
Molecular mechanisms of alveolar epithelial cell senescence and idiopathic pulmonary fibrosis: a narrative review
Introduction
Idiopathic pulmonary fibrosis (IPF) is a commonly diagnosed chronic, progressive, and fibrotic interstitial pneumonia that accounts for 20–30% of interstitial lung diseases. It usually occurs in middle-aged and elderly individuals (1). The most common features observed on high-resolution computed tomography (HRCT) of the chest in patients with IPF are ground-glass opacity (GGO), reticular structures, traction bronchiectasis, and honeycomb-like structures (2). It is now generally accepted that persistent alveolar epithelial damage and repair dysregulation are the principal mechanisms leading to progressive pulmonary fibrosis. Repetitive epithelial cell injury and deficiencies in regeneration result in the release of mediators, including cytokines, chemokines, fibrogenic factors, coagulant proteins, oxidants, and regulators of apoptosis. This leads to the recruitment, proliferation, and activation of interstitial fibroblasts to form fibrotic foci (3,4). Additionally, excessive deposition of the extracellular matrix leads to destruction of lung parenchymal structures (5). Interestingly, a variety of cells, including alveolar epithelial type II cells (ATII) and fibroblasts, can drive IPF (6,7). Regardless of the driver cell types, senescence leads to a decrease in the repair capacity of damaged alveolar epithelium. As a result, fibrous tissue replaces the damaged alveolar epithelium (8).
From a histopathological point of view, IPF formation is a dynamic process involving complex interactions among epithelial cells, fibroblasts, immune cells (such as macrophages and T lymphocytes), and endothelial cells (9). Alveolar epithelial cells undergo cytoskeletal remodeling and acquire a mesenchymal phenotype through epithelial-mesenchymal transition (EMT), in which epithelial cells lose intercellular attachment, polarity, and epithelial-specific markers, leading to fibrosis (10). Some investigators have identified ATII as a major player in the synthesis of transforming growth factor-beta (TGF-β) and tumor necrosis factor-alpha (TNF-α) in lung biopsies from patients with IPF (11). In the process of organ fibrosis formation, including pulmonary fibrosis, TGF-β acts as the master switch for the induction of the EMT process (12). In particular, TGF-β mediates fibrous proliferative effects by inducing apoptosis in alveolar epithelial type I (ATI) cells (13,14). However, there is no direct evidence that TGF-β promotes IPF by inducing senescence in the alveolar epithelial cells. In the lungs of patients with IPF, the ability of ATII cells to transdifferentiate into ATI cells is diminished. Emerging evidence also suggests that triggering ATII senescence can promote IPF (6). Therefore, studying the mechanisms of cellular senescence in the lung microenvironment is crucial to understand IPF pathogenesis and progression.
Notable progress has been made by clinicians and researchers worldwide in uncovering the pathogenic mechanisms and treatment strategies for IPF. For instance, pirfenidone (PFD) is a pleiotropic pyridine compound that improves fibrosis, inflammatory responses, and oxidative stress (15). In addition, Nintedanib is an intracellular tyrosine kinase inhibitor that inhibits the progression of pulmonary fibrosis. In line with these observations, in the 2015 Official Clinical Practice Guidelines for IPF, Raghu et al. proposed that PFD and nintedanib may be used to treat IPF (16). Considering that PFD and nintedanib have serious adverse effects such as photosensitivity and diarrhea, discovery of novel antifibrotic drugs still deserves priority research. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-886/rc).
Methods
PubMed, Web of Science, and Google Scholar databases were searched using the following terms to identify relevant papers for this review (Table 1): “cell senescence AND idiopathic pulmonary fibrosis”, “aging AND idiopathic pulmonary fibrosis”, “alveolar epithelial cell AND cell senescence AND idiopathic pulmonary fibrosis”, “alveolar epithelial cell AND cell senescence AND lung disease”, “alveolar epithelial cell AND cell senescence”, “alveolar epithelial cell AND idiopathic pulmonary fibrosis”, “cell senescence”, “WNT/β-catenin AND cell senescence AND pulmonary fibrosis”, “PI3K/Akt AND cell senescence AND pulmonary fibrosis”, “mTOR AND cell senescence AND pulmonary fibrosis”, “NF-κB AND cell senescence AND pulmonary fibrosis”, “WNT/β-catenin AND cell senescence”, “PI3K/Akt AND cell senescence”, “mTOR AND cell senescence”, “NF-κB AND cell senescence”. Owing to the lack of studies on the correlation between IPF and aging in the database before 2000, we prioritized articles published between 2000 and 2022. Clinical trials, research articles, and review articles were examined. Publications identified as associated with both IPF and aging were further examined in detail to identify previously unidentified related articles. Partial anti-aging agents and telomere protectors have been researched primarily in relation to Alzheimer’s disease or other age-related disorders, whereas no studies have shown their associations with IPF; thus, they are not discussed in this narrative review.
Table 1
| Items | Specification |
|---|---|
| Date of search | January 1st, 2021–May 1st, 2022 |
| Databases and other sources searched | PubMed, Web of Science, Google Scholar |
| Search terms used | Search terms included “cell senescence AND idiopathic pulmonary fibrosis”, “aging AND idiopathic pulmonary fibrosis”, “alveolar epithelial cell AND cell senescence AND idiopathic pulmonary fibrosis”, “alveolar epithelial cell AND cell senescence AND lung disease”, “alveolar epithelial cell AND cell senescence”, “alveolar epithelial cell AND idiopathic pulmonary fibrosis”, “cell senescence”, “WNT/β-catenin AND cell senescence AND pulmonary fibrosis”, “PI3K/Akt AND cell senescence AND pulmonary fibrosis”, “mTOR AND cell senescence AND pulmonary fibrosis”, “NF-κB AND cell senescence AND pulmonary fibrosis”, “WNT/β-catenin AND cell senescence”, “PI3K/Akt AND cell senescence”, “mTOR AND cell senescence”, “NF-κB AND cell senescence” |
| Timeframe | 2000–2022 |
| Inclusion and exclusion criteria | Inclusion criteria: lung diseases associated with aging |
| Exclusion criteria: research with similar conclusions | |
| Selection process | Mingjin Tu independently selected and reviewed all initial articles, with additional review by Ting Wei and Yufang Jia. Ultimate final article inclusion was determined by all authors |
PI3K/Akt, phosphatidylinositol-3-kinase/protein kinase B; NF-κB, nuclear factor kappa B.
