reflux disease and cough have impaired laryngopharyngeal mechanosensitivity. Thorax 2005;60:488-91. [Crossref] [PubMed]
- Keller JA, McGovern AE, Mazzone SB. Translating Cough Mechanisms Into Better Cough Suppressants. Chest 2017;152:833-41. [Crossref] [PubMed]
- Hutchings HA, Morris S, Eccles R, et al. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med 1993;87:379-82. [Crossref] [PubMed]
- Cho PSP, Fletcher HV, Turner RD, et al. Impaired cough suppression in chronic refractory cough. Eur Respir J 2019;53:1802203. [Crossref] [PubMed]
- Cho PSP, Fletcher HV, Patel IS, et al. Cough hypersensitivity and suppression in COPD. Eur Respir J 2021;57:2003569. [Crossref] [PubMed]
- Wallace E, Guiu Hernandez E, Ang A, et al. Quantifying test-retest variability of natural and suppressed citric acid cough thresholds and urge to cough ratings. Pulm Pharmacol Ther 2019;58:101838. [Crossref] [PubMed]
- Loued-Khenissi L, Döll O, Preuschoff K. An Overview of Functional Magnetic Resonance Imaging Techniques for Organizational Research. Organ Res Methods 2019;22:17-45.
- Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin N Am 2011;22:133-9. vii. [Crossref] [PubMed]
- Mazzone SB, McLennan L, McGovern AE, et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med 2007;176:327-32. [Crossref] [PubMed]
- Mazzone SB, McGovern AE, Koo K, et al. Mapping supramedullary pathways involved in cough using functional brain imaging: comparison with pain. Pulm Pharmacol Ther 2009;22:90-6. [Crossref] [PubMed]
- Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 2014;44:1149-55. [Crossref] [PubMed]
- Mazzone SB, Cole LJ, Ando A, et al. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci 2011;31:2948-58. [Crossref] [PubMed]
- Farrell MJ, Cole LJ, Chiapoco D, et al. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. Neuroimage 2012;61:1324-35. [Crossref] [PubMed]
- Farrell MJ, Koch S, Ando A, et al. Functionally connected brain regions in the network activated during capsaicin inhalation. Hum Brain Mapp 2014;35:5341-55. [Crossref] [PubMed]
- Leech J, Mazzone SB, Farrell MJ. Brain activity associated with placebo suppression of the urge-to-cough in humans. Am J Respir Crit Care Med 2013;188:1069-75. [Crossref] [PubMed]
- Ando A, Smallwood D, McMahon M, et al. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax 2016;71:323-9. [Crossref] [PubMed]
- Ando A, Mazzone SB, Farrell MJ. Altered neural activity in brain cough suppression networks in cigarette smokers. Eur Respir J 2019;54:1900362. [Crossref] [PubMed]
- Namgung E, Song WJ, Kim YH, et al. Structural and Functional Correlates of Higher Cortical Brain Regions in Chronic Refractory Cough. Chest 2022;162:851-60. [Crossref] [PubMed]
- Vertigan AE, Bone SL, Gibson PG. Development and validation of the Newcastle laryngeal hypersensitivity questionnaire. Cough 2014;10:1. [Crossref] [PubMed]
- Vertigan AE, Kapela SL, Birring SS, et al. Feasibility and clinical utility of ambulatory cough monitoring in an outpatient clinical setting: a real-world retrospective evaluation. ERJ Open Res 2021;7:e00319-2021. [Crossref] [PubMed]
- Sinha A, Lee KK, Rafferty GF, et al. Predictors of objective cough frequency in pulmonary sarcoidosis. Eur Respir J 2016;47:1461-71. [Crossref] [PubMed]
- Nordin S, Millqvist E, Löwhagen O, et al. A short Chemical Sensitivity Scale for assessment of airway sensory hyperreactivity. Int Arch Occup Environ Health 2004;77:249-54. [Crossref] [PubMed]
