
Survival benefit of resection surgery for lung adenocarcinoma with bone metastases and a post-operative prognosis nomogram establishment and validation
Highlight box
Key findings
• We revealed that surgery could improve the prognosis of lung adenocarcinoma (LUAD) patients with bone metastases. And we developed a nomogram model for these patients who underwent surgery to predict the survival rates after surgery.
What is known and what is new?
• Although currently available treatment options have improved clinical benefits, the prognosis of lung LUAD patients with bone metastases is still poor, and surgery is not usually recommended for these patients.
• We firstly demonstrated that surgery prolongs the prognosis of LUAD patients with bone metastases and firstly constructed and validated a new prognostic model for patients underwent surgery.
What is the implication, and what should change now?
• Our findings have showed new light on the potential benefits of surgery for LUAD patients with bone metastases. And our prognostic model will provide individualized assessment for clinical decision making in the future.
Introduction
Lung cancer is one of the most common malignancies worldwide and the main cause of cancer-related deaths (1), accounting for approximately one tenth (11.4%) of total diagnosed cancers and one fifth (18.0%) of total cancer deaths, respectively (2,3). Among lung cancer patients, more than 85% are diagnosed with non-small cell lung cancer (NSCLC), with lung adenocarcinoma (LUAD) the most common NSCLC subtype (4,5). Early-stage symptoms of lung cancer may be asymptomatic or atypical, rendering a diagnosis difficult, and when symptoms such as hemoptysis, chest pain, and chest tightness appear, many patients have progressed to advanced disease and distant metastasis (6). Bone is an important metastatic site for bloodborne metastasis of lung cancer and is present in about 30–40% of patients throughout the course of the disease (7,8). The highest proportion of adenocarcinoma among lung cancer patients with bone metastases is about 50.3%, and the proximal spine and trunk bones are the most common sites (9). Most patients will suffer skeletal-related events (SREs) such as bone pain, pathological fractures, and spinal cord compression, which greatly affects their survival time and quality of life.
For patients with stage IV lung cancer, gender, histological type, number of metastatic organs, continued effective chemotherapy, and targeted therapy are important prognostic factors (10,11). Surgery is a common treatment for early-stage lung cancer. However, for patients with stage IV disease, resection of the primary tumor is not usually considered as a first treatment option. This is because even if surgery is chosen, the rate of recurrence and distant metastasis remains high after surgery, and the local recurrence and distant metastasis of the tumor make surgery limited (12,13). As the goal of treatment for these patients is to reduce pain while trying to prevent complications and prolong and improve their life, systemic therapy is usually chosen (14). Several studies have revealed the epidemiological characteristics and prognosis of patients with bone metastases of lung cancer, but have not illustrated the survival benefit of surgery for patients (15-17), while some retrospective studies have shown primary tumor resection could provide a survival benefit in metastatic NSCLC (18-20). With the development of surgical techniques and advances in multidisciplinary treatment, some IV stage patients, such as NSCLC patients with a single brain or adrenal metastases, can live longer with surgery (21,22). Therefore, there is a great need to gather evidence for the benefit of cancer-directed surgery (CDS) for patients with bone metastases of lung cancer and to construct an easily implemented model to predict survival after surgery to provide more personalized treatment.
Only by focusing on a more specific and limited disease can we better understand the potential benefits of surgery and make individualized assessments. Therefore, this study limited patients to LUAD with bone metastases, as it occurred in a large proportion of patients (9). We aimed to use data from the Surveillance, Epidemiology and End Results (SEER) database to investigate the value of CDS for LUAD patients with bone metastases and to identify independent prognostic factors associated with survival in those who underwent surgery. Based on this, we developed a nomogram to predict postoperative survival in LUAD patients with bone metastases. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1514/rc).
Methods
Patient selection
Patient information for this retrospective population-based study was obtained from the SEER database. The SEER database is a large oncology database created by the National Cancer Institute that contains patient clinical information, tumor characteristics, treatment, and survival information covering approximately 28% of the U.S. population (23). The data was extracted by using SEER*Stat (version 8.4.0.1) software. Our inclusion criteria were as follows: (I) year of diagnosis from 2010 to 2015; (II) primary site was lung and bronchus, and the metastatic site was bone; (III) adenocarcinoma diagnosed under microscope. Histological code (ICD-O-3): 8140-8147, 8255, 8260, 8310, 8323, 8480, 8481, 8490, 8550, and 8572. Exclusion criteria were: (I) patients with other metastatic sites such as liver, lung, and brain; (II) patients whose diagnosis was not confirmed by microscopy; (III) unknown surgical status; (IV) patient survival months or tumor size was “0”. Variables including age, sex, race, marital status, tumor site, laterality (lateral or bilateral), histologic grade, T stage, N stage, radiotherapy status, chemotherapy status, CDS status, survival time, tumor-specific death, and all-cause death were collected. All cases in this study were staged using the 7th edition TNM staging system.
