
Robotic lung resection: a narrative review of the current role on primary lung cancer treatment
Introduction
The first robotic lung lobectomy was reported in mid-2000 (1). After two decades, several researchers have investigated the feasibility, safety, and surgical outcomes of robotic lung resections (2,3). The oncological resections were the most important field for the application of this technique. The impulse generated by the outstanding postoperative outcomes related to the minimally invasive approach by video-assisted thoracic surgery (VATS) served as a solid starting point to introduce the robotic platform worldwide (4,5). The so-far supposed benefits generated by the machine-surgeon interactions would still have to be proved superior to negative factors such as cost, operation room management complexity, and the breaking paradigm of the surgeons outside the surgical field.
The years have passed, and thoracic robotic surgery experiments great spread over the world (6). According to a report from the U.S. Department of Health and Human Services, from 2009 to 2013, the percentage of robot-assisted lobectomies increased from 1% to 11% of all lobectomies, while VATS remained at 33% and open lobectomies decreased 20% (7,8). Some important factors responsible for the rapid dissemination of robotic-assisted thoracic surgery (RATS) is the great acceptance of the platform capable to bring all the consolidated benefits of minimally invasive approach without technical difficulties imposed by VATS. The migration from an open technique to a VATS approach requires radical changes in surgeons’ strategies and skills. The loss of precision due to the lack of wristed instruments, the antiparallel optic position in the multiport approach, or the restricted working space on the uniportal technique are some of the difficulties experienced by surgeons when performing VATS procedures. These problems prolong the learning curve and make it difficult for centers with lower surgical volumes to adopt the technique (9). A few reports on the learning curve for robotic lobectomies signalize 20–30 procedures to achieve proficiency while the literature about VATS lobectomy varies from 50 procedures for multiport approach going to 140 for uniportal surgery (10-12). That shorter learning curve may be due to more intuitive surgical field manipulation provided by the 3D visualization, and a more ergonomic surgeon position. Also, the parallel instrument-optic position and the wristed instruments permit an easier migration from open to minimally invasive since the surgeon is capable to mimic the open approach. However, after the establishment of RATS as a safe technique for oncological early-stage lung resections, there is a necessity to analyze the performance in key points of oncological treatment.
Therefore, we analyzed the literature about the performance of the robotic platform on the trend migration from lobectomy to sublobar resections, lymphadenectomy, and complex lung surgery to understand the impact of RATS on surgical lung cancer treatment for the next years. The aim of this paper is to review the actual statement of robotic thoracic surgery for lung cancer treatment and focus on the most recent questions about sublobar resections, lymphadenectomy, complex lung resections, and future perspectives. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-635/rc).
Methods
We performed a narrative review with the current published data regarding robotic surgery for primary lung cancer treatment. The major points to be revised were the impact of the robotic platform on the sublobar resection indication, the outcomes of lymphadenectomy, the statement on complex lung resections by robotic approach, and the next frontiers for robotic lung cancer surgery.
The authors conducted a literature search to identify all English-written published papers on the digital platforms PubMed, Google Scholar, and Scielo. For that, the authors used the terms: “Robotic” and “Lung Cancer” with “AND” divisor as detailed in Table 1. Those terms resulted in 1,186 papers that after applying the filters for inclusion and exclusion criteria resulted in 211 papers. Finally, after review and selection consensus, 59 papers completely analyzed by the authors were included in this narrative review.
Table 1
| Items | Specification |
|---|---|
| Date of Search | February 1st, 2022 to August 1st, 2022 |
| Databases and other sources searched | PubMed, Google Scholar, and Scielo |
| Search terms used | “Robotic and Lung Cancer”, “RATS and Lung Cancer” |
| Timeframe | May 1st, 2002 to April 30th, 2022 |
| Inclusion and exclusion criteria | English-written published clinical trials, meta-analyses, randomized controlled trials, reviews, and systematic reviews. Publications that presented just with technique description, anesthetic considerations, “tips and tricks” or that does not meet the scope proposed by the review were excluded after the title and abstract reading |
| Selection process | The two authors conducted equally the paper selection and the full-text review of the final relevant literature to reach a consensus and to discuss the data results |
The changing trend: from lobectomy to segmentectomy
It is known that anatomical segmentectomy requires greater skill from the surgeon in bronchovascular dissection when compared to lobectomy. The reasons for that increased complexity are the progressive reduction in vessels and bronchial caliber, more frequent anatomical variations, oncologically need to correctly define the intersegmental margins, and a more restricted surgical working field due to the intraparenchymal dissection. The robotic platform seems to perfectly fit the needs required to improve surgical precision in sublobar procedures bypassing the inherent difficulties previously mentioned. The ability to visualize three-dimensionally, the greater precision and refinement of movements, the ability to angulate the forceps with multiple degrees of freedom, the stability and control of the optics by the surgeon himself, and multiple instruments manipulation, masterfully contemplate the needs required for procedures with such a degree of precision.
The reliable performance of the platform in sublobar resections has already been reported by Cerfolio et al. in their initial experience with the first one hundred cases planned for robotic anatomic segmentectomy (13). Most patients were submitted to curative lung cancer surgery (79%) by a completely robotic four-arm approach and all achieved R0 resection with a median of 19 lymph nodes dissected. There were no conversions for the open approach and 7% needed intraoperative change for a lobectomy due to inadequate margin or nodule localization issues. The results of that work were similar to those observed in our published experience with the first 49 patients operated (14). At that time, we used a three-arm approach described by Ninan and Dylewski with some minor adaptations due to the initial implementation status and cost issues in Brazil (15). There were also neither conversions to an open procedure nor lobectomy. The median operative time was 160 minutes and the postoperative complication rate was 18.3%. The most frequent complication was prolonged air leak (8.1%) however, the median pleural drainage time was 2 days with a length of stay of 3. Those papers just reported isolated results of technique performance without the intent to compare with the more consolidated access by VATS. Some retrospective database analysis was performed to compare the early outcomes of RATS segmentectomy with VATS. Zhang et al. showed results of a large retrospective analysis with 774 patients, 298 operated by RATS and 476 by VATS, for early-stage lung cancer treatment (16). The groups were matched by propensity score and results showed no significant difference in operative time (RATS 147.91 min/VATS 149.23 min; P=773), blood loss (RATS 50 mL/VATS 100 mL, P=0.177), overall complications (RATS 17.9/VATS 14.8%; P=0.340), and length of stay (RATS 4 days/VATS 4 days; P=0.417). Nevertheless, the RATS group presented a significantly higher number of lymph nodes and stations dissected (P<0.01) compared with VATS, which is an important trend to define better the pathological stage and adjuvant strategy. A meta-analysis published by Ma et al. with publications comparing postoperative outcomes of anatomical resections by RATS and VATS performed a subgroup analysis dividing lobectomy and sublobar resections. Although in the lobectomy group the RATS approach was associated with lower conversion rate, a great number of harvested lymph nodes and dissected stations, shorter postoperative chest tube duration, lower overall complication rate, and recurrence, in the sublobar resection group those differences were not significant (17). More recently Kneuertz et al. published data from the National Society of Thoracic Surgeons General Thoracic Surgery Database, comparing early outcomes of sublobar resections for stage I disease performed by VATS, RATS, and open approach (18). Despite the minimally invasive group (VATS and RATS) presented with a significantly lower postoperative complication rate (RATS 31.3%, VATS 28.8%, open 38.3%; P<0.001) and length of stay (RATS 4.3 days, VATS 4.4 days, open 5.2 days; P<0.001) the RATS group showed a higher rate of home discharge with chest tube and pneumothorax after chest tube removal. Those results showed a non-inferiority of the RATS approach over VATS and reassure the benefit of minimally invasive procedures.