- Johansson A, Millqvist E, Nordin S, et al. Relationship between self-reported odor intolerance and sensitivity to inhaled capsaicin: proposed definition of airway sensory hyperreactivity and estimation of its prevalence. Chest 2006;129:1623-8. [Crossref] [PubMed]
- Sykes DL, Morice AH. The Cough Reflex: The Janus of Respiratory Medicine. Front Physiol 2021;12:684080. [Crossref] [PubMed]
- Park JS, Burton L, Van der Wall H, et al. Modified Reflux Scintigraphy Detects Pulmonary Microaspiration in Severe Gastro-Esophageal and Laryngopharyngeal Reflux Disease. Lung 2021;199:139-45. [Crossref] [PubMed]
- Morice AH. On Chronic Cough Diagnosis, Classification, and Treatment. Lung 2021;199:433-4. [Crossref] [PubMed]
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020;55:1901136. [Crossref] [PubMed]
- Zhang M, Chen Q, Dong R, et al. Prediction of therapeutic efficacy of gabapentin by Hull Airway Reflux Questionnaire in chronic refractory cough. Ther Adv Chronic Dis 2020;11:2040622320982463. [Crossref] [PubMed]
- Morice A, Birring S, Dicpinigaitis P, et al. Cough Triggers and Symptoms Among Patients with Refractory or Unexplained Chronic Cough in Two Phase 3 Trials of the P2X3 Receptor Antagonist Gefapixant (COUGH-1 and COUGH-2). J Allergy Clin Immunol 2021;147:AB61.
- La-Crette J, Lee K, Chamberlain S, et al. P150 The Development of a Cough Hypersensitivity Questionnaire (CHQ). Thorax 2012;67:A127.
- Won HK, Kang SY, Kang Y, et al. Cough-Related Laryngeal Sensations and Triggers in Adults With Chronic Cough: Symptom Profile and Impact. Allergy Asthma Immunol Res 2019;11:622-31. [Crossref] [PubMed]
- Gibson PG, Vertigan AE. Gabapentin in chronic cough. Pulm Pharmacol Ther 2015;35:145-8. [Crossref] [PubMed]
- Nordin S, Palmquist E, Bende M, et al. Normative data for the chemical sensitivity scale for sensory hyperreactivity: the Västerbotten environmental health study. Int Arch Occup Environ Health 2013;86:749-53. [Crossref] [PubMed]
- Farrell MJ, Mazzone SB. Are neural pathways processing airway inputs sensitized in patients with cough hypersensitivity? Pulm Pharmacol Ther 2019;57:101806. [Crossref] [PubMed]
- Dicpinigaitis PV, Rhoton WA, Bhat R, et al. Investigation of the urge-to-cough sensation in healthy volunteers. Respirology 2012;17:337-41. [Crossref] [PubMed]
- Hilton E, Marsden P, Thurston A, et al. Clinical features of the urge-to-cough in patients with chronic cough. Respir Med 2015;109:701-7. [Crossref] [PubMed]
- Janssens T, Silva M, Davenport PW, et al. Attentional modulation of reflex cough. Chest 2014;146:135-41. [Crossref] [PubMed]
- Smith JA, Owen EC, Jones AM, et al. Objective measurement of cough during pulmonary exacerbations in adults with cystic fibrosis. Thorax 2006;61:425-9. [Crossref] [PubMed]
- Windmon A, Minakshi M, Bharti P, et al. TussisWatch: A Smart-Phone System to Identify Cough Episodes as Early Symptoms of Chronic Obstructive Pulmonary Disease and Congestive Heart Failure. IEEE J Biomed Health Inform 2019;23:1566-73. [Crossref] [PubMed]
- Crooks MG, den Brinker AC, Thackray-Nocera S, et al. Domiciliary Cough Monitoring for the Prediction of COPD Exacerbations. Lung 2021;199:131-7. [Crossref] [PubMed]
- den Brinker AC, van Dinther R, Crooks MG, et al. Alert system design based on experimental findings from long-term unobtrusive monitoring in COPD. Biomedical Signal Processing and Control 2021;63:102205.