The data used in this study are publicly available and do not include identifying information for individual patients. Therefore, written informed consent from patients or institutional review board approval was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Propensity score-matching (PSM)
The study sample was divided into a CDS group and a non-CDS group according to the CDS status of the patients. To reduce the effects of data bias and confounding variables in the groups, PSM was performed (24,25). That is because in observational studies, study subjects are non-randomly assigned, which can cause confounding factors to be unevenly distributed between the two groups, resulting in confounding interference in the relationship between treatment factors and outcomes. And PSM method reduces intergroup bias by balancing the distribution of characteristic variables between the experimental and control groups (26). Variables that may affect treatment outcome were used to generate propensity scores by logistic regression, including age, sex, race, marital status, tumor site, laterality, histologic grade, T stage, N stage, radiotherapy status, and chemotherapy status. Patients in the two groups were matched 1:1 using the closest propensity score on the logit scale. After PSM, the significance of the differences in all variables were tested by chi-square test.
Survival analysis
Overall survival (OS) and cancer-specific survival (CSS) were estimated using Kaplan-Meier Method and compared with the log-rank test. OS was defined as the time from diagnosis to death from any cause (patients still alive at the end of the study were excluded). CSS was calculated from the date of diagnosis to the date of death due to cancer. Deaths attributed to cancer were considered events, and deaths from causes unrelated to cancer were censored at the last follow-up. The variables included in the multivariate Cox proportional risk model were determined by a forward-stepwise-selection method based on the smallest Akaike Information Criterion (AIC) values, which indicated the minimal loss of prognostic information (27,28). A multivariate Cox proportional risk model was used to identify independent prognostic factors, and 95% confidence intervals (CIs) and risk ratios (HRs) were calculated for the variables. We also compared the survival benefit of CDS in a subgroup analysis and generated forest plots with HR and 95% CI.
Development and validation of a nomogram in CDS group patients
Patients who underwent CDS were randomized in a 7:3 ratio into a training cohort and a validation cohort. The training cohort was used to develop the column line graph and the validation cohort was used to verify the model. To determine the independent prognostic factors for patients who underwent surgical treatment, the smallest AIC based forward-stepwise-selection method was used to select variables into the multivariate Cox proportional risk regression model. The variables with P<0.05 were identified as independent prognostic factors. The nomogram prediction model was then developed based on the Cox regression model to predict OS probability at 1-, 2-, and 3-year, and the concordance index (C-index) was calculated to evaluate the discriminative power of the nomogram. The calibration plots for the 1-, 2-, and 3-year OS probability were plotted to compare the predicted and actual events. In addition, the time independent receiver operating characteristic curve (ROC) and the area under the curve (AUC) were used to evaluate the ability of the model to discriminate between events and non-events at 1-, 2-, and 3-year. The sensitivity and specificity of the nomogram were also evaluated based on the best cutoff of ROC. Finally, patients were divided into high-risk and low-risk groups according to the best cutoff value of the risk score (based on the total score of each patient in the nomogram), and the log-rank test of the Kaplan-Meier survival curve was performed to verify its prognostic value.
Statistical analysis
Categorical variables were analyzed separately by chi-square test. All statistical calculations were performed using R software version 4.2.1 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). PSM was performed using the “MatchIt” package, and the smallest AIC value was calculated by the “MuMIn” and “survival” packages. The nomogram and calibration plots were produced using the “rms” package. ROC curves were plotted by the “survivalROC” package, and the best cutoff value was determined by the “surv_cutpoint” function of the “survminer” package. In all statistical tests, P<0.05 was considered statistically significant, and all tests were two-sided.
Results
Clinicopathological characteristics of the selected patients before and after PSM
We identified 22,998 patients with microscopically confirmed LUAD with bone metastases in the SEER database between 2010 and 2015, of which 7,984 patients met the inclusion criteria (Figure 1). Among these eligible patients, 200 (2.5%) underwent CDS of the primary tumor, while the remaining 7,784 (97.5%) did not. The clinicopathological characteristics of patients are presented in Table 1. Before PSM, there was a significant difference between the CDS and non-CDS groups in terms of age, marriage, histological grade, T stage, N stage and radiotherapy (P<0.05), which was due to an imbalance in the baseline characteristics of the two groups.