Discussion
Cell senescence
Cellular senescence, a hallmark of the aging process, plays an important role in the pathogenesis of IPF (17). Senescent cells possess a phenotype in which cell growth stops permanently but cell death does not occur. Cellular senescence can occur at any point, from the embryonic developmental stage to the adult stage. Cellular senescence can be classified as either replicative senescence (RS) or stress-induced premature senescence (SIPS), depending on the factors that induce aging (18). Cellular senescence caused by telomere shortening is known as RS, whereas cellular senescence induced by exogenous stresses, such as oxidative stress, DNA damage, and proto-oncogene activation is called premature senescence. A common feature of senescent cells is irreversible cell cycle arrest, whereby senescent blockade is regulated by the p53-p21 and p16-retinoblastoma protein (Rb) signaling pathways (19). Senescent cells also express several cytokines, growth factors, and proteases that maintain cellular growth arrest and promote the degeneration and proliferation of neighboring cells. Given its importance, cellular senescence is involved in the development and progression of various aging-related diseases.
Aging of alveolar epithelial cells and IPF
Cellular senescence can contribute to the development of IPF through multiple mechanisms, including senescence-associated secretory phenotype (SASP) (20), telomere dysfunction (21), mitochondrial dysfunction (22), DNA damage (23), epigenetic alterations (24), inflammatory response (25), and protein homeostatic imbalance (26). Abnormal telomere shortening in IPF lungs leads to cellular senescence in the alveolar epithelial cells (27). Additionally, ATII cells in IPF lungs exhibit significant cellular senescence features such as mitochondrial malformations and dysfunction (28).
Recent studies have shown that mitochondrial dysfunction and metabolic reprogramming are distinctive features of IPF lungs (Figure 1). Mitochondria consume oxygen and produce reactive oxygen species (ROS), while producing the majority of cellular ATP. ROS has a fundamental signaling role and can increase the antioxidant capacity of cells through mitotic excitation processes (29). Along with cellular senescence, mitochondria accumulate abnormalities, including morphological changes (rounded appearance, cristae, and inner membrane absence), reduced biogenesis, and decreased mitochondrial DNA copy number. Furthermore, increased mitochondrial DNA mutations, leads to a failure in respiratory chain and ATP production (30). In lung tissue of IPF patients, it has been found that ATP production is reduced and mitochondrial ROS production is increased (31). When the concentration of ROS exceeds physiological levels, it can leak into the cytosol and activate excessive inflammatory mediators such as nuclear factor kappa B (NF-κB) (32).
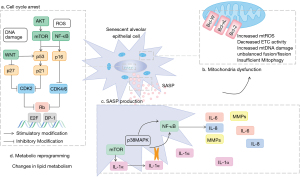
Mitochondrial biogenesis is controlled by the peroxisome proliferator-activated receptor γ coactivator-1alpha (PGC-1α) and PGC-1β signaling pathways. The activities of PGC-1α/β upstream activators, such as AMP-activated protein kinase (AMPK) and SIRT1, decrease as an organism age, leading to a decline in mitochondrial biogenesis (33,34).
Dysfunctional mitochondria are less efficient in oxidizing NADH to NAD+, resulting in a lower NAD+/NADH ratio, with the activation of AMPK and p53 leading to a senescent phenotype (35). Waters et al. demonstrated that knockdown of PGC-1α in lung fibroblasts effectively reduced the expression of cellular senescence markers in IPF fibroblasts (36). In a bleomycin-induced mouse model of senescence, senescent alveolar epithelial cells exhibited increased mTOR/PGC-1α/β activation, which is also associated with increased mitochondrial mass and upregulation of oxidative phosphorylation (37).
The lung tissue in IPF has been shown to increase metabolic activity (38). Lung samples from IPF have altered metabolite production and downregulated expression of key enzymes involved in multiple metabolic pathways, including glycolysis and key mitochondrial-related metabolic pathways, such as mitochondrial β-oxidation and the tricarboxylic acid cycle (39). Similarly, alveolar macrophages isolated from bleomycin-treated mice showed glycolytic reprogramming and increased fatty acid (FA) oxidation (40). Moreover, IPF myofibroblasts have a higher glycolytic enzyme expression and lactate content (41).
Metabolic homeostasis is the basis of physiological state maintenance in cells, tissues, and organs. Not surprisingly, various metabolic pathways are involved in structural remodeling of the lung. In particular, lipid synthesis is essential for the production of pulmonary surfactants. Single-cell RNA sequence data in alveolar epithelial cells indicated low expression of enzymes required for lipid metabolism (42). However, aging modifies lipid metabolism by modulating several important pathways including: adipose tissue lipolysis, lipoprotein, and triglyceride metabolism, as well as shifts in lipid transport proteins (43,44). During β-oxidation, FAs attach to coenzyme A for transport to the mitochondria via the carnitine shuttle. These intermediates are oxidized to produce NADH and FADH2, which generates ATP in the electron transport chain. This process occurs within the mitochondrial matrix. In IPF lungs, long- and medium-chain FA (caproic, caprylic, myristic, and palmitic acids) levels were elevated, and carnitine and medium-chain acyl carnitine (caproic, caprylic, palmitoyl, and succinyl carnitine) levels were significantly reduced (39). FAs allow β-oxidation via the carnitine shuttle transport to the mitochondrial matrix. This finding suggests a mechanism for lipid accumulation and downregulation of β-oxidation in the IPF lungs. In addition to these alterations in metabolic processes, changes in organelles are associated with aging and lipid metabolism, such as mitochondrial dysfunction.
Mitochondria are the primary sites of lipid metabolism. Mitofusins, including Mitofusin1 (MFN1) and Mitofusin2 (MFN2), are GTPase proteins that coordinate outer mitochondrial membrane fusion. In bleomycin-induced mice, Mfn1 or Mfn2 deficiency disrupts lipid metabolism in AEC2 cells. Fatty acid synthase (FASN) is a key enzyme in lipid metabolism. Through AEC2 cell-specific deletion of FASN Chung et al. demonstrated that loss of lipid synthesis in AEC2 cells exacerbated pulmonary fibrosis in a mouse model after mitochondrial injury (45). Interestingly, alveolar macrophages expressing a dominant negative mitochondrial calcium uniporter (MCU) diverted glycolysis to fatty acid oxidation (FAO) through metabolic reprogramming, increasing MCU and mitochondrial calcium, resulting in protective effects in a mouse model of IPF (46). As alluded above, during aging, FA uptake increases, adipogenesis from the head decreases, and FA oxidation processes decrease, leading to ectopic lipid accumulation. Decreased lipid catabolism is primarily caused by mitochondrial dysfunction. All of these changes further lead to lipotoxicity in cells, exhaustion of energy in tissues, and alteration of cell signaling, accelerating the onset of IPF.
Alveolar epithelial cell senescence, cell cycle arrest, and the SASP may contribute to the development and perpetuation of fibrotic scarring. In a mouse model of bleomycin-induced pulmonary fibrosis, alveolar epithelial cells exhibited increased expression of senescence-associated markers, including β-galactosidase activity, p16, p21, pRb (47,48).