- Fontana GA, Widdicombe J. What is cough and what should be measured? Pulm Pharmacol Ther 2007;20:307-12. [Crossref] [PubMed]
- Kelsall A, Decalmer S, Webster D, et al. How to quantify coughing: correlations with quality of life in chronic cough. Eur Respir J 2008;32:175-9. [Crossref] [PubMed]
- Urban C, Kiefer A, Conradt R, et al. Validation of the LEOSound(R) monitor for standardized detection of wheezing and cough in children. Pediatr Pulmonol 2022;57:551-9. [Crossref] [PubMed]
- Lee GO, Comina G, Hernandez-Cordova G, et al. Cough dynamics in adults receiving tuberculosis treatment. PLoS One 2020;15:e0231167. [Crossref] [PubMed]
- Radine A, Werner C, Raidt J, et al. Comparison of Nocturnal Cough Analysis in Healthy Subjects and in Patients with Cystic Fibrosis and Primary Ciliary Dyskinesia: A Prospective Observational Study. Respiration 2019;97:60-9. [Crossref] [PubMed]
- Badri H, Gibbard C, Denton D, et al. A double-blind randomised placebo-controlled trial investigating the effects of lesogaberan on the objective cough frequency and capsaicin-evoked coughs in patients with refractory chronic cough. ERJ Open Res 2022;8:e00546-2021. [Crossref] [PubMed]
- Turner RD, Bothamley GH. How to count coughs? Counting by ear, the effect of visual data and the evaluation of an automated cough monitor. Respir Med 2014;108:1808-15. [Crossref] [PubMed]
- Smith JA, Earis JE, Woodcock AA. Establishing a gold standard for manual cough counting: video versus digital audio recordings. Cough 2006;2:6. [Crossref] [PubMed]
- Pavesi L, Subburaj S, Porter-Shaw K. Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta-analysis. Chest 2001;120:1121-8. [Crossref] [PubMed]
- Lee KK, Birring SS. Cough and sleep. Lung 2010;188:S91-4. [Crossref] [PubMed]
- Birring SS, Fleming T, Matos S, et al. The Leicester Cough Monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J 2008;31:1013-8. [Crossref] [PubMed]
- McGuinness K, Morice A, Woodcock A, et al. The Leicester Cough Monitor: a semi-automated, semi-validated cough detection system? Eur Respir J 2008;32:529-30; author reply 530-1. [Crossref] [PubMed]
- Cho PSP, Shearer J, Simpson A, et al. Healthcare utilization and costs in chronic cough. Curr Med Res Opin 2022;38:1251-7. [Crossref] [PubMed]
- Jackson AR, Hull JH, Hopker JG, et al. The impact of a heat and moisture exchange mask on respiratory symptoms and airway response to exercise in asthma. ERJ Open Res 2020;6:e00271-2019. [Crossref] [PubMed]
- Fukuhara A, Saito J, Birring SS, et al. Clinical Characteristics of Cough Frequency Patterns in Patients with and without Asthma. J Allergy Clin Immunol Pract 2020;8:654-61. [Crossref] [PubMed]
- Vertigan AE, Kapela SL, Gibson PG. Chronic cough in Vocal Cord Dysfunction: Description of a clinical entity. Respir Med 2020;168:105990. [Crossref] [PubMed]
- Birring SS, Wijsenbeek MS, Agrawal S, et al. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir Med 2017;5:806-15. [Crossref] [PubMed]
- Lee KK, Matos S, Evans DH, et al. A longitudinal assessment of acute cough. Am J Respir Crit Care Med 2013;187:991-7. [Crossref] [PubMed]