At the same time, we see the emergence of a trend towards the application of sublobar resections as the standard treatment for early lung lesions. This trend arises with the evolution of tomographic imaging techniques that made the diagnosis of early lung lesions possible and with the evolution of resection techniques that allow superior performance of anatomical sublobar surgeries by the oncological rigors. This evolution comes to oppose the dogmas established almost two decades ago by the Lung Cancer Study Group (LCSG), which in another scenario, established lobectomy as the gold standard treatment for lung cancer (19). The recently published results from JCOG0802 comes to rectify the previous studies by showing consistent improvement in overall survival after sublobar anatomical resections for patients with stage IA (≤2.0 cm and consolidation-to-tumor ratio of 0.25 or more) NSCLC (20). However, it is a fact that this paradigm shift brings surgeons closer to minimally invasive and more precise surgery and the number of robotic sublobar resections must increase in the next years. Confirming this, Gergen et al. reported an increased number of segmentectomies for stage I lung cancer patients after the first-year introduction of a robotic program. The overall number of sublobar anatomic resections raised from 8.6% to 25% (P=0.01) and the rate of segmentectomies indicated for RATS approach raised from 16.7% to 88.2% (P=0.003) (21). Zhou et al. also presented similar results with a significant increase not only in total numbers of sublobar anatomic resections but also a significant rise in number of the considered complex sublobar resections: any segmentectomy excluding lingular-sparing (Left S1+S2+S3), lingulectomy, S6 and, basilar segmentectomy (22). In the period from 2015–2019 they performed 222 anatomic segmentectomies (37% RATS; 18% VATS and 47% open) with a subgroup analysis showing that the incidence of complex resection in RATS group was 45% vs. 22% in VATS group (P<0.001) (22).
The presented literature on this topic represents the actual scenario of lung-sparing curative surgery for lung cancer (Table 2). We believe, by data support, that the robotic approach has an important role in that framing change by providing more precision, and comfort for the surgeon with no inferior postoperative results when compared to VATS. Those qualities are important to provide surgeons with more trust to perform challenging anatomically resections. Also, the possibility to improve precision by including new technologies such as multimodal imaging segmentectomy which involves intraoperative navigation with 3D tomography reconstructions and indocyanine green fluorescence to define the intersegmental plane, steps up the role of robotic platforms and will be discussed further in this article (Figure 1).
Table 2
| Study | Number of patients | Comparative intent | RATS approach | Operative time (minutes) | Conversion rate | Prolonged air leak | Complication rate | Length of stay (days) |
|---|---|---|---|---|---|---|---|---|
| Cerfolio 2015 (13) | Total: 100, NSCLC: 79 (79%) | None | 4-arm complete portal | 88 (46–205) | No conversion was needed | Not reported | 9% | 3 [2–9] |
| Terra 2019 (14) | Total: 49, NSCLC: 34 (69.3%) | None | 3-arm complete portal | 160 (60–313) | No conversion was needed | 8.1% | 18.3% | 3 [1–30] |
| Zhang 2019 (16) | 774, just NSCLC patients | RATS: 257 | 4-arm complete portal | RATS: 147.91±52.42 | RATS: 0.4% | Not reported | RATS: 17.9% | RATS: 4 [3–5] |
| VATS: 257 | VATS: 149.23±49.66 | VATS: 1.2% | VATS: 14.8% | VATS: 4 [3–5] | ||||
| Propensity score matching |
P=0.773 | P=0.624 | P=0.340 | P=0.417 | ||||
| Kneuertz 2022 (18) | 3,680, just NSCLC patients | RATS: 737 | Not reported | RATS: 238.61 (92.08) | Not reported | RATS: 8.1% | RATS: 31.3% | RATS: 4.3 |
| VATS: 2,279 | VATS: 220.63 (75.17) | VATS: 6.6% | VATS: 28.8% | VATS: 4.4 | ||||
| Open: 664 | Open: 227.01 (79.87) | Open: 7.0% | Open: 38.3% | Open: 5.2 | ||||
| IPTW | P=0.40 | P<0.001 | P<0.001 |
This table summarizes the papers that analyses the performance of robotic segmentectomies. RATS, robotic assisted thoracic surgery; NSCLC, non-small cell lung cancer; VATS, video assisted thoracic surgery; IPTW, Inverse probability of treatment weighting.
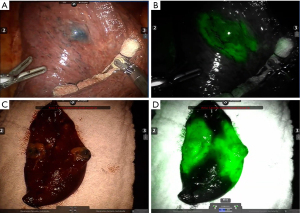
The quality of lymphadenectomy
Lymphadenectomy is a key point for prognostic definition in non-small cell lung cancer. An adequate lymph node dissection allows the correct pathological staging and the ideal choice of postoperative therapy, impacting overall survival and disease-free survival (23). With the advancement of chemotherapeutic and immunotherapeutic clinical treatments within different adjuvant and neoadjuvant strategies, the need for an ideal and extended lymphadenectomy has been increasingly discussed (24,25). Additionally, unsettling data was published by Edwards et al. showing that just 46% of resected lung cancer has adequate lobe-specific systematic nodal dissection following the guidelines recommended by the International Association for the Study of Lung Cancer (IASLC) (26). In view of this, the comparison between the different minimally invasive techniques and their ability to facilitate access to lymph node stations has become the focus of several studies. Results such as lymph node upstaging rate, number of resected lymph nodes, and number of dissected stations are used as indicators to compare the quality of lymphadenectomy by RATS, VATS, and thoracotomy. Although all these outcomes are extensively used in the papers, it is worth mentioning that the number of dissected lymph nodes is subject to some biases, mainly those related to the dissection of the specimen and lymph node count by the pathologist. However, the number of lymph nodes removed and examined after surgery is historically related to survival. Higher number of resected lymph nodes provides a greater staging accuracy by a lower chance of missing metastatic disease (22,23). Even though lymph node upstaging is more objective since it directly represents the potential change in disease stage.