Signaling pathways in alveolar epithelial cell senescence
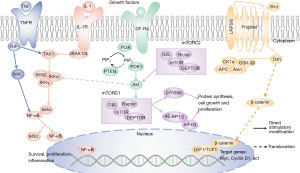
Aging, like many other biological processes, is regulated by classical signaling pathways and transcription factors. Scientists have altered the aging process in various animal models by intervening in different biological systems and signaling pathways to delay the onset of various aging-associated diseases. Numerous crucial signaling pathways associated with aging have been identified, including the insulin/insulin-like growth factor 1 (IGF-1) (49), JAK-STAT (50), WNT/β-catenin (51), phosphatidylinositol-3-kinase (PI3K)/protein kinase B (PKB/Akt) (52), mTOR (53), AMPK (54), NF-κB (55), RhoA/ROCK (56), Notch (57), and sirtuin (58) pathways. Our study revealed that several signaling pathways, including WNT, PI3K/Akt, mTOR, and NF-κB, are not only involved in the process of cellular senescence, but are also closely related to IPF (Figure 2).
WNT/β-catenin signaling pathway
The WNT signaling pathway has critical regulatory roles in early development, organogenesis, tissue regeneration, and other physiological processes in animal embryos.
The WNT/β-catenin signaling pathway is composed of the secreted WNT protein family, frizzled protein receptor family, casein kinase 1 (CK1), dishevelled (Dsh or Dvl), glycogen synthase kinase-3β (GSK-3β), adenomatous polyposis coli (APC), Axin, β-catenin, and the T cell factor/lymphatic enhancer factor family (TCF/LEF). In the absence of WNT, β-catenin binds to a cytoplasmic complex containing CK1α, GSK-3β, Axin, and APC proteins. In turn, this promotes the phosphorylation of β-catenin and its interaction with β-transducin repeat-containing proteins (β-TRCP). Subsequently, β-TRCP recognizes and ubiquitinates the phosphorylation of β-catenin, leading to its subsequent degradation by the proteasome (59). When the WNT/β-catenin signal is activated, WNT is secreted into the extracellular space, where it binds to the transmembrane receptor Frizzled, which in turn activates intracellular Dsh. Activated Dsh protein subsequently enhances the phosphorylation of GSK-3β. As a result, phosphorylation of GSK-3β inhibits the formation of β-catenin degradation complex and β-catenin phosphorylation. Eventually, β-catenin accumulated in the cytoplasm. When cytoplasmic β-catenin reaches a certain concentration, it translocates to the nucleus, where it binds TCF/LEF to form a transcriptional activation complex, which further activates downstream target genes (60). Therefore, administration of the GSK3-β inhibitor CHIR99021 (CHIR) can lead to the direct accumulation of β-catenin.
Role of WNT/β-catenin signaling pathway in alveolar epithelial cell senescence
The WNT/β-catenin signaling pathway is activated when IPF is induced in rats using bleomycin. WNT components, including WNT3a, β-catenin, and pGSK-3β proteins, are increased during IPF development, and decreased GSK-3β expression has also been observed in mouse fibrotic lungs (61,62). Therefore, inhibition of the WNT/β-catenin signaling pathway leads to attenuation of IPF (63). Lehman et al. reported that WNT/β-catenin activity was increased in ATII cells of aged mice (16–24 months) compared to that in young mice (3 months), whereas chronic typical WNT/β-catenin activation for seven days induced cellular senescence (64).
Lehmann et al. have demonstrated that CHIR, an inhibitor of GSK3-β, induces the accumulation of β-catenin, thereby increasing the expression of the WNT target gene Axin2 in mouse ATII cell line (MLE12 cells) (64). Interestingly, Damalas et al. showed that activated WNT/β-catenin signaling leads to p53 accumulation (65). Moreover, p21, a downstream target of p53, directly induces cellular senescence (66). Similarly, senescence of alveolar epithelial cells can be induced by increasing GSK-3β phosphorylation (67). In addition, activated β-catenin signaling triggers DNA damage response (DDR) (68). DDR induces the expression of p16 (ink4a) (69), a gene that directly induces cellular senescence (70). In summary, activated WNT/β-catenin signaling can induce cellular senescence via DDR and p53/p21 pathways.
WNT-secreted proteins are cysteine-rich glycosylated proteins that activate either the β-catenin-dependent (typical) WNT pathway (e.g., WNT3a) or the β-catenin-independent (atypical) WNT pathway (e.g., WNT5a). Co-treatment of cells with WNT3a and WNT5a revealed that the non-standard ligand WNT5a reduces the ability of WNT3a to induce cellular senescence (64). Treatment of cells with WNT3a increases the expression of cell cycle inhibitors p16, p21, and p53, but decreased the expression of p27 (71). Moreover, p27 can block the cell cycle transition from G1 to S phase, thereby inducing cell quiescence (72). Impaired cell cycle progression, decreased DNA repair gene expression, and SASP are essential aspects of senescence (73,74). It has been demonstrated that WNT/β-catenin signaling inhibits SASP factors and prevents paracrine senescence (51). In addition, WNT7a induces cellular senescence by promoting inactivation of S-phase kinase-associated protein 2 (SKP2) in a β-catenin-independent manner. Furthermore, deficiency of WNT7a decreases the number of senescent alveolar epithelial cells (75). Like other members of the LGR family, LGR6 serves as a promoter that regulates WNT signaling (76). In addition to increased expression of LGR6 in alveolar epithelial cells of IPF tissues, higher SA-β-Gal activity and increased p16 and p21 in LGR6-expressing cells have been observed by Cortesi et al. (77). This suggests that LGR6 mediates the activation of the canonical WNT/β-catenin protein pathway, which ultimately leads to chronic signaling and promotes the acquisition of a senescence phenotype involved in IPF (Figure 1).
WNT/β-catenin as a therapeutic target in IPF: Citrus alkaline extract (CAE)
Citrus plants are an essential source of herbal medicines (78). Active ingredients from dried citrus peel have been shown to lower blood lipid levels and exert anti-tumor (79), anti-inflammatory (80), antioxidant (81), and anti-fibrosis (82) effects. CAE prepared from 75% ethanol extract is an active ingredient in the prevention of pulmonary fibrosis (83). CAE reduces pulmonary fibrosis in vivo and in vitro by suppressing fibroblast senescence (84). Moreover, CAE inhibited alveolar epithelial cell senescence through the β-catenin/p53 pathway. After 24 h of CAE treatment in Adriamycin RD (ARD)-induced A549 cells, the level of β-catenin decreased, and the expression level of its downstream target GSK-3β gradually increased; however, the expression levels of p53 and downstream factor p21 tended to decrease. In another study, the supernatant obtained after centrifuging CAE-treated A549 cells was transferred to cultures of MRC-5 cells for three days, and the protein levels of alpha smooth muscle actin (α-SMA), collagen I, and collagen II gradually decreased with increasing CAE concentrations (85). Therefore, CAE inhibited the expression of α-SMA, collagen I, and collagen II in fibroblasts, thereby alleviating pulmonary fibrosis.