- Crooks MG, Hayman Y, Innes A, et al. Objective Measurement of Cough Frequency During COPD Exacerbation Convalescence. Lung 2016;194:117-20. [Crossref] [PubMed]
- Spinou A, Lee KK, Sinha A, et al. The Objective Assessment of Cough Frequency in Bronchiectasis. Lung 2017;195:575-85. [Crossref] [PubMed]
- Turner RD, Birring SS, Darmalingam M, et al. Daily cough frequency in tuberculosis and association with household infection. Int J Tuberc Lung Dis 2018;22:863-70. [Crossref] [PubMed]
- Williams CM, Abdulwhhab M, Birring SS, et al. Exhaled Mycobacterium tuberculosis output and detection of subclinical disease by face-mask sampling: prospective observational studies. Lancet Infect Dis 2020;20:607-17. [Crossref] [PubMed]
- Birring SS, Floyd S, Reilly CC, et al. Physiotherapy and Speech and Language therapy intervention for chronic cough. Pulm Pharmacol Ther 2017;47:84-7. [Crossref] [PubMed]
- Chamberlain Mitchell SA, Garrod R, Clark L, et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax 2017;72:129-36. [Crossref] [PubMed]
- Slinger C, Mehdi SB, Milan SJ, et al. Speech and language therapy for management of chronic cough. Cochrane Database Syst Rev 2019;7:CD013067. [Crossref] [PubMed]
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [Crossref] [PubMed]
- Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and Speech Pathology Combination Therapy for Refractory Chronic Cough: A Randomized Controlled Trial. Chest 2016;149:639-48. [Crossref] [PubMed]
- Smith JA, Holt K, Dockry R, et al. Performance of a digital signal processing algorithm for the accurate quantification of cough frequency. Eur Respir J 2021;58:2004271. [Crossref] [PubMed]
- Barton A, Gaydecki P, Holt K, et al. Data reduction for cough studies using distribution of audio frequency content. Cough 2012;8:12. [Crossref] [PubMed]
- McGuinness K, Holt K, Dockry R, et al. P159 Validation of the VitaloJAK™ 24 Hour Ambulatory Cough Monitor. Thorax 2012;67:A131.1.
- Smith JA, McGarvey LPA, Badri H, et al. Effects of a novel sodium channel blocker, GSK2339345, in patients with refractory chronic cough . Int J Clin Pharmacol Ther 2017;55:712-9. [Crossref] [PubMed]
- Ludbrook VJ, Hanrott KE, Kreindler JL, et al. Adaptive study design to assess effect of TRPV4 inhibition in patients with chronic cough. ERJ Open Res 2021;7:e00269-2021. [Crossref] [PubMed]
- Morice A, Smith JA, McGarvey L, et al. Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study. Eur Respir J 2021;58:2004240. [Crossref] [PubMed]
- Muccino DR, Morice AH, Birring SS, et al. Design and rationale of two phase 3 randomised controlled trials (COUGH-1 and COUGH-2) of gefapixant, a P2X3 receptor antagonist, in refractory or unexplained chronic cough. ERJ Open Res 2020;6:e00284-2020. [Crossref] [PubMed]
- Singh D, Beier J, Astbury C, et al. The novel bronchodilator navafenterol: a phase 2a, multicentre, randomised, double-blind, placebo-controlled crossover trial in COPD. Eur Respir J 2022;59:2100972. [Crossref] [PubMed]
- Key AL, Holt K, Hamilton A, et al. Objective cough frequency in Idiopathic Pulmonary Fibrosis. Cough 2010;6:4. [Crossref] [PubMed]