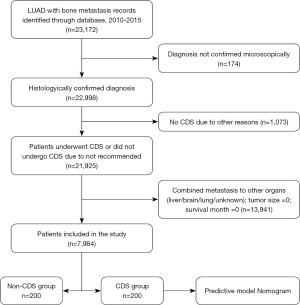
Table 1
| Characteristics | CDS (N=200) (%) | Non-CDS (N=7,784) (%) | χ2 | P |
|---|---|---|---|---|
| Age, years | 18.00 | <0.001 | ||
| <65 | 96 (48.0) | 2,858 (36.7) | ||
| 65–74 | 70 (35.0) | 2,590 (33.3) | ||
| ≥75 | 34 (17.0) | 2,336 (30.0) | ||
| Sex | 1.02 | 0.312 | ||
| Female | 97 (48.5) | 3,475 (44.6) | ||
| Male | 103 (51.5) | 4,309 (55.4) | ||
| Race | 4.19 | 0.123 | ||
| Black | 13 (6.5) | 841 (10.8) | ||
| Other | 17 (8.5) | 730 (9.4) | ||
| White | 170 (85.0) | 6,213 (79.8) | ||
| Marital status | 12.52 | 0.014 | ||
| Divorced/widowed | 32 (16.0) | 2,030 (26.1) | ||
| Married | 132 (66.0) | 4,285 (55.0) | ||
| Unmarried | 36 (18.0) | 1,469 (18.9) | ||
| Primary site | 9.45 | 0.093 | ||
| Main bronchus | 5 (2.5) | 234 (3.0) | ||
| Upper lobe | 103 (51.5) | 4,048 (52.0) | ||
| Middle lobe | 13 (6.5) | 362 (4.7) | ||
| Lower lobe | 61 (30.5) | 2,033 (26.1) | ||
| Overlapping lesion | 3 (1.5) | 61 (0.8) | ||
| Lung, NOS | 15 (7.5) | 1,046 (13.4) | ||
| Laterality | 1.91 | 0.167 | ||
| Bilateral | 3 (1.5) | 279 (3.6) | ||
| Unilateral | 197 (98.5) | 7,505 (96.4) | ||
| Grade | 149.88 | < 0.001 | ||
| I | 8 (4.0) | 204 (2.6) | ||
| II | 60 (30.0) | 913 (11.7) | ||
| III | 85 (42.5) | 1,625 (20.9) | ||
| IV | 1 (0.5) | 40 (0.5) | ||
| Unknown | 46 (23.0) | 5,002 (64.3) | ||
| T stage | 10.47 | 0.033 | ||
| T1 | 42 (21.0) | 1,334 (17.1) | ||
| T2 | 63 (31.5) | 2,257 (29.0) | ||
| T3 | 42 (21.0) | 1,466 (18.8) | ||
| T4 | 38 (19.0) | 1,538 (19.8) | ||
| TX | 15 (7.5) | 1,189 (15.3) | ||
| N stage | 38.52 | <0.001 | ||
| N0 | 84 (42.0) | 1,986 (25.5) | ||
| N1 | 25 (12.5) | 710 (9.1) | ||
| N2 | 66 (33.0) | 3,288 (42.2) | ||
| N3 | 14 (7.0) | 1,319 (16.9) | ||
| NX | 11 (5.5) | 481 (6.2) | ||
| Radiotherapy | 5.77 | 0.016 | ||
| No/unknown | 113 (56.5) | 3,709 (47.6) | ||
| Yes | 87 (43.5) | 4,075 (52.4) | ||
| Chemotherapy | 0.75 | 0.387 | ||
| No/unknown | 64 (32.0) | 2,741 (35.2) | ||
| Yes | 136 (68.0) | 5,043 (64.8) |
LUAD, lung adenocarcinoma; CDS, cancer-directed surgery; NOS, not otherwise specified; PSM, propensity score-matching.
After 1:1 PSM, 200 matched patients in both groups were matched, and the baseline characteristics table showed no significant differences between the two in terms of age, gender, race, marriage, primary site, laterality, histologic grade, T stage, N stage, radiotherapy and chemotherapy (P>0.05), as detailed in Table 2, indicating minimal potential bias.