Toker et al. reported equivalent results comparing RATS, VATS, and open in the dissection of N1 and N2 stations (27). RATS yielded significantly more lymph nodes in total (mean 14.9±6.5; P=0.0007) and a significant difference in the number of dissected N1 in the RATS (mean 6.8±3.7; P<0.0001). Wilson et al. evaluated the rate of lymph node upstaging as an outcome indicator of the quality of robotic lymphadenectomy (28). In this multicentric database analysis, pathological upstaging was evidenced in 10.9% of the 302 patients operated on early-stage NSCLC, 6.6% for pN2, and 4.4% for pN1. Zirafa et al. reported analysis of 212 patients (106 RATS, 106 open) submitted to lobectomy and hilar-mediastinal dissection observing no difference in the number of lymph nodes dissected or upstaging rate between groups (29). Although, in this study, the RATS group presents a higher rate of N0 to N2 upstaging (RATS 9.4%; Open 2.8% P=0.045). However, the analysis of the upstaging rate should consider not only the access route but also consider possible confounding factors such as the T stage and the clinical staging protocols to which the patient was submitted. For this, studies using propensity-matching scores were carried out. Kneuertz et al. reported the results of 911 patients (254 robotic, 296 VATS, and 261 open) evidencing an upstaging rate of 21.8% for open surgery, 16.2% for RATS and 12.3% for VATS (P=0.03) (30). The number of resected stations (N1+N2) was similar between open surgery (4.0) and RATS (3.8) but lower in VATS (3.6) (P=0.001). The significant difference in the frequency of upstaging observed was only for N1 stations, with no difference for N2. However, in the multivariate analysis considering the invasive mediastinal staging and the clinicopathological characteristics as covariates, the upstaging rate by RATS was equivalent to open staging while VATS continued with a lower frequency of upstaging when compared to the conventional access with an OR of 0.50 (95% CI: 0.29–0.85). Tang et al. performed a large database analysis including 7,452 pairs of patients matched between open surgery and RATS for treatment of early-stage lung cancer, showing no significant difference in the upstaging rate between the two modalities (11.0% and 11.6% respectively, P=0.28) (31). However, patients undergoing the RATS technique had a greater number of resected lymph nodes when compared to open surgery (10 vs. 8, P=0.001). The ROMAN study, an Italian randomized multicentric trial comparing VATS vs. RATS on surgery outcomes observed a significant improvement in the number of hilar lymph nodes (7, IQR 5–10 vs. 4, IQR 2–7; P=0.0003) and lymph node station (6, IQR 4–6 vs. 4, IQR 3–5; P=0.0002) resected on RATS group (32). However, no technique was superior in terms of nodal upstaging (P=0.72), and we also should remember that those lymph node evaluations were secondary outcome analyses.
A meta-analysis published by Zhang et al. evaluated twenty-six studies comparing 45,733 patients (14,271 by RATS and 31,462 by VATS) submitted to lobectomy or segmentectomy for lung cancer treatment. There was no difference in the number of lymph station (P=0.73; 95% CI: −0.98 to 0.68; I2=99%) dissected. However, the number of lymph node dissected was higher by RATS them VATS (P=0.0006; 95% CI: 0.51 to 1.86; I2=98%) (3). Aiolfi et al., in another meta-analysis including thirty-four studies and 183,426 patients (88,865 open, 79,171 VATS, and 15,390 RATS) reported similar results with a significantly higher number of lymph nodes and lymph nodes stations by RATS when compared to VATS (mean difference =2.18, 95% CI: 0.52–3.92), but no difference between RATS and Open (mean difference =0.73, 95% CI: −1.06–2.55) (33). No meta-analysis of upstaging rate data was found in the search for this review which may be the result of the high degree of heterogeneity evidenced among the papers included in this type of study. Although the data show a greater number of resected lymph nodes and sampled stations in patients operated by RATS, these results do not translate into gains in late oncological outcomes. It is noteworthy that evidence of long-term outcomes depends on a long period of follow-up, which limits the analysis in most studies related to lymph node sampling. Another important point when correlating lymph node sampling data and survival outcomes are the lag between the advances in adjuvant clinical therapy and the time of surgery in study analysis. The increasing possibilities of clinical treatment for postoperative node-positive patients and the changes in clinical staging reinforce the lymph node upstaging following surgery as a good predictor of the effectiveness of complete medical care.
These papers serve at least to prove the non-inferiority of RATS lymphadenectomy over an open approach. Regarding the discussion about which would be the best minimally invasive route for better results in lymph node dissection, we can observe a trend towards a greater number of lymph nodes dissected robotically. However, studies with greater power of evidence and robust statistical methods to eliminate bias are needed. The fact is that the quality of lymph node dissection does not seem to depend primarily on the access route used, but on the surgical experience and mindset regarding the need for a wide lymphadenectomy for better oncological results and adjuvant treatment possibilities. Okusanya et al. evaluate the association between robotic lobectomy hospital volume and nodal upstaging using the National Cancer Data Base (NCDB) from the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons (ACS) (34). They observed a trend in the number of lymph node harvests and upstaging by the hospital’s robotic surgery volume. On multivariable analysis, high-volume robotic centers (≥53 procedures) had higher nodal harvest (12.6 vs. 6.8, P<0.001) and nodal-upstaging rates (13.4 vs. 10.3, P<0.001) than low-volume hospitals (≤12 procedures).
It’s said that the main message of that revision (Table 3) is that surgeons should value a widely adequate lymphadenectomy regardless of the access route (18,27,29-32,35). The benefits of the robotic platform regarding the quality of lymphadenectomy compared to VATS require more time to be effectively proven, but the technique already has solid results when compared to the current standard of care.