- Marsden PA, Satia I, Ibrahim B, et al. Objective Cough Frequency, Airway Inflammation, and Disease Control in Asthma. Chest 2016;149:1460-6. [Crossref] [PubMed]
- Barry SJ, Dane AD, Morice AH, et al. The automatic recognition and counting of cough. Cough 2006;2:8. [Crossref] [PubMed]
- Crooks MG, den Brinker A, Hayman Y, et al. Continuous Cough Monitoring Using Ambient Sound Recording During Convalescence from a COPD Exacerbation. Lung 2017;195:289-94. [Crossref] [PubMed]
- Zhang M, Brindle K, Robinson M, et al. Chronic cough in cystic fibrosis: the effect of modulator therapy on objective 24-h cough monitoring. ERJ Open Res 2022;8:00031-2022. [Crossref] [PubMed]
- Proaño A, Bravard MA, Tracey BH, et al. Protocol for studying cough frequency in people with pulmonary tuberculosis. BMJ Open 2016;6:e010365. [Crossref] [PubMed]
- Larson S, Comina G, Gilman RH, et al. Validation of an automated cough detection algorithm for tracking recovery of pulmonary tuberculosis patients. PLoS One 2012;7:e46229. [Crossref] [PubMed]
- Proaño A, Bravard MA, López JW, et al. Dynamics of Cough Frequency in Adults Undergoing Treatment for Pulmonary Tuberculosis. Clin Infect Dis 2017;64:1174-81. [Crossref] [PubMed]
- Proaño A, Bui DP, López JW, et al. Cough Frequency During Treatment Associated With Baseline Cavitary Volume and Proximity to the Airway in Pulmonary TB. Chest 2018;153:1358-67. [Crossref] [PubMed]
- Koehler U, Hildebrandt O, Weissflog A, et al. LEOSound - A new device for long-term recording of wheezing and cough in pediatric and adult patients with asthma (during sleep). Clinical Investigation 2018;8:103-7.
- Krönig J, Hildebrandt O, Weissflog A, et al. Long-term Recording of Night-Time Respiratory Symptoms in Patients with Stable COPD II-IV. COPD 2017;14:498-503. [Crossref] [PubMed]
- Fischer P, Gross V, Kroenig J, et al. Description of nighttime cough epochs in patients with stable COPD GOLD II-IV. Int J Chron Obstruct Pulmon Dis 2018;13:1071-8. [Crossref] [PubMed]
- Schwarz SB, Windisch W, Majorski DS, et al. Long-Term Auscultation in Chronic Obstructive Pulmonary Disease: Renaissance of an Ideograph of Medical Care. Respiration 2021; Epub ahead of print. [Crossref]
- Doenges J, Kuckuck E, Cassel W, et al. Disease control in patients with asthma and respiratory symptoms (wheezing, cough) during sleep. Asthma Res Pract 2020;6:9. [Crossref] [PubMed]
- Pekacka-Egli AM, Kazmierski R, Lutz D, et al. Predictive Value of Cough Frequency in Addition to Aspiration Risk for Increased Risk of Pneumonia in Dysphagic Stroke Survivors: A Clinical Pilot Study. Brain Sci 2021;11:847. [Crossref] [PubMed]
- Amiriparian S, Hübner T, Karas V, et al. DeepSpectrumLite: A Power-Efficient Transfer Learning Framework for Embedded Speech and Audio Processing From Decentralized Data. Front Artif Intell 2022;5:856232. [Crossref] [PubMed]
- Vedaei SS, Fotovvat A, Mohebbian MR, et al. COVID-SAFE: An IoT-Based System for Automated Health Monitoring and Surveillance in Post-Pandemic Life. IEEE Access 2020;8:188538-51.