PI3K/Akt signaling pathway
The PI3K/Akt signaling pathway, involving PI3K and its downstream molecule Akt, regulates various cellular functions such as proliferation, differentiation, apoptosis, and glucose transport.
Role of PI3K/Akt signaling pathway in alveolar epithelial cell senescence
Under pathological conditions, alveolar epithelial cells release IGF-1, which activates the IGF-1 receptor (IGF-1R) on the surface of adjacent normal alveolar epithelial cells, further activating intracellular downstream PI3K and Akt (86-88). Activated PI3K/Akt participates in alveolar epithelial cell senescence and IPF progression through the release of connective tissue growth factor (CTGF), TGF-β, and matrix metalloproteinases (MMPs) (89-91). Additionally, activation of PI3K/Akt can be involved in pulmonary fibrosis by regulating its downstream pathways, such as mammalian target of rapamycin (mTOR), hypoxia-inducible factor-1a (HIF-1a), and forkhead box (FOX) family.
Phosphatase and tensin homolog (PTEN) is a tumor suppressor with bispecific phosphatase activity (92). PTEN is hypothesized to function primarily via the PI3K/Akt pathway. PTEN encodes a protein with lipid phosphatase activity that dephosphorylates PIP3 [phosphatidylinositol (3,4,5)-trisphosphate] to form PIP2 [phosphatidylinositol (4,5)-bisphosphate], thereby blocking the growth factor-signaling pathway regulated by PI3K/Akt (93,94). Fibroblasts in IPF fibrotic lesions express low levels of PTEN and high levels of Akt (95). Downregulation of PTEN can accelerate the premature senescence of alveolar epithelial cells by activating the PI3K/Akt/mTOR pathway (96). In line with these observations, PTEN inhibitors activate the PI3K/Akt pathway and induce IPF in animal models (97). Nonetheless, the application of PI3K and PTEN inhibitors in the treatment of IPF in humans requires further investigation.
Three members of the Akt kinase family exist: AKT1, AKT2, and AKT3. AKT1 and AKT2 are widely expressed in many tissues and cell types, whereas AKT3 is predominantly expressed in the brain tissue. Each isoform of Akt has distinct but overlapping functions in proliferation, apoptosis, protein synthesis, and cell cycle regulation (98). Activated Akt activates or inhibits its downstream target proteins, Bad, caspase-9, NF-κB, GSK-3, p21, and p27, which in turn regulate cell proliferation, differentiation, apoptosis, and senescence. Knocking down AKT2 expression in A549 cells significantly reduced the rate of bleomycin-stimulated alveolar epithelial cell senescence. Similarly, Akt pathway inhibitors (LY294002 and MK2206) dramatically reduced the expression of senescence-associated marker p21 and attenuated SA-β-Gal activity in bleomycin-stimulated alveolar epithelial cells (99). Moreover, it reduced the expression levels of α-SMA, fibronectin, collagen I, and collagen II by lowering the phosphorylation levels of Akt in Bleomycin (BLM)-stimulated mice (100). Thus, Akt inhibition effectively diminishes alveolar epithelial cell senescence and subsequently alleviates IPF.
PI3K/Akt as a therapeutic target in IPF: quercetin
Quercetin, a member of the flavonoid family, is a dietary antioxidant widely found in vegetables, fruits, tea, and wines (101). Quercetin exerts its antioxidant and anti-inflammatory effects by eliminating oxidants. Quercetin has been shown to reduce oxidative stress and inflammatory markers in IPF (102,103). Boots et al. showed that quercetin exerts antifibrotic and anti-inflammatory effects in bleomycin-induced lung injury in mice (104). Quercetin functions by regulating the activity of protein kinases including PI3K (105) and Akt (106). Hohmann et al. reported that quercetin attenuates bleomycin-induced pulmonary fibrosis by restoring senescent fibroblast sensitivity to pro-apoptotic stimuli through the activation of Akt in aged mice (107). Moreover, a recent study showed that the combination of quercetin in combination with the SRC/ABL protein kinase inhibitor dasatinib ameliorates lung function by reducing the expression of various aging markers to reverse bleomycin-induced IPF in aged mice (108). The beneficial effects of quercetin and dasatinib in eliminating cellular senescence during IPF were also demonstrated by Lehmann et al., who reported that quercetin and dasatinib depleted senescent cells by inducing apoptosis and reducing the SASP in a bleomycin-induced alveolar epithelial cell fibrosis model (109). In addition, in an in vivo open-label trial in humans, the combination of quercetin and dasatinib alleviated physical dysfunction in IPF patients as measured using several tests, such as six-minute walking distance, four-meter gait speed, and five repeated chair-stand times (110). In summary, targeting the PI3K/Akt signaling pathway may similarly deplete senescent alveolar epithelial cells in IPF; however, the exact mechanism requires further investigation.
mTOR signaling pathway
mTOR is a central regulator of cellular metabolism, growth, proliferation, and cell survival. mTOR consists of two complex subunits: mTORC1 and mTORC2. The mTORC1 complex is a ternary complex composed of mTOR, Raptor (mTOR regulator related protein), and GβL (G-protein-β subunit-like protein), whereas the mTORC2 complex is composed of mTOR, GβL, and Rictor (111). mTORC1 directly regulates protein synthesis, participates in lipid and nucleotide metabolism, and negatively regulates catabolic processes (112). mTORC2 mainly regulates cell proliferation, survival, cytoskeletal remodeling, and cell migration (113).
Role of mTOR signaling pathway in alveolar epithelial cell senescence
Downregulation of mTOR counteracts the signs of aging, including nutrient dysregulation, mitochondrial dysfunction, loss of proteostasis, cellular senescence, and stem cell failure (114). mTOR can be elicited by removing PTEN or Akt to induce mTOR activation in normal mice or senescent human cells (115,116).
Cellular senescence is a characteristic feature of IPF. Both lung fibroblasts and alveolar epithelial cells show evidence of SASPs acquired from the lungs of patients with IPF (108,117). SASP is characterized by the secretion of a series of pro-inflammatory cytokines, chemokines, matrix remodeling proteases, and growth factors [including TGF-β1, interleukin (IL)-6, and MMP-12]. The secretion of these cytokines is regulated by mTORC1 signaling in senescent cells (118).
