
Clinical significance of mean corpuscular volume as a prognostic indicator of radiotherapy for locally advanced lung cancer: a retrospective cohort study
Highlight box
Key findings
• MCV can roughly predict the survival time of patients with locally advanced lung cancer after radiotherapy.
What is known and what is new?
• MCV can predict the survival of many solid tumors.
• We found that MCV can predict the survival time of locally advanced lung cancer after radiotherapy.
What is the implication, and what should change now?
• High MCV is an unfavorable predictor of OS, which can be used to roughly predict its survival time.
Introduction
Lung cancer is currently the most common cancer in China and the leading cause of cancer death (1). While the incidence and mortality of other cancers have declined, the incidence of lung cancer has gradually increased (2). About 30% of patients with non-small cell lung cancer are diagnosed with stage III disease, which is usually unresectable. Radiotherapy and concurrent radiotherapy have become the standard treatment for locally advanced lung cancer, but prognosis is poor, with a 5-year survival rate of »15–30% (3-5).
So far, traditional tumor-based histopathological risk factors such as tumor and lymph node stages, tumor differentiation, and resection margin status are the main clinical prognostic factors but can only be determined from a biopsy. Furthermore, these factors are often influenced by neoadjuvant therapy. Therefore, additional prognostic indicators available preoperatively are urgently needed.
Recent findings suggest that mean corpuscular volume (MCV) is associated with prognosis in head and neck tumors, colorectal cancer, gastroesophageal adenocarcinoma, esophageal cancer, and liver cancer (6-10). Alcoholism can cause elevated MCV and folic acid deficiencies (11), and lung cancer is highly associated with alcohol and nicotine abuse (12). In addition, folic acid and vitamin B12 deficiencies, oxidative stress, and chemotherapy are known causes of elevated and/or altered MCV levels in cancer patients (10,13-16).
Currently, there is no research on the prognostic value of MCV in patients with locally advanced lung cancer, so we conducted a retrospective cohort study of patients with locally advanced lung cancer undergoing radiotherapy for the potential prognostic value of MCV. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1684/rc).
Methods
Patients
Patients with locally advanced lung cancer, including those clinically or pathologically diagnosed, who underwent intensity-modulated radiotherapy in the Department of Radiation Oncology at The First Affiliated Hospital of Shandong First Medical University between January 2013 and April 2017 were enrolled. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of The First Affiliated Hospital of Shandong First Medical University (No. 2022-S607). Individual consent for this retrospective analysis was waived.
Eligible patients were: ≥18 years old, male or female, with locally advanced lung cancer confirmed by cytology or histology and without a history of malignant tumor, radiotherapy, serious cardiopulmonary, liver, or kidney disease, immune deficiency, or other complications. The hematological and routine biochemical results of the patients within 1 week prior to radiotherapy were collected, including red blood cells (RBC), white blood cells (WBC), platelets (PLT), hemoglobin (Hb), MCV, mean corpuscular hemoglobin (MCH), hematocrit (HCT), red blood cell distribution width index standard deviation value (RDW-SD) and RBC coefficient of variation (RDW-CV). In addition, basic tumor characteristics, including tumor-node-metastasis (TNM) stage and pathological type, and baseline information such as patient age, sex, smoking and drinking history, and ECOG score were collected.
A standardized monitoring protocol followed patients for at least 5 years with clinical assessments every 3 months. Data collected after radiotherapy included clinical assessment, laboratory tests, and whole body computed tomography. Evidence of recurrence was acquired from the patients’ medical records. Overall survival (OS) was defined as the time from diagnosis to death from any cause or to the last follow-up.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences version 25.0 (SPSS Inc., Chicago, IL, USA). The optimal cutoff value for each metric was selected using the subject receiver operating characteristic (ROC) curve, and the metrics were stratified. Survival curves were drawn using the Kaplan-Meier method; differences in survival were assessed using the log-rank test; multivariate analyses were performed using Cox proportional hazards models to assess the effect of prognostic factors on survival. P<0.05 was considered statistically significant.