Table 2
| Characteristics | CDS (N=200) (%) | Non-CDS (N=200) (%) | χ2 | P |
|---|---|---|---|---|
| Age, years | 3.05 | 0.218 | ||
| <65 | 96 (48.0) | 112 (56.0) | ||
| 65–74 | 70 (35.0) | 55 (27.5) | ||
| ≥75 | 34 (17.0) | 33 (16.5) | ||
| Sex | 0.04 | 0.841 | ||
| Female | 97 (48.5) | 94 (47.0) | ||
| Male | 103 (51.5) | 106 (53.0) | ||
| Race | 0.34 | 0.844 | ||
| Black | 13 (6.5) | 14 (7.0) | ||
| Other | 17 (8.5) | 14 (7.0) | ||
| White | 170 (85.0) | 172 (86.0) | ||
| Marital status | 4.20 | 0.379 | ||
| Divorced/widowed | 32 (16.0) | 41 (20.5) | ||
| Married | 132 (66.0) | 114 (57.0) | ||
| Unmarried | 36 (18.0) | 45 (22.5) | ||
| Primary site | 2.57 | 0.767 | ||
| Main bronchus | 5 (2.5) | 4 (2.0) | ||
| Upper lobe | 103 (51.5) | 115 (57.5) | ||
| Middle lobe | 13 (6.5) | 15 (7.5) | ||
| Lower lobe | 61 (30.5) | 51 (25.5) | ||
| Overlapping lesion | 3 (1.5) | 4 (2.0) | ||
| Lung, NOS | 15 (7.5) | 11 (5.5) | ||
| Laterality | 0.25 | 0.615 | ||
| Bilateral | 3 (1.5) | 1 (0.5) | ||
| Unilateral | 197 (98.5) | 199 (99.5) | ||
| Grade | 1.11 | 0.893 | ||
| I | 8 (4.0) | 7 (3.5) | ||
| II | 60 (30.0) | 60 (30.0) | ||
| III | 85 (42.5) | 85 (42.5) | ||
| IV | 1 (0.5) | 0 (0) | ||
| Unknown | 46 (23.0) | 48 (24.0) | ||
| T stage | 0.82 | 0.936 | ||
| T1 | 42 (21.0) | 37 (18.5) | ||
| T2 | 63 (31.5) | 70 (35.0) | ||
| T3 | 42 (21.0) | 43 (21.5) | ||
| T4 | 38 (19.0) | 35 (17.5) | ||
| TX | 15 (7.5) | 15 (7.5) | ||
| N stage | 1.43 | 0.838 | ||
| N0 | 84 (42.0) | 78 (39.0) | ||
| N1 | 25 (12.5) | 29 (14.5) | ||
| N2 | 66 (33.0) | 73 (36.5) | ||
| N3 | 14 (7.0) | 11 (5.5) | ||
| NX | 11 (5.5) | 9 (4.5) | ||
| Radiotherapy | 1.49 | 0.221 | ||
| No/unknown | 113 (56.5) | 126 (63.0) | ||
| Yes | 87 (43.5) | 74 (37.0) | ||
| Chemotherapy | 0.22 | 0.914 | ||
| No/unknown | 64 (32.0) | 62 (31.0) | ||
| Yes | 136 (68.0) | 138 (69.0) |
LUAD, lung adenocarcinoma; CDS, cancer-directed surgery; NOS, not otherwise specified; PSM, propensity score-matching.
Impact of CDS on survival outcomes
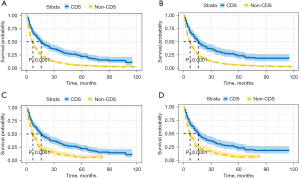
After summarizing the baseline characteristics, we used Kaplan-Meir analysis and log-rank test to investigate the prognostic impact of CDS. Notably, patients who underwent CDS had a longer OS and CSS than those who did not, regardless of PSM (Figure 2, P<0.0001). For example, after PSM, the 1-year OS rates were 30.1% and 56.9% in the non-CDS and CDS groups, respectively, and the 3-year OS rates were 11.2% and 32.5%, respectively. The CSS rates of CDS groups were also higher than that of non-CDS groups (1-year: 56.5% vs. 30.4%, 3-year: 31.4% vs. 12.4%). In addition, we found that patients who underwent CDS also had a higher median survival time than those who did not, with a median survival of 16 months OS in the CDS group versus 7 months in the non-CDS group (P<0.001). We then examined the effect of CDS on the prognosis of each subgroup, and found that after CDS, the prognosis was improved in most subgroups compared to patients with non-CDS. We found CDS did not improve OS in patients with a primary site of bronchus or overlapping lesion, TNM stage T4, TX or, N3 (Figure 3, P>0.05), and CSS in patients with a primary site of bronchus or overlapping lesion, unmarried, stage T4, or N3 (Figure S1, P>0.05).