Moreover, mitochondrial dysfunction in the alveolar epithelial cells of IPF lungs has been recognized as an important contributor to cellular senescence. The mitochondrial biogenesis pathway downstream of the mTOR/PGC-1α/β axis was significantly upregulated in senescent lung epithelial cells. Using rapamycin, an inhibitor of mTORC1, bleomycin-induced cellular senescence was reduced by restoring mitochondrial homeostasis in lung epithelial cells (37).
Short-term rapamycin exposure blocks the activity of mTORC1, but not mTORC2, whereas long-term exposure inhibits the activity of both complexes (119). mTOR kinase, a major component of mTORC1 and mTORC2, directly binds p53 and increases its stability by phosphorylating p53 at serine 15. It then induces cellular senescence through accumulation of the cell cycle inhibitor p21 (120). Therefore, mTOR kinase may be a promising target for the treatment of pulmonary fibrosis, as it acts by targeting the mTOR pathway to inhibit cellular senescence.
The mTORC1 inhibitor rapamycin slightly increases p53-mediated Akt Ser473 phosphorylation, whereas the mTORC1/mTORC2 inhibitor Torin1 inhibits Akt Ser473 phosphorylation (121). Moreover, silencing Rictor suppresses phosphorylation of the AKT1 Ser473 site, myofibroblast differentiation, CDKN1A and CDKN2A expression, and SA-GLB1/β-gal activity (122). This suggests that mTORC2 controls the expression of senescence markers and myofibroblast differentiation. In contrast, Raptor silencing does not inhibit AKT1 phosphorylation or ACTA2 overexpression, nor does it reduce CDKN1A and CDKN2A levels. However, silencing Raptor resulted in inhibition of SA-GLB1/β-gal activity. mTORC1 and mTORC2 regulate senescence through different downstream signaling pathways.
mTOR as a therapeutic target in IPF: Rapamycin
Rapamycin, an mTOR-mediated inhibitor of mammalian targets, is a potent antiproliferative agent that was originally introduced to the clinic to prevent transplant rejection (123). Rapamycin is known for its wide-ranging biological effects, including prolonging lifespan and inhibition or reversal of cellular senescence in vitro (124,125). In addition, rapamycin has demonstrated potent antifibrotic effects in animal models of liver (126), kidney (127), and lung fibrosis (128). In a mouse model of bleomycin-induced lung fibrosis, rapamycin attenuated IPF by inhibiting E-cadherin downregulation and fibronectin upregulation (129). Further studies have shown that the combination of rapamycin and PFD exerts antifibrotic effects on primary fibroblasts and human alveolar epithelial cells (130). Rapamycin decreases the secretion of SASP-related cytokines and alleviates ATII cell senescence by suppressing mTORC1 to an overall decrease in IPF (131). Furthermore, Herranz et al. found that rapamycin reduces SASP secretion from senescent cells (132). Rapamycin may also delay senescence by inhibiting mTOR to improve the mitochondrial function (133). Inhibition of the mTOR pathway by rapamycin has profound effects on aging-associated phenotypes. Therefore, rapamycin is a promising therapeutic agent for aging-related IPF.
NF-κB signaling pathway
The NF-κB family includes five transcription factors: NF-κB1 (p50), NF-κB2 (p52), Rel A (p65), Rel B, and c-Rel (134). NF-κB proteins bind to the κB site as dimers, affecting target gene transcription (135). Activation of the NF-κB signaling pathway is achieved through phosphorylation of NF-κB.
When cells are subjected to various extracellular and intracellular stimuli, the IKK complex is activated to phosphorylate the IκB protein and induce its ubiquitination. Proteasomes degrade ubiquitinated IκB proteins, leading to the release of a heterodimer composed of p65 and p50. p65 and p50 are then activated via post-translational modifications (phosphorylation, acetylation, and glycosylation). Activated p65 and p50 then migrate into the nucleus to bind specific DNA sequences and promote the transcription of target genes (136). Thus, NF-κB proteins and the IKK complex play central roles in regulating the NF-κB pathway.
Role of NF-κB signaling pathway in alveolar epithelial cell senescence
The NF-κB signaling pathway is considered a key regulator of the SASP (137). Hyperactivation of NF-κB has been observed in various mouse models of premature and normal aging, including Sirt6-/-, Ercc1-/Δ, and Zmpste24-/- mice with premature aging (138-140). The inhibition or knockdown of several components that regulate NF-κB signaling, such as p65, has been shown to inhibit SASP (141).
Phosphorylation of NF-κB, IKKα/β, and IκBα was increased in IPF lung tissues. However, PTEN levels were decreased. Knockdown of the PTEN gene in A549 cells led to activation of the NF-κB pathway and significant upregulation of senescence-related markers such as p21, p16, and SASP. This confirmed that PTEN deletion accelerates alveolar epithelial cell senescence by activating the NF-κB pathway (142). Iannetti et al. demonstrated that NF-κB2/RelB regulates Rb activity to regulate EZH2 expression, thereby controlling the stability of p21WAF1 and p53 in primary human fibroblasts (143). In addition, CCAAT/enhancer binding protein (C/EBP) homolog (CHOP) activates the downstream NF-κB pathway by promoting ROS production, leading to ER stress-induced alveolar epithelial cell senescence and IPF (144). These findings suggest that sustained activation of NF-κB is not only associated with cellular senescence but also promotes the aging process (145).
NF-κB as a therapeutic target in IPF: Fisetin (FIS)
FIS is a novel dietary agent present in a range of plants, fruits, and vegetables. FIS improves pulmonary inflammation by downregulating the p-STAT-1 and NF-κB signaling pathways via heme oxygenase-1 (146). It can effectively alleviate pulmonary oxidative stress induced by strong oxidants (147). Moreover, it can reduce the number of senescent mesenchymal stem cells/progenitor cells, immune cells, and endothelial cells (148). Recently, researchers found that FIS can effectively mitigate the aging of alveolar epithelial cells by inhibiting NF-κB. It was shown to improve the SASP of senescent alveolar epithelial cells, thereby reducing the transdifferentiation of fibroblasts into myofibroblasts and collagen deposition in fibroblasts (149). Previous studies have reported that administration of FIS can prevent liver fibrosis in mice by inhibiting COL1, MMP2, MMP3, and MMP9 gene expression as well as collagen accumulation (150). Furthermore, FIS can significantly inhibit fibrosis-related gene expression and prevent or mitigate myocardial fibrosis by inactivating the TGF-β1/SMADS/ERK1/2 signaling pathway (151). Notably, no side effects of FIS have been reported. Therefore, FIS may be an effective treatment option for IPF patients.