Table 3
| Study | Patients | Number of stations dissected | Number of lymph nodes dissected | Overall upstaging | N1 upstaging | N2 upstaging |
|---|---|---|---|---|---|---|
| Toker 2016 (27) | RATS: 106 | RATS: 4.9±1.5 | RATS: 14.9±6.5 | Not reported | Not reported | Not reported |
| VATS: 68 | VATS: 4.6±1.2 | VATS: 11.7±4.7 | ||||
| Open: 96 | Open: 4.6±1.4 | Open: 12.0±6.4 | ||||
| P=0.32 | P=0.0007 | |||||
| Zirafa 2018 (29) | RATS: 106 | RATS: 4.95 ±1.2 | RATS: 14.42±6.99 | RATS: 20.8% | RATS: 11.3% | RATS: 9.4% |
| Open: 106 | Open: 4.22 ±1.58 | Open: 14.32±7.34 | Open: 17.9% | Open: 15.1% | Open: 2.8% | |
| P=0.001 | P=0.926 | P=0.602 | P=0.417 | P=0.045 | ||
| Kneuertz 2019 (30) | RATS: 391 | RATS: 3.8±0.07 | RATS: 11.8±0.4 | RATS: 16.2% | RATS: 10% | RATS: 5% |
| VATS: 338 | VATS: 3.6±0.08 | VATS: 11.8±0.4 | VATS: 12.3% | VATS: 8% | VATS: 3% | |
| Open: 324 | Open: 4.0±0.08 | Open: 11.9±0.5 | Open: 21.8% | Open: 15% | Open: 7% | |
| P=0.001 | P=0.95 | P=0.03 | ||||
| Tang 2020 (31) | RATS: 7,452 | Not reported | RATS: 10 | RATS: 11% | Not reported | Not reported |
| VATS: none | Open: 8 | Open: 11.6% | ||||
| Open: 50,186 | P<0.001 | P=0.28 | ||||
| Gallina 2021 (35) | RATS: 237 | Exact values not reported however | RATS: 15±7.01 | RATS: 21.1% | RATS: 10.1% | RATS: 11.0% |
| VATS: 110 | P>0.5 | VATS: 9±5.7 | VATS: 13.6% | VATS: 7.3% | VATS: 6.4% | |
| Open: 158 | Open: 13±11.6 | Open: 25.3% | Open: 9.6% | Open: 15.9% | ||
| P=0.0001 | P=0.1 | P=0.2 | P=0.04 | |||
| Veronesi 2021 (32) | RATS: 38 | RATS: 6 (IQR 4–6) | N1: VATS: 4 (IQR 2–7), RATS: 7 (IQR 5–10) (P=0.0003) | RATS: 11.4% | RATS: 5.7% | RATS: 5.7% |
| VATS: 39 | VATS: 4 (IQR 3–5) | VATS: 14.3% | VATS: 5.7% | VATS: 8.6% | ||
| Open: none | P=0.0002 | N2: VATS: 5 (IQR 3–7), RATS: 7 (IQR 5–10) (P=0.0001) |
P=0.72 | P>0.5 | P>0.5 | |
| Kneuertz 2022 (18) | RATS: 737 | RATS: 4.80 | RATS: 10.14±7.24 | RATS: 6.2% | No significant difference after multivariate analysis | RATS: 2.9% |
| VATS: 2,279 | VATS: 3.80 | VATS: 8.43±6.82 | VATS: 5.6% | VATS: 2.2% | ||
| Open: 664 | Open: 4.02 | Open: 8.07±6.40 | Open: 8.6%, | Open: 3.4% | ||
| P=0.001 | P<0.001 | P=0.05 | P=0.15 |
This table summarizes the papers that performed comparative analyses about lymph node dissection. VATS, video assisted thoracic surgery; RATS, robotic assisted thoracic surgery; IQR, interquartile range.
Robotic surgery for complex resections
As the experience of the robotic surgeon community advances, robotic-assisted lung resections have increasingly been established as a reliable option for early-stage lung cancer resections. The expertise gained in standard lobectomies and others medium-complexity surgeries that are typically indicated for VATS brings the possibility of pushing forward the limits of robotic surgery indications for procedures that otherwise would be indicated for open surgery. Based on the already known benefits of the robotic platform to increased precision and lung manipulation in restricted operatory fields, we are seeing reports with great results in sleeve resections, stage III disease after neoadjuvant treatment, and other complex resection (Figure 2).
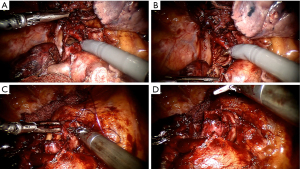
Central lesions are often considered major challenges in oncological lung surgery. In a single surgeon experience, Cosgun et al. reported the sleeve lung resection as a benchmark procedure to evaluate the proficiency in lung resections by robotic approach (36). Setting the first successful sleeve resection as the benchmark to divide early and late procedures on a series of 197 cases of lung resection they observe a significant improvement in the mean number of dissected lymph nodes and cases of extended resections (central lesions, mediastinal invasion, sleeve resection, and chest wall invasion) in the late procedure group. Jiao, et al. has the largest reported experience with sleeve lobectomy resections using a robotic approach (37). The technique used in 67 patients consisted of a three-arm approach without gas inflation and a utility incision of 3 cm. The overall operative morbidity was 20.9% and atelectasis was the most common complication. No mortality was reported, and the multivariate analysis showed that age >70 years, comorbidities, and surgeon’s early experience were significant morbidity risk factors (P=0.011; P=0.002 and P=0.007 respectively). Geraci, et al. published a series of 23 consecutive cases submitted to sleeve resections for lung cancer treatment by a totally robotic approach (38). In that series, most patients need a lobe resection (82%) and two were submitted to a bronchial and artery sleeve resection (double-sleeve). A very low morbidity rate (9%), no death, and a 100% rate of R0 resection with just one conversion were reported. However, the authors call attention to strict selection criteria with an extensive preoperative evaluation leading to a sleeve indication rate of just 2.1% (22 of 1,061) of the total surgical group series. Therefore, consistently positive results presented in those series supports the safety application of robotic platform on sleeve resections.
Another challenge is lung resections after neoadjuvant therapy. The post-induction inflammatory state creates a hazardous surgical field with bleeding dissection, fibrosis, and dense pleuropulmonary adhesions. Some data exists comparing VATS with the open approach after induction therapy showing so significant differences in major endpoints like survival and morbidity. However, the VATS group was associated with a fewer number of lymph nodes dissected, which is a major concern in that population (39). Cerfolio et al. looked at 223 patients with N2 disease operated by RATS (40). Just 15.2% received neoadjuvant therapy. In that cohort, the complete resection rate was 98.1% in the neoadjuvant group with no mortality, and the major complications (10.1%) were comparable with those in adjuvant patients. The conversion rate was 15% on the neoadjuvant resections, but most were for oncologic reasons, due to extensive mediastinal lymph node involvement. In a larger series Park et al. reviewed 428 patients with Stage II-IIIA NSCLC who underwent lobectomy after neoadjuvant therapy (41). Of those patients, 31 were submitted for minimally invasive surgery 17 by robotic approach. No comparison results between VATS and RATS were made, and there was no significant difference between the minimally invasive group and open on rates of operative complications, the extent of resection, and the final pathologic stage.