CDS as an independent prognostic factor for survival in matched patients
We used the AIC method to assess the impact of CDS on prognosis in a multivariate setting and to identify other factors associated with prognosis. When we included seven variables (age, gender, race, histological grade, N stage, chemotherapy, and CDS) in a multivariate Cox regression analysis of OS, the AIC value was the smallest (AIC =3,602.7), and the smallest AIC value (AIC =2,994.5) also occurred when we included these variables in the multivariate Cox regression analysis of CSS (Table 3). In multivariate Cox analysis, CDS was independently associated with better OS (HR =0.47, 95% CI: 0.39–0.57, P<0.001) and CSS (HR =0.51, 95% CI: 0.42–0.63, P<0.001), while age, gender, race, histological grade, N stage, and chemotherapy were all independent factors affecting survival in LUAD patients with bone metastases (Table 3).
Table 3
| Factors | OS | CSS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age, years | |||||
| <65 | Reference | Reference | |||
| 65–74 | 0.96 (0.78–1.18) | 0.729 | 0.91 (0.72–1.13) | 0.465 | |
| ≥75 | 1.40 (1.09–1.81) | 0.028 | 1.62 (1.22–2.13) | 0.004 | |
| Sex | |||||
| Female | Reference | Reference | |||
| Male | 1.56 (1.30–1.88) | <0.001 | 1.65 (1.35–2.02) | <0.001 | |
| Race | |||||
| Non-white | Reference | Reference | |||
| White | 1.45 (1.09–1.91) | 0.027 | 1.57 (1.16–2.12) | 0.013 | |
| Grade | |||||
| I-II | Reference | Reference | |||
| III-IV | 1.43 (1.16–1.76) | 0.004 | 1.51 (1.21–1.89) | 0.002 | |
| Unknown | 1.12 (0.87–1.44) | 0.461 | 1.08 (0.82–1.42) | 0.663 | |
| N stage | |||||
| N0-1 | Reference | Reference | |||
| N2 | 1.54 (1.26–1.88) | <0.001 | 1.55 (1.25–1.93) | <0.001 | |
| N3 | 1.80 (1.19–2.72) | 0.019 | 1.90 (1.20–3.01) | 0.021 | |
| NX | 2.21 (1.45–3.34) | 0.002 | 2.65 (1.62–4.36) | 0.001 | |
| Chemotherapy | |||||
| No/unknown | Reference | Reference | |||
| Yes | 0.47 (0.38–0.58) | <0.001 | 0.45 (0.35–0.56) | <0.001 | |
| CDS | |||||
| Non-CDS | Reference | Reference | |||
| CDS | 0.47 (0.39–0.57) | <0.001 | 0.51 (0.42–0.63) | <0.001 | |
PSM, propensity score-matching; AIC, Akaike Information Criterion; OS, overall survival; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval; CDS, cancer-directed surgery.
Nomogram variables screening
We randomly divided the 200 patients in the CDS group into a training cohort and a validation cohort in a 7:3 ratio. To determine independent prognostic factors in the CDS group, we performed an AIC method analysis on the training cohort and found the smallest AIC value occurred when we incorporated six factors into the multivariate Cox analysis: Age, sex, race, histological grade, N stage, and chemotherapy (AIC =955.9) (Table 4). These factors were identified as independent prognostic factors after surgery.
Table 4
| Factors | HR (95% CI) | P |
|---|---|---|
| Age, years | ||
| <65 | Reference | |
| 65–74 | 1.10 (0.72–1.68) | 0.653 |
| ≥75 | 1.76 (1.04–2.98) | 0.036 |
| Sex | ||
| Female | Reference | |
| Male | 1.48 (1.01–2.16) | 0.044 |
| Race | ||
| Non-white | Reference | |
| White | 2.06 (1.16–3.67) | 0.013 |
| Grade | ||
| I-II | Reference | |
| III-IV | 1.89 (1.22–2.95) | 0.005 |
| Unknown | 1.87 (1.10–3.18) | 0.021 |
| N stage | ||
| N0-1 | Reference | |
| N2 | 3.26 (2.01–5.29) | <0.001 |
| N3 | 5.22 (2.48–10.98) | <0.001 |
| NX | 2.12 (0.92–4.91) | 0.079 |
| Chemotherapy | ||
| No/unknown | Reference | |
| Yes | 0.38 (0.24–0.61) | <0.001 |
OS, overall survival; AIC, Akaike Information Criterion; HR, hazard ratio; CI, confidence interval.
Nomogram construction
We next constructed a nomogram based on selected independent prognostic factors for LUAD patients with bone metastases who underwent CDS using the six variables listed in the previous section. Following this, the nomogram was constructed based on the training cohort for predicting 1-, 2-, and 3-year OS (Figure 4).