Results
Patients’ characteristics
A total of 112 patients with locally advanced lung cancer diagnosed between January 2012 and April 2017 were identified: 89 were male (79.5%), 23 (20.5%) were female, 46 (41.1%) were >65 years old, and 66 (58.9%) were £65 years old. The median follow-up period for the entire cohort was 24 months. The specific physiological and pathological characteristics of the patients are shown in Table 1, and the pretreatment clinicopathological parameters are shown in Table 2.
Table 1
| Characteristics | Value (N=112) |
|---|---|
| Gender, n (%) | |
| Male | 89 (79.5) |
| Female | 23 (20.5) |
| Age, years, median [range] | 62 [37–83] |
| Age, n (%) | |
| ≥65 years | 46 (41.1) |
| <65 years | 66 (58.9) |
| History of smoking, n (%) | |
| Smoking | 78 (69.6) |
| No smoking | 34 (30.4) |
| Drinking history, n (%) | |
| Drinking | 73 (65.2) |
| No drinking | 39 (34.8) |
| T stage*, n (%) | |
| T1 + T2 | 63 (63.3) |
| T3 + T4 | 49 (43.8) |
| N stage*, n (%) | |
| N0 + N1 + N2 | 53 (47.3) |
| N3 | 59 (52.7) |
| ECOG score, n (%) | |
| 0 | 56 (50.0) |
| 1+2 | 56 (50.0) |
| Pathological type, n (%) | |
| Squamous cell carcinoma | 42 (37.5) |
| Adenocarcinoma | 41 (36.6) |
| Small cell lung cancer | 29 (25.9) |
*, according to the 7th AJCC/International Union against Cancer staging system. ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee for Cancer.
Table 2
| Parameters | Mean ± SD |
|---|---|
| RBC (×1012/L) | 4.1±0.69 |
| WBC (×109/L) | 6.41±2.57 |
| PLT (×109/L) | 226.82±84.28 |
| Hb (g/L) | 124.41±19.52 |
| MCV (fL) | 91.43±6.21 |
| MCH (pg) | 30.5±2.41 |
| MCHC (g/L) | 333.67±16.77 |
| HCT | 0.373±0.06 |
| RDW-CV (%) | 13.86±1.84 |
| RDW-SD (fL) | 48.68±20.52 |
MCV, mean red blood cell volume; MCHC, mean hemoglobin concentration; HCT, hematocrit; MCH, mean corpuscular hemoglobin; RDW-CV, red blood cell width CV; RDW-SD, red blood cell width SD value; CV, coefficient of variation; SD, standard deviation.
Relationship between pretreatment hematology and basic physiological characteristics and OS
To clarify whether hematologic indexes and basic physiological characteristics of the patients were related to OS, the correlation between the values obtained before radiotherapy and OS was analyzed. T stage, N stage, ECOG score, HCT, MCV, MCH, and RBC-SD were all negatively correlated with OS (P<0.05) (Figure 1).
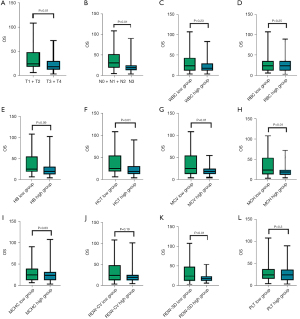
Critical value of each parameter in patients with locally advanced lung cancer before radiotherapy
From the correlation analysis, the relevant parameters were included in the ROC curve analysis, and the optimal critical point of each index parameter was obtained. MCV area under the curve (AUC) =0.683, and the best cutoff value was 93.65 fL; HCT AUC =0.565, and the best cutoff value was 0.35; MCH AUC =0.692, and the best cutoff value was 31.15; RDW-SD AUC =0.64, and the best cutoff value was 48.75 (Figure 2).