Recently, neoadjuvant treatment with immunotherapy begins to be a new trend. Bott et al. looked at the outcomes of 20 stage I–IIIA patients who received neoadjuvant Nivolumab (42). A minimally invasive approach was initially attempted on 13 patients however just 6 (46%) were completed by the proposed access (3 VATS and 3 RATS). There was no perioperative mortality and 50% morbidity, the most common of which was atrial fibrillation. The primary causes for conversion were hemorrhage, dense adhesions, and inflamed mediastinal and hilar nodal stations.
Those reviews signalize the increasing application of the robotic approach on complex lung resections. As long as the surgeon team becomes more confident and comfortable with the platform the benefits imposed by the technological improvement begin to supplant the fear of bleeding in those minimally invasive complex surgeries (Figure 3) (43). However, it is important to emphasize that the selection of robotic cases must consider the surgery team’s learning curve. Initial experience is usually adopted with simpler procedures such as thymectomies or lower lobectomies. The performance of complex procedures such as arterioplasties, complex segmentectomies, bronchoplasty and tumors with chest-wall invasion should be reserved for teams with experienced surgeons. Another key point is joint training with the bed-side team for possible emergency situations. In this regard, training with catastrophe simulation involving cardiac arrest, massive hemorrhage and situations that require rapid undocking and surgery conversion is vital to improve safety.
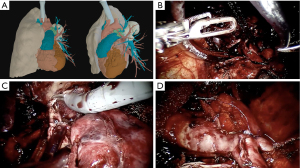
Postoperative outcomes
Since the spread of robotic lung cancer resections, we observe arisen concern about perioperative complications directly caused or facilitated by the technique. The interposition of a machine between the once inseparable binomial “surgeon-patient”, justifies the need for a carefully protocolized technique learning workflow and clinical studies evaluation. The technique standardization for anatomic lung resections and the initial industry regulation on the platform use were critical to improving the reliability among the surgical society. However, once intraoperative safety was granted, the results on postoperative outcomes become the focus of academic research. Therefore, some retrospective analyses were produced in recent years evaluating major and minor complications, and oncological outcomes.
Our initial experience in Brazil was recently published after the outcome analysis of 154 patients treated for non-small cell lung cancer by a robotic approach (44). Most of the patients were stage I disease and were submitted to a lobe resection (86.3%). Postoperative complications (major and minor) occurred in 20.4% and prolonged air leak was the most frequent (9.5%), however, we had a short median pleural drainage time of 2 days. There was one death (0.5%), 12 days after a lobectomy due to complicated pneumonia. Although its series includes most of the patients from the initial robotic surgery experience, the numbers are similar to those presented in worldwide literature. A large retrospective, multicenter, analysis was performed by Cao et al. with 1264 patients and the Clavien-Dindo grade III or higher events were analyzed (45,46). The overall major complication rate was 4.3% and the most frequently observed was pneumonia (1.2%) followed by prolonged air leak (0.9%). The entire study’s mortality rate was 0.6%, and among patients that developed major complications, the 30-day mortality rate was 15%. The characteristics associated with an increased risk for major complications were male sex, FEV1, DLCO, neoadjuvant therapy, and extended resections. The disease stage was not identified as a major risk factor for complications, however, 74.1% of patients were in clinical stage I. Li et al. published results of 121 patients analyzed with clinical stage IIB–IIIA (47). The comparative results between VATS [85] and RATS [36] showed a similar morbidity rate between the groups (13.9% for RATS vs. 15.3% for VATS; P=0.84). However, the RATS group presented a shorter length of stay (4 vs. 5 days, P<0.01) and a higher number of lymph nodes harvested (13 vs. 10, P<0.01).
Reddy et al. selected 23779 patients (9360 VATS; 2994 RATS) who received lobectomy from Premier Healthcare database (48). More than 80% of data was from primary lung cancer treatment and all patients come from high-volume centers (more than 20 minimally invasive lobectomies per year). After applying propensity-matching they observed that the RATS group performed with significantly better postoperative (30.7% vs. 36.6%, P=0.0097), and 30-day (33.4% vs. 39.3%, P=0.0128) complication rate. It’s important to mention that in this study the conversion rate to open surgery was significantly higher in VATS group (8.0% vs. 4.8%, P=0.007). Servais et al. analyzed the conversion rate issue using The Society of Thoracic Surgeons General Thoracic Surgery Database (49). A total of 27,695 minimally invasive lobectomies were analyzed and the conversion rate from VATS group was 11% against 6% of RATS group (P<0.001). The major reason for conversion was anatomy issues which may indicate greater ease in dealing with anatomy using the robotic platform. However, emergency conversions were significantly more frequent in the RATS group, with a greater need for intraoperative blood transfusion. Despite negative outcomes, these data may also reflect the surgeon’s greater safety and confidence in insisting on a minimally invasive approach when using the robotic platform.
A systematic review with metanalysis made by Agzarian et al. selected 20 papers that compare the surgical outcomes between VATS and RATS (50). It found no difference regards blood loss, prolonged air leak, length of stay, conversion to thoracotomy rates, and nevertheless the operative time was quite long for RATS (64.28 vs. 61.69 min) when considering the learning curve, the early RATS experience was like early VATS cases. An earlier metanalysis performed by Wu et al. compilated 25 studies comparing RATS vs. VATS outcomes for lung cancer resection culminating in a pool of 50.404 patients (51). The RATS group had significantly lower 30-day mortality (OR: 0.55; 95% CI: 0.38–0.81; P=0.002), but also no difference in overall postoperative complication rate was observed (OR: 1.01; 95% CI: 0.87–1.16; P=0.94) (Table 4) (3,17,50-53).