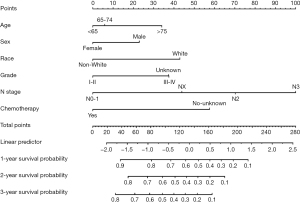
The OS nomogram indicated N stage was the strongest prognostic factor, followed by chemotherapy status and race with a greater impact on the nomogram. The probability of survival of a particular patient can be calculated by adding the scores of the selected variables. Each variable on the nomogram was assigned a point, and a vertical line was drawn to the top points row to determine the number of points received for each variable value. The number of points from each variable was then added together and a vertical line dropped from the total points row to obtain the likelihood of OS time (1-, 2-, and 3-year OS probability) (Figure 4). For example, for a 50-year-old white male patient who was diagnosed with LUAD with bone metastases and N2 stage was histologically graded as moderately differentiated, and had already undergone surgery and chemotherapy scored 136, and the model predicts the probability of his survival for 1 year as 56%.
Nomogram validation and calibration
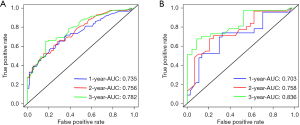
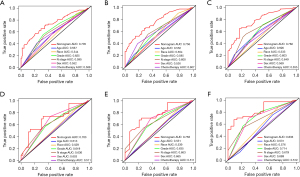
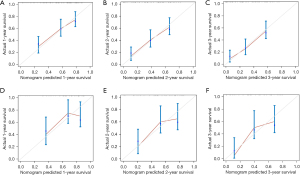
The nomogram was validated both in the training cohort and in the validation cohort and showed the C-index was 0.692 in the training cohort and 0.710 in the validation cohort for OS. Based on the ROC curve analyses, the time dependent AUC values of 1, 2, and 3 years were 0.735, 0.756, and 0.782 in the training cohort and 0.703, 0.758, and 0.836 in the verification cohort, respectively (Figure 5). The corresponding sensitivity and specificity of the time dependent ROC at 1, 2, and 3 years were evaluated in training (sensitivity: 0.654, 0.522 and 0.652; specificity: 0.692, 0.828 and 0.805) and validation (sensitivity: 0.739, 0.677 and 0.676; specificity: 0.650, 0.759 and 0.869) cohort. The nomogram and independent prognostic factors were then compared and showed the AUC values of the former were significantly higher than all independent prognostic factors for 1-, 2-, and 3-year OS in the training cohort, and the same result was confirmed in the validation cohort (Figure 6). These results confirm the C-index and AUC values had good discriminative and accurate prediction capabilities. Furthermore, we generated calibration curves for possible 1-, 2-, and 3-year OS (Figure 7), and the calibration curves for survival probability indicated the nomogram has an optimal correlation between OS prediction and observation in both training cohort and validation cohort. Our newly developed nomogram reliably predicted survival in LUAD patients with bone metastases after CDS.
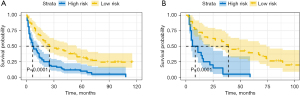
The prognostic nomogram in clinical practice
We performed an exploratory examination of the predicted values of the nomogram for risk stratification. First, the total risk score for each patient in the training cohort was determined, and the cutoff value for the total score was calculated to be 105. Therefore, we categorized patients with scores greater than or equal to 105 as a high-risk group, and those with scores less than 105 as a low-risk group. The Kaplan-Meier OS curves showed that in the training cohort, patients in the low-risk group had a better prognosis than those in the high-risk group (log-rank, P<0.0001) (Figure 8A), and the same cutoff value for OS also distinguished high- and low-risk groups in the validation cohort (log-rank, P<0.0001) (Figure 8B). These results indicate patients classified in the low-risk group could derived the greatest survival benefit from CDS and the risk classification system based on the nomogram was a valid predictor of survival in patients with bone metastases of LUAD after surgery.
Discussion
In this retrospective study, we screened cases from the SEER database to analyze the efficacy of CDS with primary tumor resection and other treatment modalities in patients with bone metastases from LUAD. By using PSM to reduce potential confounding in the CDS and non-CDS groups, we found a positive prognostic effect of CDS on LUAD patients with bone metastases. In addition, we developed a prognostic nomogram to predict OS at 1, 2, and 3 years postoperatively in patients undergoing surgery and validated the accuracy of the nomogram by developing ROC and calibration curves, and the results indicate this could be a meaningful evaluation tool for clinicians.