The development of IPF relation to alveolar epithelial cellular senescence
Alveolar epithelial cell senescence plays a central role in IPF development. Alveolar epithelial cell senescence can occur through the WNT/β-catenin, PI3K/Akt, mTOR, and NF-κB signaling pathways, all of which are involved in the occurrence and development of IPF. It is important to determine the role of inhibitors of various signaling pathways in the regulation of fibrosis. This can be achieved by studying the function of various signaling pathways in alveolar epithelial cell senescence, thus improving our understanding of the cellular and molecular mechanisms that regulate alveolar epithelial cell senescence and lung injury repair.
As summarized in this review, it is now generally accepted that damage to alveolar epithelial cells and the interaction between alveolar epithelial cells and fibroblasts are fundamental to the pathogenesis of IPF. For instance, senescence of alveolar epithelial cells can impair the function of ATII cells (152). Yao et al. demonstrated that senescence of ATII cells is sufficient to drive progressive IPF (6). Rapamycin, CAE, and FIS alleviated IPF by reducing alveolar epithelial cell senescence. Quercetin can alleviate IPF by reducing the number of senescent alveolar epithelial cells and fibroblasts. In addition, several drugs that target cellular senescence in IPF are shown in Tables 2,3. These drugs show broad potential in the prevention and treatment of IPF.
Table 2
| Target | Compound | Function | Ref |
|---|---|---|---|
| WNT | HBEC EVs | Reduces WNT3A, WNT5A, and WNT10B expression | (153) |
| ZNF24 | Inhibits cylinD1, c-MYC, cJUN, fra-1, WISP1, and MMIP7 | (154) | |
| Klotho | Inhibits WNT1- and WNT9a-induced mitochondrial injury | (155,156) | |
| Ginsenoside Rg1 | Inhibits TCF, LEF, p-GSK-3β, and c-MYC but activates GSK-3β | (157,158) | |
| Betulinic acid | Increases the phospho-β-catenin ratio (S33/S37/T41 and S45), inhibits the phosphorylation of DVL2 and LRP, and decreases the levels of Wnt3a and LRP6 | (159) | |
| PRI-724 | Reduces CBP protein and increases p300 protein binding to β-catenin in the nucleus of lung fibroblasts | (160) | |
| Dickkopf 1 | A more specific WNT inhibitor and the mitochondria-targeted antioxidant mitoquinone | (161,162) | |
| Baicalein | Weaken the phosphorylation of GSK3β (S9) and alleviate the senescence of alveolar epithelial cells | (67) | |
| Akt/mTOR | SHQA | Inhibits the phosphorylation of Akt, mTOR, and downstream targets of mTOR, such as p-S6K | (163) |
| Resveratrol | Suppresses ROS generation and increases the activity of the PI3K/Akt pathway | (164,165) | |
| NF-κB | ICA | Decreases the protein levels of p50 and p65 | (166) |
| SR12343 | Inhibits NF‐κB activation by disrupting the association between IKKβ and NEMO | (55) | |
| metformin | Increases production of collagen I–III and decreases activation of NF-κB(p65) activity under the high glucose conditions | (167) | |
| Avenanthramide C | Suppress SASP production by activating AMPK and inhibiting p38/NF-κB signaling pathway | (168) | |
| SIRT1 activators | Inhibits NF‐κB activation by deacetylating the RelA/p65 component of NF-κB complex | (58,169) |
Akt, protein kinase B; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; HBEC EVs, human bronchial epithelial cell-derived EVs; ZNF24, Zinc finger transcription factor 24; SHQA, sargahydroquinoic acid; ICA, icariin; TCF, transcription factor T cytokine; LEF, lymphatic enhancer factor; LRP, lipoprotein receptor-related protein; CBP, CREB binding protein; ROS, reactive oxygen species; PI3K/Akt, phosphatidylinositol-3-kinase/protein kinase B; NF-κB, nuclear factor kappa B; IKK, IkappaB kinase; NEMO, NF-kappaB essential modulator; SASP, senescence-associated secretory phenotype; AMPK, AMP-activated protein kinase.
Table 3
| Agent | Pharmacological class | Effective target | Applicable area | Ref |
|---|---|---|---|---|
| Dasatinib | Tyrosine kinase inhibitors | It has a significant high affinity for BCR/ABL kinases, inhibits many kinases, including Src family kinases | Alveolar epithelial cells | (109) |
| Roxithromycin | Macrolide antibiotic | It inhibits NOX4-mediated ROS | Fibroblasts | (170) |
| Liproxstatin-1 | Radical-trapping antioxidant | It reduces the levels of ROS and MDA | Alveolar epithelial cells | (25) |
| PTUPB | COX-2/sEH dual inhibitor | It regulates COX-2/CYP-mediated ARA metabolic imbalance | Alveolar epithelial cells | (171) |
| STA-21 | STAT3 inhibitor | It inhibits STAT3 activity, attenuates IL-6 production, reduces p21 levels and restores normal mitochondrial function | Fibroblasts | (172) |
| Spermidine | naturally occurring polyamine | It reduces endoplasmic reticulum stress-mediated apoptosis and activates autophagy in primary lung fibroblasts and in vivo | Alveolar epithelial cells and fibroblasts | (173) |
| TM5275 | PAI-1 inhibitor | It blocks TGF-β1-induced p16 expression and the secretion of SASP | Alveolar epithelial cells | (90) |
| IL-18BP | IL-18 binding protein | It inhibits lung fibroblast senescence by neutralizing IL-18 and promoting Klotho expression | Fibroblasts | (174) |
| Navitoclax (ABT263) | BCL-2 family inhibitors | It targets both the antiapoptotic proteins BCL-xL and BCL-2 | Alveolar epithelial cells | (175) |
| A1331852 and A1155463 | BCL-xL inhibitors | They bind to BCL-xL with high selectivity for closely related proteins such as BCL-2, BCL-W and MCL-1 | Fibroblasts | (176) |
| CPT1C | A key regulator of senescence | It reverses cellular senescence through the regulation of lipid metabolism and mitochondrial function | Fibroblasts | (177) |
IL, interleukin; CPT1C, carnitine palmitoyltransferase 1C; COX-2, cyclooxygenase-2; sEH, soluble epoxide hydrolase; PAI-1, plasminogen activator inhibitor 1; ROS, reactive oxygen species; NOX4, Nicotinamide adenine dinucleotide phosphate oxidase 4; ROS, reactive oxygen species; MDA, methane dicarboxylic aldehyde; CYP, cytochrome P450; ARA, arachidonic acid; TGF, transforming growth factor; SASP, senescence-associated secretory phenotype; BCL-xL, B-cell lymphoma-extra-l; BCL-2, B-cell lymphoma 2; MCL-1, myeloid cell leukemia-1.