Table 4
| Study | Conversion rate | Operative time | Morbidity | Length of stay | 30-day mortality | Overall survival | Disease free survival |
|---|---|---|---|---|---|---|---|
| Agzarian 2016 (50) | No significant difference | Mean longer operative time for RATS group compared to VATS and open | Not reported | Significant shorter on RATS when compared to open but no difference when compared to VATS | Not reported | Not reported | Not reported |
| Liang 2018 (52) | Lower in RATS group. 10.3% vs. 11.3% (OR 0.57, 95% CI: 0.48–0.67; P=0.000) (I2=39, P=0.119) | No significant difference. RATS: 176.63 and VATS: 162.74 min (P=0.086) | No difference (OR 0.95, 95% CI: 0.83–1.08, P=0.431) (I2=26.2, P=0.172) | No significant difference. RATS: 4.89 days; VATS: 5.23 days (P=0.292) | Lower in RATS: 0.7% vs. 1.1% (OR 0.53, 95% CI: 0.29–0.99, P=0.045) (I2=0, P=0.998) | Not reported | Not reported |
| Wu 2019 (51) | No difference (OR: 0.92; 95% CI: 0.56–1.52; P=0.75) (I2=33%, P=0.11) | No difference identified. Data was not pooled due to insufficient data and high heterogeneity | No difference (OR: 1.01; 95% CI: 0.87–1.16; P=0.94) (I2=1%, P=0.45) | No difference identified. Data were not pooled due to insufficient data and high heterogeneity | RATS presented a significant lower incidence: OR: 0.55; 95% CI: 0.38–0.81; P=0.002 (I2=0%, P=0.98) | No difference between RATS and VATS (HR: 0.77, 95% CI: 0.57–1.05; P=0.10) (I2=0%; P=0.98) | RATS showed a longer DFS (HR: 0.76, 95% CI: 0.59–0.97; P=0.03) |
| Mao 2021 (53) | No significant difference (OR =1.42, 95% CI: 0.70–2.88, P=0.336) (I2=85.2%) | RATS group was longer (standardized mean difference; =0.671, 95% CI: 0.462–0.880, P=0.00) (I2=91.3%) | No significant difference (OR =0.947, 95% CI: 0.79–1.14, P=0.564) (I2=61.5%) |
No significant difference (standardized mean difference =0.003, 95% CI: −0.10–0.11), P=0.957) (I2=90.6%) | No significant difference in postoperative hospital (OR =0.72, 95% CI: 0.47–1.11, P=0.139) (I2=65.4%) | Not evaluated | Not evaluated |
| Ma 2021 (17) | Lower in RATS group (OR =0.50, 95% CI: 0.43–0.60, P<0.001, I2 =37%) | No significant difference. (Weighted mean difference =−0.79, 95% CI: −15.65–14.06, P=0.920, I2=97%) | Lower in RATS than VATS (OR =0.90, 95% CI: 0.83–0.99, P=0.020, I2=11%) | Shorter in RATS than that of VATS (weighted mean difference =−1.12, 95% CI: −1.58 to −0.66, P<0.001, I2=80%) |
No significant difference (OR =0.99, 95% CI: 0.68–1.45, P=0.970, I2=37%) | No significant difference (HR =1.02, 95% CI: 0.82–1.26, P=0.880, I2=28%) | No significant difference. (HR =0.03, 95% CI: 0.66–1.61, P=0.890) (I2=0%) |
| Aiolfi 2021 (33) | No statistical difference. (RR =0.55; 95% CI: 0.10–0.277) | Significant higher in RATS groups. (Mean difference =56.4; 95% CI: 47.3–65.5) | No significant difference | No significant difference | No significant difference | No difference (HR 1.53; 95% CI: 0.87–2.88) | No reported |
| Zhang 2022 (3) | Lower in RATS group (P=0.004; 95% CI: 0.45 to 0.85) | No difference identified (P=0.94; 95% CI: −16.86 to 15.64) | No difference (P=0.15; 95% CI: 0.88 to 1.02; I2=31%) | Slightly longer in the VATS group (P=0.02; 95% CI: −0.60 to −0.04; I2=99%) |
No difference between groups | No difference (P=0.22; 95% CI: 0.90 to 1.02; I2=0%) | Slightly better in RATS than in VATS (P=0.01; 95% CI: 1.11 to 2.57; I2=23%) |
This table summarizes the currently existing meta-analysis on postoperative outcomes in thoracic robotic surgery. RATS, robotic assisted thoracic surgery; VATS, video assisted thoracic surgery; OR, odds-ratio; CI, confidence interval; HR, hazard ratio; DFS, disease-free survival; RR, risk ratio.
Regarding major oncological long terms outcomes, Cerfolio et al. report data from a multi-institutional retrospective review involving 1,339 patients from important centers for the diffusion of robotics technique (54). The 5-year stage-specific survival rate was: 83% for stage IA, 77% for stage IB, 68% for IIA, 70% percent IIB, 62% IIIA, and 31% for stage IIIB disease. All survival rates were significantly higher than those reported in the literature (IASLC), which can be explained by the fact that only centers with high volume and expertise in thoracic oncology were included in this study. However, it is worth mentioning the high 5-year survival for patients with stage IIIA disease which may reflect a better intraoperative staging, allowing the identification of patients who benefit from complementary treatment. The systematic review made by Wu et al. showed a significant longer disease-free survival (HR: 0.76; 95% CI: 0.59–0.97; P=0.03) and lower 30-days mortality for RATS surgery (OR: 0.55; 95% CI: 0.38–0.81; P=0.002) with no significant heterogeneity detected on both analyses. However, the overall survival rate was similar for both techniques (HR: 0.77; 95% CI: 0.57–1.05; P=0.10) (51). Zhang et al. published similar results of long-term outcomes in a more recent meta-analysis. There was also no difference in overall-survival (P=0.22; 95% CI: 0.90–1.02; I2=0%) and a slightly better disease free survival in RATS group (P=0.01; 95% CI: 1.11–2.57; I2=23% (3). It’s important to mention that the papers included in those metanalyses consisted of retrospective analysis and most of them represent an important part of data from early experience RATS cases mainly when long-term outcomes are analyzed.
Next frontier in robotic surgery
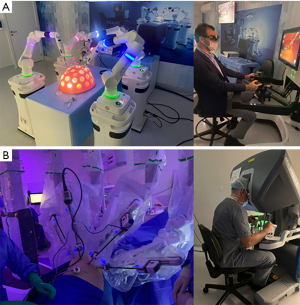
The actual robotic surgery practice is based on the use of the da Vinci® platform (Intuitive Surgical Inc. Sunnyvale, CA, USA) (55). Recently we are witnessing the emergence of many new players in this hectic technological market. The CMR Surgical (Cambridge, United Kingdom) platform named Versius® is already approved for use in Europe, Australia, and Brazil (Figure 4). The giant of healthcare, Medtronic (Minneapolis, Minnesota, EUA), is in final staging testing HUGO®. However, despite minor differences regarding the construction of the arms and distribution of the parts, all platforms share the same basic principle: the intermediary of a machine between the surgeon and the patient. That singularity represents the major disruption of robotic surgery in healthcare. A mechanical assistant in the operating room opens possibilities for data collection and analysis in inhuman proportions, making it possible to integrate with artificial intelligence systems, intraoperative navigation technologies, and even automation of surgical processes.