Bone is one of the main sites of hematogenous metastasis in advanced lung cancer, and the incidence of bone metastasis in LUAD is the highest, followed by small cell lung cancer and squamous lung cancer (29). The 1-year survival rate after the occurrence of bone metastases from lung cancer is 5.3%, the 2-year survival rate is 2.1%, and the median survival time is only 6–10 months (30). Traditionally, the treatment of bone metastases was not curative, and patients were treated with a multidisciplinary approach based on systemic therapy combined with optimal local treatment, including radiotherapy, targeted therapy, immunotherapy, surgery, and symptomatic pain relief (31). Among these treatment modalities, surgery was used not to target the primary lesion but to resect isolated bone metastases with the aim of preventing and treating pathological fractures (especially weight-bearing bone) and reducing bone pain and spinal cord compression to improve the quality of life of patients (32). However, with the development of surgical techniques, advances in perioperative management, and multidisciplinary approaches to care, adverse events associated with surgical death have decreased, and resection of the primary tumor has also been reconsidered as part of the treatment of advanced LUAD (33). In recent years, several studies have shown that the prognosis of stage IV NSCLC could improve by resection of the primary lesion (18-20). Although there are no current guidelines recommencing this for patients with bone metastases from advanced NSCLC, some evidence supports surgical intervention (17,34). In our study, we focused on a more specific type of bone metastatic lung cancer as this helped make more precise individualized decisions and because LUAD has the highest incidence of bone metastases (29). We believed CDS targeting the primary tumor could slow tumor progression by reducing tumor load and decreasing the release of tumor cells into the bloodstream. Our findings indicated OS and CSS were better in the surgical group than in the non-surgical group, and that CDS could provide a survival benefit for patients with bone metastases of LUAD. However, our results also suggested not all patients could benefit from CDS, such as those with T4 or N3 stage. Therefore, we must conduct an aggressive exploration in the future to determine who is suitable for CDS and when to perform the surgery.
To our knowledge, this is the first nomogram developed and validated for predicting postoperative survival in LUAD patients with bone metastases. Nomograms have been widely used in cancer prediction as tools that combine multiple predictors of prognosis to assess it. Nomograms allow clinicians to know more intuitively the prognostic survival of patients and make individualized decisions (35). We studied LUAD patients with bone metastases who received CDS based on the SEER database. By the smallest AIC based forward-stepwise-selection method, several predictors of postoperative survival were screened out and included in a multivariate Cox regression model, and we identified survival-related independent prognostic factors including age, gender, race, N stage, histologic grade, and chemotherapy status. By combining these factors, we developed a nomogram that could accurately predict 1-, 2-, and 3-year postoperative OS in these patients. The nomogram demonstrated good discrimination and calibration in both training and validation cohorts and has the potential to be applied to realistic clinical decision making.
We found chemotherapy was an independent prognostic risk factor for LUAD patients with bone metastases, which suggested systemic therapy is required. In contrast, we did not observe a prognostic role for radiotherapy, which is puzzling. This may be because for patients with bone metastases, radiotherapy is only a local treatment used to alleviate bone pain and prevent SREs such as pathological fractures, and does not delay tumor progression (36). Another reason may be that although patients received radiotherapy, the high dose and long course resulted in severe myelosuppression, and survival time did not improve. As Wallace et al. alert, patients with bone metastases should be treated with shorter courses of radiotherapy (37), and LUAD is not particularly sensitive to radiation, resulting in tumor cells becoming tolerant to it (38).
This study has several limitations. First, the SEER database lacks basic information about patients, such as smoking history, cardiopulmonary function, and the specific site and number of metastases, which may affect the clinicians’ choice of treatment options. Second, we did not access the exact radiotherapy information, such as the site, regimen, and dose of chemotherapy, and did not access the data on targeted therapies and immunotherapy. Furthermore, as a retrospective study, selection bias is inevitable, although we have used PSM to reduce this variation. Finally, our model was not further externally validated with other datasets, which may bias the validation of model performance. In the future, large sample sizes and multicenter prospective studies are required to enable individualized assessment of surgery in patients with bone metastases of LUAD.