Correlation of parameters with OS
Kaplan-Meier survival analysis showed that the median OS of T1 + T2 patients was significantly better than that of T3 + T4 patients (24 vs. 18 months, P<0.01), and the median OS of N1 + N2 patients was significantly better than for N3 patients (30 vs. 18 months, P<0.01). Patients with MCV ≤93.65 fL had significantly better median OS than those with MCV >93.65 fL (24 vs. 18 months, P<0.01). The median OS of patients with HCT ≤0.35 was significantly better than that of patients with HCT >0.35 (24 vs. 18 months, P<0.01), the median OS of patients with MCH ≤31.15 was significantly better than that of patients with MCH >31.15 (24 vs. 18 months, P<0.01), and the median OS of patients with RDW-SD ≤48.75 was significantly better than that of patients with RDW-SD > 48.75 (24 vs. 18 months, P<0.01) (Figure 3).
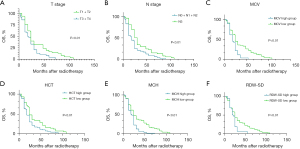
Prognostic value of MCV
To investigate the prognostic impact of MCV on OS in patients with locally advanced lung cancer, we performed a Cox regression analysis. In the univariate analysis, ECOG score (P=0.03), T stage (P=0.02), N stage (P=0.01), MCV (P=0.01), HCT (P=0.02), MCHC (P=0.01), and RDW-SD (P=0.02) (Figure 4) were significant predictors and included in the multivariate analysis results showed that MCV [odds ratio (OR) =0.534, 95% confidence interval (CI): 0.349–0.818, P=0.01], T stage (OR =0.654, 95% CI: 0.440–0.972, P=0.04) and N stage (OR =0.545, 95% CI: 0.371–0.801, P=0.01) were prognostic factors. MCV was a prognostic factor among the pretreatment hematological indices, indicating that patients with pretreatment MCV level >93.6 fL had significantly shorter OS than those with MCV <93.6 fL (Table 3).
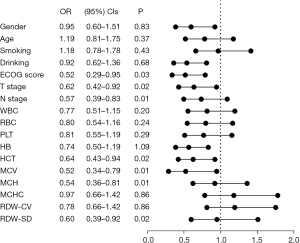
Table 3
| Factors | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| ECOG score | 0.52 | 0.29–0.95 | 0.03 | * | |||
| T stage | 0.62 | 0.42–0.92 | 0.02 | 0.65 | 0.44–0.97 | 0.04 | |
| N stage | 0.57 | 0.39–0.83 | 0.01 | 0.55 | 0.37–0.8 | 0.01 | |
| MCV | 0.52 | 0.34–0.79 | 0.01 | 0.53 | 0.35–0.82 | 0.01 | |
| HCT | 0.64 | 0.43–0.94 | 0.02 | * | |||
| MCH | 0.54 | 0.36–0.81 | 0.01 | * | |||
| RDW-SD | 0.62 | 0.39–0.92 | 0.02 | * | |||
*, not in the last step. OR, odds ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; MCV, mean red blood cell volume; HCT, hematocrit; MCH, mean corpuscular hemoglobin; RDW-SD, red blood cell width SD value; SD, standard deviation.
Discussion
In the present study, MCV was associated with OS of patients with locally advanced lung cancer undergoing radiotherapy, and a high MCV was a poor prognostic factor.
Currently, at present, the prognosis of locally advanced lung cancer mainly includes pathological type, pathological stage, molecular pathology, tumor location, treatment methods, patients’ mental state, and lifestyle, among which the TNM stage is the most effective predictor of lung cancer survival rate. In this study, T stage, N stage, and MCV all had predictive effects on long-term survival in patients with locally advanced lung cancer before radiotherapy. However, precise TNM staging usually requires postoperative or biopsy pathological evaluation, making it hard to predict survival and determine the next treatment strategies for patients with inoperable locally advanced lung cancer. For such patients, other valid predictors are urgently needed.
MCV is a relatively stable blood index in healthy crowds, which mainly reflects the degree of anemia in patients. With the advent of automatic blood counts, more and more attention has been paid to the clinical significance of its increase or decrease in predicting disease progression. Except for being used to assist in the diagnosis of hematological diseases, MCV has been reported as clinically associated with tumors such as head and neck tumors, esophageal cancer, colorectal cancer, liver cancer, etc. and a high MCV is a poor predictor of long-term survival in patients with solid tumors (6-10,17,18).