Nowadays, despite the use of the term “robotic surgery”, the real situation is not a procedure performed by an autonomous mechanical device, but a “robotic-assisted” procedure. Undoubtedly, it’s easy to imagine that one of the next evolutionary steps in robotic surgery is the automation of processes or the emergence of active interactions where the machine is no longer a mere reproducer of movements and starts to “suggest” better strategies or actively “alert” about some dangerous situations. For that, it’s necessary to improve the machine learning algorithms that make possible the development of artificial intelligence. The machine learning process used in robotic surgery is dependent on reinforcement learning processes a machine learning strategy based on training the robot to perform some surgical steps (e.g., vessel dissection) that encourage positive behavior (e.g., tumor resection) and disincentivize the negative ones (e.g., bleeding), as formalized in a reward function (56). With that, the algorithms will try different sequences of actions until the reward function is appropriately optimized and the system can achieve the best performance. However, development of that technique is dependent on a large amount of data, and surgical imaging since the great number of anatomical variations and the multiple intraoperative surgery situations (57).
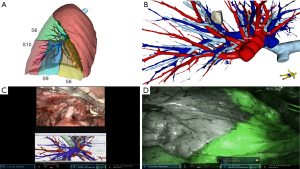
Therefore, the use of robotic platforms is the only actual way to collect all necessary data to improve its own reinforcement learning processes. Using parallel technologies, the data acquisition process became possible, and surgery took on an increasingly digital profile. Recording and systemically peer review surgery evaluation programs like C-SATS (Johnson & Johnson; New Brunswick, Nova Jersey, EUA), and Touch Surgery™ (Medtronic; Minneapolis, Minnesota, EUA) provide sufficient intraoperative videos to be applied to machine learning and algorithm development. The use of 3D reconstruction from CT scans (Figure 5) and Tilepro® (Intuitive Surgical Inc. Sunnyvale, CA, USA) promotes the creation of large image databases that could help robots improve their anatomical knowledge (58). All those technologies could be integrated with large clinical databases providing the necessary amount of integrated data to perform outcome predictions and set the best strategy for each patient.
As with all cutting-edge technology, the cost added to robotic surgeries is a negative factor. However, in micro-cost analyzes such as that performed by Shanahan et al. the cost difference is only €515 between VATS and RATS. The major contributor to cost increase is Intuitive® consumable materials (instruments, robotic staplers, etc...) which correspond to 63% of the increased value (59). In cost-effectiveness analysis made by Heiden et al. the difference between RATS and VATS when analyzed from a societal perspective was $247.77, resulting in an Incremental cost-effectiveness ratio of $113,388.80 per quality-adjusted life-years (60). In this case, RATS was cost-effective for a $150,000 willingness-to-pay (WTP) threshold or higher. They reported that RATS is cost-effective, even for lower WTP threshold ($50,000), if the rate of planned minimal invasive procedures was 73.6%. It means that considering a rate of 30% of open lobectomies, if the adoption of the RATS approach can reduce the rate of open surgery by 3.6% it becomes cost-effective. They also conclude that RATS is more cost-effective in high-volume centers since there is a diminishing per-patient-cost of the robot, reduced number of robotic instruments used, operating room time, and length of stay. All those variables make RATS lobectomy cost-effective when compared to VATS. Considering that we have an increasing number of RATS procedures and dissemination of new platforms on market, the cost reduction for RATS procedures is just a matter of time.
Summary
Robotic lung resection for primary lung cancer treatment has already established itself as a reliable approach and, above all, in a great expansion reality. The benefits of greater freedom of movement and surgical precision reflect in surgeon autonomy and, consequently, the feeling of intraoperative safety. These characteristics are transposed to the study’s main results such as a greater number of dissected lymph nodes, an increase in the number of complex surgeries, and great results in postinduction resections that demands a more laborious dissection. Furthermore, the robotic platform serves as a means by which the digitization of the surgical process will take place. Allowing the integration of other technological areas such as artificial intelligence and advanced imaging resources, the role of robotic surgery in lung cancer treatment is not only important in the present but also a fundamental part of the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-635/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-635/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-635/coif). RMT has relation as speaker for H.Strattner/ Intuitive AstraZeneca, Medtronic, MSD, and Roche. ERJ has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [Crossref] [PubMed]
- Zhang J, Feng Q, Huang Y, et al. Updated Evaluation of Robotic- and Video-Assisted Thoracoscopic Lobectomy or Segmentectomy for Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol 2022;12:853530. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [Crossref] [PubMed]
- Terra RM, Kazantzis T, Pinto-Filho DR, et al. Anatomic pulmonary resection by video-assisted thoracoscopy: the Brazilian experience (VATS Brazil study). J Bras Pneumol 2016;42:215-21. [Crossref] [PubMed]
- Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016;101:28-34. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Song G, Sun X, Miao S, et al. Learning curve for robot-assisted lobectomy of lung cancer. J Thorac Dis 2019;11:2431-7. [Crossref] [PubMed]
- Blasberg JD, Seder CW, Leverson G, et al. Video-Assisted Thoracoscopic Lobectomy for Lung Cancer: Current Practice Patterns and Predictors of Adoption. Ann Thorac Surg 2016;102:1854-62. [Crossref] [PubMed]
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Vieira A, Bourdages-Pageau E, Kennedy K, et al. The learning curve on uniportal video-assisted thoracic surgery: An analysis of proficiency. J Thorac Cardiovasc Surg 2020;159:2487-2495.e2. [Crossref] [PubMed]
- Terra RM, Haddad R, de Campos JRM, et al. Building a Large Robotic Thoracic Surgery Program in an Emerging Country: Experience in Brazil. World J Surg 2019;43:2920-6. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