Conclusions
In summary, by analyzing data from the SEER database, this study demonstrated surgery could improve the prognosis for LUAD patients with bone metastases. In addition, the nomogram we developed may be a valid model for predicting OS after surgery in LUAD patients with bone metastases and may provide individualized clinical assessment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1514/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1514/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535-46. [Crossref] [PubMed]
- Couraud S, Zalcman G, Milleron B, et al. Lung cancer in never smokers--a review. Eur J Cancer 2012;48:1299-311. [Crossref] [PubMed]
- Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer 2015;136:1921-30. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Wang G, Chen J, Ma R, et al. Effects of zoledronic acid and ibandronate in the treatment of cancer pain in rats with lung cancer combined with bone metastases. Oncol Lett 2018;16:1696-700. [Crossref] [PubMed]
- Cetin K, Christiansen CF, Jacobsen JB, et al. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer 2014;86:247-54. [Crossref] [PubMed]
- Hirashima T, Suzuki H, Okamoto N, et al. Important factors for achieving survival of five years or more in non-small cell lung cancer patients with distant metastasis. Oncol Lett 2014;8:327-34. [Crossref] [PubMed]
- Zhang R, Li P, Li Q, et al. Radiotherapy improves the survival of patients with stage IV NSCLC: A propensity score matched analysis of the SEER database. Cancer Med 2018;7:5015-26. [Crossref] [PubMed]
- Ma L, Qiu B, Zhang J, et al. Survival and prognostic factors of non-small cell lung cancer patients with postoperative locoregional recurrence treated with radical radiotherapy. Chin J Cancer 2017;36:93. [Crossref] [PubMed]
- Su Q, Sun YP, Liu YH, et al. Prognostic factors in older patients with advanced non-small cell lung cancer in China. Tumori 2014;100:69-74. [Crossref] [PubMed]
- David EA, Canter RJ, Chen Y, et al. Surgical Management of Advanced Non-Small Cell Lung Cancer Is Decreasing But Is Associated With Improved Survival. Ann Thorac Surg 2016;102:1101-9. [Crossref] [PubMed]
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among patients 65 years and above with lung cancer: A population-based analysis of U.S. Medicare beneficiaries, 1999-2006. Lung India 2013;30:20-6. [Crossref] [PubMed]
- Sun JM, Ahn JS, Lee S, et al. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer 2011;71:89-93. [Crossref] [PubMed]
- Zheng XQ, Huang JF, Lin JL, et al. Incidence, prognostic factors, and a nomogram of lung cancer with bone metastasis at initial diagnosis: a population-based study. Transl Lung Cancer Res 2019;8:367-79. [Crossref] [PubMed]
- Kawano D, Takeo S, Katsura M, et al. Surgical treatment of stage IV non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2012;14:167-70. [Crossref] [PubMed]
- Abdel-Rahman O. Outcomes of Surgery as Part of the Management of Metastatic Non-Small-Cell Lung Cancer: A Surveillance, Epidemiology and End Results Database Analysis. Cancer Invest 2018;36:238-45. [Crossref] [PubMed]
- Sun Z, Sui X, Yang F, et al. Effects of primary tumor resection on the survival of patients with stage IV extrathoracic metastatic non-small cell lung cancer: A population-based study. Lung Cancer 2019;129:98-106. [Crossref] [PubMed]
- Congedo MT, Cesario A, Lococo F, et al. Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. J Thorac Cardiovasc Surg 2012;144:444-52. [Crossref] [PubMed]
- Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg 1996;62:1614-6. [Crossref] [PubMed]
- Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 2014;120:3755-7. [Crossref] [PubMed]
- Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol 2010;172:1092-7. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27:2037-49. [Crossref] [PubMed]
- Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol 2004;53:793-808. [Crossref] [PubMed]
- Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev 2004;11:192-6. [Crossref] [PubMed]
- Oliveira MB, Mello FC, Paschoal ME. The relationship between lung cancer histology and the clinicopathological characteristics of bone metastases. Lung Cancer 2016;96:19-24. [Crossref] [PubMed]
- Hoda MA, Rozsas A, Lang E, et al. High circulating activin A level is associated with tumor progression and predicts poor prognosis in lung adenocarcinoma. Oncotarget 2016;7:13388-99. [Crossref] [PubMed]
- Manabe J, Kawaguchi N, Matsumoto S, et al. Surgical treatment of bone metastasis: indications and outcomes. Int J Clin Oncol 2005;10:103-11. [Crossref] [PubMed]
- Tsuya A, Kurata T, Tamura K, et al. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 2007;57:229-32. [Crossref] [PubMed]
- Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev 2016;42:56-72. [Crossref] [PubMed]
- Tian D, Ben X, Wang S, et al. Surgical resection of primary tumors improved the prognosis of patients with bone metastasis of non-small cell lung cancer: a population-based and propensity score-matched study. Ann Transl Med 2021;9:775. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- De Felice F, Piccioli A, Musio D, et al. The role of radiation therapy in bone metastases management. Oncotarget 2017;8:25691-9. [Crossref] [PubMed]
- Wallace AS, Fiveash JB, Williams CP, et al. Choosing Wisely at the End of Life: Use of Shorter Courses of Palliative Radiation Therapy for Bone Metastasis. Int J Radiat Oncol Biol Phys 2018;102:320-4. [Crossref] [PubMed]
- Niu H, Huang Y, Yan L, et al. Knockdown of SMAD3 inhibits the growth and enhances the radiosensitivity of lung adenocarcinoma via p21 in vitro and in vivo. Int J Biol Sci 2020;16:1010-22. [Crossref] [PubMed]
(English Language Editor: B. Draper)