In this study, we investigated the relationship between MCV and OS in patients with locally advanced lung cancer receiving radiotherapy. First, the best cutoff value of MCV was determined by ROC curve analysis, and it was 93.6 fL. Univariate and multivariate Cox regression analysis also showed that MCV is strongly associated with OS in these patients. Those with high MCV levels before treatment had significantly shorter OS than those with low MCV levels. Similarly, we found that both T and N stages were negatively correlated with OS, which concurred with the results of previous studies (6-10,17,18).
Our study of the relationship between MCV and locally advanced lung cancer found the high MCV level is a poor prognostic factor, however, we did not determine whether MCV plays a direct role in local recurrence and metastatic invasion. We hypothesize why a high MCV is a poor prognostic factor in patients with locally advanced lung cancer receiving radiotherapy.
First, MCV is an important marker of folate level, and folate deficiency usually causes an elevated MCV. Folic acid is an important carbon unit transfer carrier in human body, which plays an important role in DNA synthesis, replication, repair and methylation. Folic acid deficiency often leads to abnormal DNA methylation, which may be a bad predictor of the prognosis of lung cancer patients (19-21).
Furthermore, patients undergoing radiotherapy for lung cancer will experience loss of appetite, nausea, vomiting, dry mouth, and dry throat, resulting in poor dietary intake and reduced serum levels of Na+, K+, and other electrolytes, leading to a loss of osmotic pressure and a negative correlation with MCV level (22).
The MCV value is bound up with the HCT/L of blood and the number of RBCs/L of blood. The decrease of RBCs per unit volume involved in oxygen transport and metabolism may lead to the increase of MCV. Fewer RBCs will result in less oxygen available to the tumor, which will increase the proportion of hypoxic tumor cells. Hypoxia reduces the sensitivity of cells to radiation therapy, leading to a poor prognosis (21,23-25).
The current study has several limitations, despite repeated confirmation and calculation of our data and results. First, the number of patients was only 112, which may have led to erratic results due to small sample sizes. Secondly, this was a single-center retrospective study of patients in the same hospital, and its conclusions have not been validated by other centers. Hence, further prospective trials in multiple centers are needed to verify the reproducibility of these results in different populations. Several diseases that affect the MCV value, such as hypothyroidism, hematogic and hepatic diseases, were not screened, which could lead to selection bias. Furthermore, patients receiving concurrent chemoradiotherapy were included and represented 45.5% of the entire cohort. Concurrent chemoradiotherapy can act as an important confounding factor. Furthermore, this study was conducted over a considerable amount of time between 2012 and 2017, with historical biases regarding treatment strategies and radiotherapy management that may have determined prognostic outcomes. Despite these limitations, our findings may be clinically relevant for pre- and post-radiotherapy treatment or monitoring planning because MCV can be obtained by routine blood testing, which is readily available and inexpensive.
Conclusions
In patients with locally advanced lung cancer who received radiotherapy, high MCV was an unfavorable predictor of OS. In the clinical treatment of patients with newly diagnosed locally advanced lung cancer, MCV can be used to roughly predict their survival time. For patients with a short-expected survival period, relatively conservative treatment methods can be adopted to shorten the radiotherapy cycle, thereby reducing the toxic and side effects of treatment. For those patients who are expected to survive long term, we can use more aggressive treatment to achieve the best results. We believe our findings will trigger many basic studies to elucidate the mechanisms behind them.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of Shandong Province (No. ZR202108070028).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1684/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1684/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1684/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of The First Affiliated Hospital of Shandong First Medical University (No. 2022-S607). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- Pirker R, Wiesenberger K, Pohl G, et al. Anemia in lung cancer: clinical impact and management. Clin Lung Cancer 2003;5:90-7. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99.
- Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol 2017;8:1-20. [Crossref] [PubMed]
- Yoshida N, Kosumi K, Tokunaga R, et al. Clinical Importance of Mean Corpuscular Volume as a Prognostic Marker After Esophagectomy for Esophageal Cancer: A Retrospective Study. Ann Surg 2020;271:494-501. [Crossref] [PubMed]
- Jomrich G, Hollenstein M, John M, et al. High Mean Corpuscular Volume Predicts Poor Outcome for Patients With Gastroesophageal Adenocarcinoma. Ann Surg Oncol 2019;26:976-85. [Crossref] [PubMed]
- Borsetto D, Polesel J, Tirelli G, et al. Pretreatment High MCV as Adverse Prognostic Marker in Nonanemic Patients with Head and Neck Cancer. Laryngoscope 2021;131:E836-45. [Crossref] [PubMed]
- Nagai H, Yuasa N, Takeuchi E, et al. The mean corpuscular volume as a prognostic factor for colorectal cancer. Surg Today 2018;48:186-94. [Crossref] [PubMed]
- Yoon HJ, Kim K, Nam YS, et al. Mean corpuscular volume levels and all-cause and liver cancer mortality. Clin Chem Lab Med 2016;54:1247-57. [Crossref] [PubMed]
- Pavanello S, Snenghi R, Nalesso A, et al. Alcohol drinking, mean corpuscular volume of erythrocytes, and alcohol metabolic genotypes in drunk drivers. Alcohol 2012;46:61-8. [Crossref] [PubMed]
- Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J 2016;48:889-902. [Crossref] [PubMed]
- Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician 2009;79:203-8.
- Jung HA, Kim HJ, Maeng CH, et al. Changes in the mean corpuscular volume after capecitabine treatment are associated with clinical response and survival in patients with advanced gastric cancer. Cancer Res Treat 2015;47:72-7. [Crossref] [PubMed]
- Karvellas CJ, Sawyer M, Hamilton M, et al. Effect of capecitabine on mean corpuscular volume in patients with metastatic breast cancer. Am J Clin Oncol 2004;27:364-8. [Crossref] [PubMed]
- Wenzel C, Mader RM, Steger GG, et al. Capecitabine treatment results in increased mean corpuscular volume of red blood cells in patients with advanced solid malignancies. Anticancer Drugs 2003;14:119-23. [Crossref] [PubMed]
- Mizuno H, Yuasa N, Takeuchi E, et al. Blood cell markers that can predict the long-term outcomes of patients with colorectal cancer. PLoS One 2019;14:e0220579. [Crossref] [PubMed]
- Liu Q, Yang Y, Li X, et al. Implications of Habitual Alcohol Intake With the Prognostic Significance of Mean Corpuscular Volume in Stage II-III Colorectal Cancer. Front Oncol 2021;11:681406. [Crossref] [PubMed]
- Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 2004;80:1123-8. [Crossref] [PubMed]
- Zhao N, Ruan M, Koestler DC, et al. Methylation-derived inflammatory measures and lung cancer risk and survival. Clin Epigenetics 2021;13:222. [Crossref] [PubMed]
- Shi R, Bao X, Unger K, et al. Identification and validation of hypoxia-derived gene signatures to predict clinical outcomes and therapeutic responses in stage I lung adenocarcinoma patients. Theranostics 2021;11:5061-76. [Crossref] [PubMed]
- Barthelemy N, Streel S, Donneau AF, et al. Screening for malnutrition in lung cancer patients undergoing radiotherapy. Support Care Cancer 2014;22:1531-6. [Crossref] [PubMed]
- Brunner-Agten S, von Känel T, Röthlisberger B, et al. Hb Bakersfield (HBA1: c.151_152insGGAGCC): The Insertion of Arg-His Between Codons 49 and 50 of the α1-Globin Chain Leads to Increased Oxygen Affinity. Hemoglobin 2017;41:1-5. [Crossref] [PubMed]
- Reiss UM, Bensimhon P, Zimmerman SA, et al. Hydroxyurea therapy for management of secondary erythrocytosis in cyanotic congenital heart disease. Am J Hematol 2007;82:740-3. [Crossref] [PubMed]
- O'Donnell JL, Joyce MR, Shannon AM, et al. Oncological implications of hypoxia inducible factor-1alpha (HIF-1alpha) expression. Cancer Treat Rev 2006;32:407-16. [Crossref] [PubMed]