Conclusions
In recent years, PFD and nintedanib have been most commonly used in the clinical treatment of IPF, but their efficacy still needs to be improved, so the search for new effective drugs against IPF is still a hot topic in domestic and international research. We have summarized the molecular mechanisms associated with alveolar epithelial cell senescence and IPF to identify new therapeutic options. Recent advances in drugs targeting pulmonary epithelial cell senescence are listed in Tables 2,3. These drugs can be explored for their ability to target alveolar epithelial cell senescence-related pathways to reduce senescence because lowering senescent alveolar epithelial cells may prove to be a promising strategy for IPF treatment.
Acknowledgments
We are grateful to Dr. Haitao Zhang for his guidance in revising this manuscript. At the same time, we would like to thank Vikas Narang for his help in polishing our paper.
Funding: This work was supported by the Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010335), the Discipline Construction Project of Guangdong Medical University (Grant No. 4SG21012G), and the Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (Grant No. ZJW-2019-00).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-886/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-886/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-886/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ryu JH, Moua T, Daniels CE, et al. Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin Proc 2014;89:1130-42. [Crossref] [PubMed]
- Hochhegger B, Marchiori E, Zanon M, et al. Imaging in idiopathic pulmonary fibrosis: diagnosis and mimics. Clinics (Sao Paulo) 2019;74:e225. [Crossref] [PubMed]
- Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest 2012;122:2756-62. [Crossref] [PubMed]
- Maher TM. Beyond the diagnosis of idiopathic pulmonary fibrosis; the growing role of systems biology and stratified medicine. Curr Opin Pulm Med 2013;19:460-5. [Crossref] [PubMed]
- Selman M, King TE, Pardo A, et al. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136-51. [Crossref] [PubMed]
- Yao C, Guan X, Carraro G, et al. Senescence of Alveolar Type 2 Cells Drives Progressive Pulmonary Fibrosis. Am J Respir Crit Care Med 2021;203:707-17. [Crossref] [PubMed]
- Wiley CD, Brumwell AN, Davis SS, et al. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight 2019;4:130056. [Crossref] [PubMed]
- Liu RM, Liu G. Cell senescence and fibrotic lung diseases. Exp Gerontol 2020;132:110836. [Crossref] [PubMed]
- Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. Am J Physiol Cell Physiol 2014;306:C987-96. [Crossref] [PubMed]
- Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc 2006;3:377-82. [Crossref] [PubMed]
- Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res 2002;3:3. [Crossref] [PubMed]
- Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 2001;99:308-19. [Crossref] [PubMed]
- Lee CG, Cho SJ, Kang MJ, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 2004;200:377-89. [Crossref] [PubMed]
- Budinger GR, Mutlu GM, Eisenbart J, et al. Proapoptotic Bid is required for pulmonary fibrosis. Proc Natl Acad Sci U S A 2006;103:4604-9. [Crossref] [PubMed]
- Lopez-de la Mora DA, Sanchez-Roque C, Montoya-Buelna M, et al. Role and New Insights of Pirfenidone in Fibrotic Diseases. Int J Med Sci 2015;12:840-7. [Crossref] [PubMed]
- Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3-19. [Crossref] [PubMed]
- Gulati S, Thannickal VJ. The Aging Lung and Idiopathic Pulmonary Fibrosis. Am J Med Sci 2019;357:384-9. [Crossref] [PubMed]
- Mohamad Kamal NS, Safuan S, Shamsuddin S, et al. Aging of the cells: Insight into cellular senescence and detection Methods. Eur J Cell Biol 2020;99:151108. [Crossref] [PubMed]
- Ito Y, Hoare M, Narita M. Spatial and Temporal Control of Senescence. Trends Cell Biol 2017;27:820-32. [Crossref] [PubMed]
- Blokland KEC, Waters DW, Schuliga M, et al. Senescence of IPF Lung Fibroblasts Disrupt Alveolar Epithelial Cell Proliferation and Promote Migration in Wound Healing. Pharmaceutics 2020;12:389. [Crossref] [PubMed]
- Lee JS, La J, Aziz S, et al. Molecular markers of telomere dysfunction and senescence are common findings in the usual interstitial pneumonia pattern of lung fibrosis. Histopathology 2021;79:67-76. [Crossref] [PubMed]
- Sullivan DI, Jiang M, Hinchie AM, et al. Transcriptional and Proteomic Characterization of Telomere-Induced Senescence in a Human Alveolar Epithelial Cell Line. Front Med (Lausanne) 2021;8:600626. [Crossref] [PubMed]
- Schuliga M, Read J, Blokland KEC, et al. Self DNA perpetuates IPF lung fibroblast senescence in a cGAS-dependent manner. Clin Sci (Lond) 2020;134:889-905. [Crossref] [PubMed]
- Wang Y, Gao J, Wu F, et al. Biological and epigenetic alterations of mitochondria involved in cellular replicative and hydrogen peroxide-induced premature senescence of human embryonic lung fibroblasts. Ecotoxicol Environ Saf 2021;216:112204. [Crossref] [PubMed]
- Tao N, Li K, Liu J, et al. Liproxstatin-1 alleviates bleomycin-induced alveolar epithelial cells injury and mice pulmonary fibrosis via attenuating inflammation, reshaping redox equilibrium, and suppressing ROS/p53/α-SMA pathway. Biochem Biophys Res Commun 2021;551:133-9. [Crossref] [PubMed]
- Sellares J, Veraldi KL, Thiel KJ, et al. Intracellular Heat Shock Protein 70 Deficiency in Pulmonary Fibrosis. Am J Respir Cell Mol Biol 2019;60:629-36. [Crossref] [PubMed]
- Minagawa S, Araya J, Numata T, et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2011;300:L391-401. [Crossref] [PubMed]
- Bueno M, Lai YC, Romero Y, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 2015;125:521-38. [Crossref] [PubMed]
- Yun J, Finkel T. Mitohormesis. Cell Metab 2014;19:757-66. [Crossref] [PubMed]
- Mora AL, Bueno M, Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J Clin Invest 2017;127:405-14. [Crossref] [PubMed]
- Jaeger VK, Lebrecht D, Nicholson AG, et al. Mitochondrial DNA mutations and respiratory chain dysfunction in idiopathic and connective tissue disease-related lung fibrosis. Sci Rep 2019;9:5500. [Crossref] [PubMed]
- Piantadosi CA, Suliman HB. Mitochondrial Dysfunction in Lung Pathogenesis. Annu Rev Physiol 2017;79:495-515. [Crossref] [PubMed]
- Jäger S, Handschin C, St-Pierre J, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 2007;104:12017-22. [Crossref] [PubMed]
- Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056-60. [Crossref] [PubMed]
- Wiley CD, Velarde MC, Lecot P, et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab 2016;23:303-14. [Crossref] [PubMed]
- Waters DW, Blokland KEC, Pathinayake PS, et al. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2018;315:L162-72. [Crossref] [PubMed]
- Summer R, Shaghaghi H, Schriner D, et al. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am J Physiol Lung Cell Mol Physiol 2019;316:L1049-60. [Crossref] [PubMed]
- Justet A, Laurent-Bellue A, Thabut G, et al. [18F]FDG PET/CT predicts progression-free survival in patients with idiopathic pulmonary fibrosis. Respir Res 2017;18:74.