- Pahar M, Klopper M, Warren R, et al. COVID-19 cough classification using machine learning and global smartphone recordings. Comput Biol Med 2021;135:104572. [Crossref] [PubMed]
- Mukhtar H, Rubaiee S, Krichen M, et al. An IoT Framework for Screening of COVID-19 Using Real-Time Data from Wearable Sensors. Int J Environ Res Public Health 2021;18:4022. [Crossref] [PubMed]
- Jiang W, Majumder S, Kumar S, et al. A Wearable Tele-Health System towards Monitoring COVID-19 and Chronic Diseases. IEEE Rev Biomed Eng 2022;15:61-84. [Crossref] [PubMed]
- Monge-Alvarez J, Hoyos-Barcelo C, Lesso P, et al. Effect of importance sampling on robust segmentation of audio-cough events in noisy environments. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:3740-4. [Crossref] [PubMed]
- Infante C, Chamberlain DB, Kodgule R, et al. Classification of voluntary coughs applied to the screening of respiratory disease. Annu Int Conf IEEE Eng Med Biol Soc 2017;2017:1413-6. [Crossref] [PubMed]
- Serrurier A, Neuschaefer-Rube C, Röhrig R. Past and Trends in Cough Sound Acquisition, Automatic Detection and Automatic Classification: A Comparative Review. Sensors (Basel) 2022;22:2896. [Crossref] [PubMed]
- Janjua S, Banchoff E, Threapleton CJ, et al. Digital interventions for the management of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2021;4:CD013246. [Crossref] [PubMed]
- Hoyos-Barceló C, Monge-Álvarez J, Pervez Z, et al. Efficient computation of image moments for robust cough detection using smartphones. Comput Biol Med 2018;100:176-85. [Crossref] [PubMed]
- Kvapilova L, Boza V, Dubec P, et al. Continuous Sound Collection Using Smartphones and Machine Learning to Measure Cough. Digit Biomark 2019;3:166-75. [Crossref] [PubMed]
- Nemati E, Rahman MM, Nathan V, et al. A Comprehensive Approach for Classification of the Cough Type. Annu Int Conf IEEE Eng Med Biol Soc 2020;2020:208-12. [Crossref] [PubMed]
- Claxton S, Porter P, Brisbane J, et al. Identifying acute exacerbations of chronic obstructive pulmonary disease using patient-reported symptoms and cough feature analysis. NPJ Digit Med 2021;4:107. [Crossref] [PubMed]
- Porter P, Claxton S, Brisbane J, et al. Diagnosing Chronic Obstructive Airway Disease on a Smartphone Using Patient-Reported Symptoms and Cough Analysis: Diagnostic Accuracy Study. JMIR Form Res 2020;4:e24587. [Crossref] [PubMed]
- Gabaldón-Figueira JC, Keen E, Rudd M, et al. Longitudinal passive cough monitoring and its implications for detecting changes in clinical status. ERJ Open Res 2022;8:e00001-2022. [Crossref] [PubMed]
- Rassouli F, Tinschert P, Barata F, et al. Characteristics of Asthma-related Nocturnal Cough: A Potential New Digital Biomarker. J Asthma Allergy 2020;13:649-57. [Crossref] [PubMed]
- Tinschert P, Rassouli F, Barata F, et al. Prevalence of nocturnal cough in asthma and its potential as a marker for asthma control (MAC) in combination with sleep quality: protocol of a smartphone-based, multicentre, longitudinal observational study with two stages. BMJ Open 2019;9:e026323. [Crossref] [PubMed]
- Barata F, Tinschert P, Rassouli F, et al. Automatic Recognition, Segmentation, and Sex Assignment of Nocturnal Asthmatic Coughs and Cough Epochs in Smartphone Audio Recordings: Observational Field Study. J Med Internet Res 2020;22:e18082. [Crossref] [PubMed]
- Porter P, Brisbane J, Abeyratne U, et al. A smartphone-based algorithm comprising cough analysis and patient-reported symptoms identifies acute exacerbations of asthma: a prospective, double blind, diagnostic accuracy study. J Asthma 2022; Epub ahead of print. [Crossref]
- Yates H, Adamali HI, Maskell N, et al. Visual analogue scales for interstitial lung disease: a prospective validation study. QJM 2018;111:531-9. [Crossref] [PubMed]
- Brightling CE, Monterio W, Green RH, et al. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatability. Respir Med 2001;95:999-1002. [Crossref] [PubMed]
- Martin Nguyen A, Bacci ED, Vernon M, et al. Validation of a visual analog scale for assessing cough severity in patients with chronic cough. Ther Adv Respir Dis 2021;15:17534666211049743. [Crossref] [PubMed]
- Kwon JW, Moon JY, Kim SH, et al. Korean version of the Cough Symptom Score: clinical utility and validity for chronic cough. Korean J Intern Med 2017;32:910-5. [Crossref] [PubMed]
- Zhao T, Qiu Z, Wang L, et al. Validation of the reliability and clinical value of the simplified cough score. Chinese Journal of General Practitioners 2012;11:273-6.