- Terra RM, Lauricella LL, Haddad R, et al. Robotic anatomic pulmonary segmentectomy: technical approach and outcomes. Rev Col Bras Cir 2019;46:e20192210. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Zhang Y, Chen C, Hu J, et al. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: A multi-institutional propensity score-matched analysis. J Thorac Cardiovasc Surg 2020;160:1363-72. [Crossref] [PubMed]
- Ma J, Li X, Zhao S, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer 2021;21:498. [Crossref] [PubMed]
- Kneuertz PJ, Abdel-Rasoul M, D'Souza DM, et al. Segmentectomy for clinical stage I non-small cell lung cancer: National benchmarks for nodal staging and outcomes by operative approach. Cancer 2022;128:1483-92. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Gergen AK, White AM, Mitchell JD, et al. Introduction of robotic surgery leads to increased rate of segmentectomy in patients with lung cancer. J Thorac Dis 2021;13:762-7. [Crossref] [PubMed]
- Zhou N, Corsini EM, Antonoff MB, et al. Robotic Surgery and Anatomic Segmentectomy: An Analysis of Trends, Patient Selection, and Outcomes. Ann Thorac Surg 2022;113:975-83. [Crossref] [PubMed]
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021;16:860-7. [Crossref] [PubMed]
- Edwards JG, Chansky K, Van Schil P, et al. The IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for Residual Tumor Descriptors for Non-Small Cell Lung Cancer. J Thorac Oncol 2020;15:344-59. [Crossref] [PubMed]
- Toker A, Özyurtkan MO, Demirhan Ö, et al. Lymph Node Dissection in Surgery for Lung Cancer: Comparison of Open vs. Video-Assisted vs. Robotic-Assisted Approaches. Ann Thorac Cardiovasc Surg 2016;22:284-90. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7. [Crossref] [PubMed]
- Zirafa C, Aprile V, Ricciardi S, et al. Nodal upstaging evaluation in NSCLC patients treated by robotic lobectomy. Surg Endosc 2019;33:153-8. [Crossref] [PubMed]
- Kneuertz PJ, Cheufou DH, D'Souza DM, et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1457-1466.e2. [Crossref] [PubMed]
- Tang A, Raja S, Bribriesco AC, et al. Robotic Approach Offers Similar Nodal Upstaging to Open Lobectomy for Clinical Stage I Non-small Cell Lung Cancer. Ann Thorac Surg 2020;110:424-33. [Crossref] [PubMed]
- Veronesi G, Abbas AE, Muriana P, et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (ROMAN Study). Front Oncol 2021;11:726408. [Crossref] [PubMed]
- Aiolfi A, Nosotti M, Micheletto G, et al. Pulmonary lobectomy for cancer: Systematic review and network meta-analysis comparing open, video-assisted thoracic surgery, and robotic approach. Surgery 2021;169:436-46. [Crossref] [PubMed]
- Okusanya OT, Lutfi W, Baker N, et al. The association of robotic lobectomy volume and nodal upstaging in non-small cell lung cancer. J Robot Surg 2020;14:709-15. [Crossref] [PubMed]
- Gallina FT, Melis E, Forcella D, et al. Nodal Upstaging Evaluation After Robotic-Assisted Lobectomy for Early-Stage Non-small Cell Lung Cancer Compared to Video-Assisted Thoracic Surgery and Thoracotomy: A Retrospective Single Center Analysis. Front Surg 2021;8:666158. [Crossref] [PubMed]
- Cosgun T, Kaba E, Ayalp K, et al. Successful Sleeve Resection as a Marker for Proficiency for Robotic Pulmonary Resection. Thorac Cardiovasc Surg 2021;69:551-6. [Crossref] [PubMed]
- Jiao W, Zhao Y, Qiu T, et al. Robotic Bronchial Sleeve Lobectomy for Central Lung Tumors: Technique and Outcome. Ann Thorac Surg 2019;108:211-8. [Crossref] [PubMed]
- Geraci TC, Ferrari-Light D, Wang S, et al. Robotic Sleeve Resection of the Airway: Outcomes and Technical Conduct Using Video Vignettes. Ann Thorac Surg 2020;110:236-40. [Crossref] [PubMed]
- Fang L, Wang L, Wang Y, et al. Video assisted thoracic surgery vs. thoracotomy for locally advanced lung squamous cell carcinoma after neoadjuvant chemotherapy. J Cardiothorac Surg 2018;13:128. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406-13. [Crossref] [PubMed]
- Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. [Crossref] [PubMed]
- Rocha Júnior E, Mingarini Terra R, Guerreiro Cardoso PF, et al. Robotic Lung Volume Reduction Surgery With Extracorporeal Membrane Oxygenation. Ann Thorac Surg 2022;114:e351-4. [Crossref] [PubMed]
- Terra RM, Bibas BJ, Haddad R, et al. Robotic thoracic surgery for non-small cell lung cancer: initial experience in Brazil. J Bras Pneumol 2020;46:e20190003. [Crossref] [PubMed]
- Cao C, Louie BE, Melfi F, et al. Outcomes of major complications after robotic anatomic pulmonary resection. J Thorac Cardiovasc Surg 2020;159:681-6. [Crossref] [PubMed]
- Katayama H, Kurokawa Y, Nakamura K, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 2016;46:668-85. [Crossref] [PubMed]
- Li C, Hu Y, Huang J, et al. Comparison of robotic-assisted lobectomy with video-assisted thoracic surgery for stage IIB-IIIA non-small cell lung cancer. Transl Lung Cancer Res 2019;8:820-8. [Crossref] [PubMed]
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- Servais EL, Miller DL, Thibault D, et al. Conversion to Thoracotomy During Thoracoscopic vs Robotic Lobectomy: Predictors and Outcomes. Ann Thorac Surg 2022;114:409-17. [Crossref] [PubMed]
- Agzarian J, Fahim C, Shargall Y, et al. The Use of Robotic-Assisted Thoracic Surgery for Lung Resection: A Comprehensive Systematic Review. Semin Thorac Cardiovasc Surg 2016;28:182-92. [Crossref] [PubMed]
- Wu H, Jin R, Yang S, et al. Long-term and short-term outcomes of robot- versus video-assisted anatomic lung resection in lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2021;59:732-40. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- Mao J, Tang Z, Mi Y, et al. Robotic and video-assisted lobectomy/segmentectomy for non-small cell lung cancer have similar perioperative outcomes: a systematic review and meta-analysis. Transl Cancer Res 2021;10:3883-93. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Ismail M, Swierzy M, Ulrich M, et al. Application of the da Vinci robotic system in thoracic surgery. Chirurg 2013;84:643-50. [Crossref] [PubMed]
- Bellini V, Valente M, Del Rio P, et al. Artificial intelligence in thoracic surgery: a narrative review. J Thorac Dis 2021;13:6963-75. [Crossref] [PubMed]
- Ostberg NP, Zafar MA, Elefteriades JA. Machine learning: principles and applications for thoracic surgery. Eur J Cardiothorac Surg 2021;60:213-21. [Crossref] [PubMed]
- Le Moal J, Peillon C, Dacher JN, et al. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: a pilot study. J Thorac Dis 2018;10:196-201. [Crossref] [PubMed]
- Shanahan B, Kreaden US, Sorensen J, et al. Is robotic lobectomy cheaper? A micro-cost analysis. J Robot Surg 2022;16:1441-50. [Crossref] [PubMed]
- Heiden BT, Mitchell JD, Rome E, et al. Cost-Effectiveness Analysis of Robotic-assisted Lobectomy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2022;114:265-72. [Crossref] [PubMed]