- Shembel AC, Rosen CA, Zullo TG, et al. Development and validation of the cough severity index: a severity index for chronic cough related to the upper airway. Laryngoscope 2013;123:1931-6. [Crossref] [PubMed]
- Martin Nguyen A, Bacci E, Dicpinigaitis P, et al. Quantitative measurement properties and score interpretation of the Cough Severity Diary in patients with chronic cough. Ther Adv Respir Dis 2020;14:1753466620915155. [Crossref] [PubMed]
- Baddini-Martinez J, Chinarelli T, Orlandini CB, et al. The Multidimensional Cough Index: A Practical Scale to Measure Cough and Sputum. Am J Med Sci 2021;362:396-402. [Crossref] [PubMed]
- Zhan W, Zhang L, Jiang M, et al. A new simple score of chronic cough: cough evaluation test. BMC Pulm Med 2020;20:68. [Crossref] [PubMed]
- Kum E, Guyatt GH, Munoz C, et al. Assessing cough symptom severity in refractory or unexplained chronic cough: findings from patient focus groups and an international expert panel. ERJ Open Res 2022;8:e00667-2021. [Crossref] [PubMed]
- Boulet LP, Coeytaux RR, McCrory DC, et al. Tools for assessing outcomes in studies of chronic cough: CHEST guideline and expert panel report. Chest 2015;147:804-14. [Crossref] [PubMed]
- Judson MA, Chopra A, Conuel E, et al. The Assessment of Cough in a Sarcoidosis Clinic Using a Validated instrument and a Visual Analog Scale. Lung 2017;195:587-94. [Crossref] [PubMed]
- Hsu JY, Stone RA, Logan-Sinclair RB, et al. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J 1994;7:1246-53. [Crossref] [PubMed]
- Bradley JP, Gross J, Paniello RC. Superior laryngeal nerve transection for neuropathic cough: A pilot study. Auris Nasus Larynx 2020;47:837-41. [Crossref] [PubMed]
- Vernon M, Leidy NK, Nacson A, et al. Measuring cough severity: Perspectives from the literature and from patients with chronic cough. Cough 2009;5:5. [Crossref] [PubMed]
- Vernon M, Kline Leidy N, Nacson A, et al. Measuring cough severity: development and pilot testing of a new seven-item cough severity patient-reported outcome measure. Ther Adv Respir Dis 2010;4:199-208. [Crossref] [PubMed]
- Birring SS, Dicpinigaitis P, McGarvey L, et al. Baseline cough severity and quality of life in patients with chronic cough participating in two global phase 3 randomized controlled clinical trials. American Journal of Respiratory and Critical Care Medicine 2021;203:A2359.
- Birring SS, Smith JA, Morice A, et al. The Cough Severity Diary in a pooled analysis of two, phase 3 trials of gefapixant in chronic cough. Eur Respir J 2021;58:OA4060.
- Kum E, Guyatt GH, Devji T, et al. Cough symptom severity in patients with refractory or unexplained chronic cough: a systematic survey and conceptual framework. Eur Respir Rev 2021;30:210104. [Crossref] [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Raj AA, Pavord DI, Birring SS. Clinical cough IV:what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol 2009;311-20. [Crossref] [PubMed]
- Fletcher KE, French CT, Irwin RS, et al. A prospective global measure, the Punum Ladder, provides more valid assessments of quality of life than a retrospective transition measure. J Clin Epidemiol 2010;63:1123-31. [Crossref] [PubMed]
- French CT, Irwin RS, Fletcher KE, et al. Evaluation of a cough-specific quality-of-life questionnaire. Chest 2002;121:1123-31.
- Lechtzin N, Hilliard ME, Horton MR. Validation of the Cough Quality-of-Life Questionnaire in patients with idiopathic pulmonary fibrosis. Chest 2013;143:1745-9. [Crossref] [PubMed]
- Baiardini I, Braido F, Fassio O, et al. A new tool to assess and monitor the burden of chronic cough on quality of life: Chronic Cough Impact Questionnaire. Allergy 2005;60:482-8. [Crossref] [PubMed]
- Koo HK, Jeong I, Kim JH, et al. Development and validation of the COugh Assessment Test (COAT). Respirology 2019;24:551-7. [Crossref] [PubMed]
- Slovarp L, Loomis BK, Glaspey A. Assessing referral and practice patterns of patients with chronic cough referred for behavioral cough suppression therapy. Chron Respir Dis 2018;15:296-305. [Crossref] [PubMed]
- Slovarp LJ, Jetté ME, Gillespie AI, et al. Evaluation and Management Outcomes and Burdens in Patients with Refractory Chronic Cough Referred for Behavioral Cough Suppression Therapy. Lung 2021;199:263-71. [Crossref] [PubMed]
- Park HJ, Park YM, Kim JH, et al. Effectiveness of proton pump inhibitor in unexplained chronic cough. PLoS One 2017;12:e0185397. [Crossref] [PubMed]
- Bowen AJ, Nowacki AS, Contrera K, et al. Short- and Long-term Effects of Neuromodulators for Unexplained Chronic Cough. Otolaryngol Head Neck Surg 2018;159:508-15. [Crossref] [PubMed]
- Morice AH, Menon MS, Mulrennan SA, et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med 2007;175:312-5. [Crossref] [PubMed]
- Shi G, Shen Q, Zhang C, et al. Efficacy and Safety of Gabapentin in the Treatment of Chronic Cough: A Systematic Review. Tuberc Respir Dis (Seoul) 2018;81:167-74. [Crossref] [PubMed]
- Chen Q, Zhang M, Si F, et al. Flupentixol/melitracen for chronic refractory cough after treatment failure with other neuromodulators. Int J Tuberc Lung Dis 2021;25:648-54. [Crossref] [PubMed]
- Rebelo P, Oliveira A, Paixão C, et al. Minimal Clinically Important Differences for Patient-Reported Outcome Measures of Cough and Sputum in Patients with COPD. Int J Chron Obstruct Pulmon Dis 2020;15:201-12. [Crossref] [PubMed]
- Berkhof FF, Boom LN, ten Hertog NE, et al. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes 2012;10:4. [Crossref] [PubMed]
- Fathi H, Moon T, Donaldson J, et al. Cough in adult cystic fibrosis: diagnosis and response to fundoplication. Cough 2009;5:1. [Crossref] [PubMed]
- Kalpaklioglu AF, Kara T, Kurtipek E, et al. Evaluation and impact of chronic cough: comparison of specific vs generic quality-of-life questionnaires. Ann Allergy Asthma Immunol 2005;94:581-5. [Crossref] [PubMed]
- Schmit KM, Coeytaux RR, Goode AP, et al. Evaluating cough assessment tools: a systematic review. Chest 2013;144:1819-26. [Crossref] [PubMed]
- Polley L, Yaman N, Heaney L, et al. Impact of cough across different chronic respiratory diseases: comparison of two cough-specific health-related quality of life questionnaires. Chest 2008;134:295-302. [Crossref] [PubMed]
- Braido F, Baiardini I, Tarantini F, et al. Chronic cough and QoL in allergic and respiratory diseases measured by a new specific validated tool-CCIQ. J Investig Allergol Clin Immunol 2006;16:110-6.
- Koo HK, Bae W, Moon JY, et al. Differential features of chronic cough according to etiology and the simple decision tree for predicting causes. Sci Rep 2021;11:10326. [Crossref] [PubMed]