- Zhao YD, Yin L, Archer S, et al. Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study. BMJ Open Respir Res 2017;4:e000183. [Crossref] [PubMed]
- Xie N, Cui H, Ge J, et al. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2017;313:L834-44. [Crossref] [PubMed]
- Xie N, Tan Z, Banerjee S, et al. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. Am J Respir Crit Care Med 2015;192:1462-74. [Crossref] [PubMed]
- Xu Y, Mizuno T, Sridharan A, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 2016;1:e90558. [Crossref] [PubMed]
- Toth MJ, Tchernof A. Lipid metabolism in the elderly. Eur J Clin Nutr 2000;54:S121-5. [Crossref] [PubMed]
- Mancuso P, Bouchard B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front Endocrinol (Lausanne) 2019;10:137. [Crossref] [PubMed]
- Chung KP, Hsu CL, Fan LC, et al. Mitofusins regulate lipid metabolism to mediate the development of lung fibrosis. Nat Commun 2019;10:3390. [Crossref] [PubMed]
- Gu L, Larson Casey JL, Andrabi SA, et al. Mitochondrial calcium uniporter regulates PGC-1α expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biol 2019;26:101307. [Crossref] [PubMed]
- Shivshankar P, Brampton C, Miyasato S, et al. Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am J Respir Cell Mol Biol 2012;47:28-36. [Crossref] [PubMed]
- Muthuramalingam K, Cho M, Kim Y. Cellular senescence and EMT crosstalk in bleomycin-induced pathogenesis of pulmonary fibrosis-an in vitro analysis. Cell Biol Int 2020;44:477-87. [Crossref] [PubMed]
- Xie L, Zhang J, Yan H, et al. β-elemene induced apoptosis and senescence of triple-negative breast cancer cells through IGF1/IGF1R pathway. Tissue Cell 2022;79:101914. [Crossref] [PubMed]
- Ji T, Chen M, Sun W, et al. JAK-STAT signaling mediates the senescence of cartilage-derived stem/progenitor cells. J Mol Histol 2022;53:635-43. [Crossref] [PubMed]
- Lehmann J, Narcisi R, Franceschini N, et al. WNT/beta-catenin signalling interrupts a senescence-induction cascade in human mesenchymal stem cells that restricts their expansion. Cell Mol Life Sci 2022;79:82. [Crossref] [PubMed]
- Kma L, Baruah TJ. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol Appl Biochem 2022;69:248-64. [Crossref] [PubMed]
- Chrienova Z, Nepovimova E, Kuca K. The role of mTOR in age-related diseases. J Enzyme Inhib Med Chem 2021;36:1679-93. [Crossref] [PubMed]
- Ge Y, Zhou M, Chen C, et al. Role of AMPK mediated pathways in autophagy and aging. Biochimie 2022;195:100-13. [Crossref] [PubMed]
- Zhang L, Zhao J, Mu X, et al. Novel small molecule inhibition of IKK/NF-κB activation reduces markers of senescence and improves healthspan in mouse models of aging. Aging Cell 2021;20:e13486. [Crossref] [PubMed]
- Ning L, Gao L, Zhang F, et al. Mechanical Stretch Induces Annulus Fibrosus Cell Senescence through Activation of the RhoA/ROCK Pathway. Biomed Res Int 2021;2021:5321121. [Crossref] [PubMed]
- Dong C, Wang X, Sun L, et al. ATM modulates subventricular zone neural stem cell maintenance and senescence through Notch signaling pathway. Stem Cell Res 2022;58:102618. [Crossref] [PubMed]
- Han X, Ding C, Sang X, et al. Targeting Sirtuin1 to treat aging-related tissue fibrosis: From prevention to therapy. Pharmacol Ther 2022;229:107983. [Crossref] [PubMed]
- Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol 2006;21:103-24. [Crossref] [PubMed]
- Shi C, Lv T, Xiang Z, et al. Role of Wnt/β-Catenin Signaling in Epithelial Differentiation of Lung Resident Mesenchymal Stem Cells. J Cell Biochem 2015;116:1532-9. [Crossref] [PubMed]
- Lv Q, Wang J, Xu C, et al. Pirfenidone alleviates pulmonary fibrosis in vitro and in vivo through regulating Wnt/GSK-3β/β-catenin and TGF-β1/Smad2/3 signaling pathways. Mol Med 2020;26:49. [Crossref] [PubMed]
- Gao F, Zhang Y, Yang Z, et al. Arctigenin Suppressed Epithelial-Mesenchymal Transition Through Wnt3a/β-Catenin Pathway in PQ-Induced Pulmonary Fibrosis. Front Pharmacol 2020;11:584098. [Crossref] [PubMed]
- Yang F, Hou ZF, Zhu HY, et al. Catalpol Protects Against Pulmonary Fibrosis Through Inhibiting TGF-β1/Smad3 and Wnt/β-Catenin Signaling Pathways. Front Pharmacol 2020;11:594139. [Crossref] [PubMed]
- Lehmann M, Hu Q, Hu Y, et al. Chronic WNT/β-catenin signaling induces cellular senescence in lung epithelial cells. Cell Signal 2020;70:109588. [Crossref] [PubMed]
- Damalas A, Kahan S, Shtutman M, et al. Deregulated beta-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J 2001;20:4912-22. [Crossref] [PubMed]
- Ju Z, Choudhury AR, Rudolph KL. A dual role of p21 in stem cell aging. Ann N Y Acad Sci 2007;1100:333-44. [Crossref] [PubMed]
- Xu X, Sun X, Wan X, et al. Mitomycin induces alveolar epithelial cell senescence by down-regulating GSK3β signaling. Toxicol Lett 2021;352:61-9. [Crossref] [PubMed]
- Xu M, Yu Q, Subrahmanyam R, et al. Beta-catenin expression results in p53-independent DNA damage and oncogene-induced senescence in prelymphomagenic thymocytes in vivo. Mol Cell Biol 2008;28:1713-23. [Crossref] [PubMed]
- Kosar M, Bartkova J, Hubackova S, et al. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a). Cell Cycle 2011;10:457-68. [Crossref] [PubMed]
- Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 2006;443:421-6. [Crossref] [PubMed]
- Xu Y, Tian Y, Tong D, et al. Wnt Signaling Inhibits High-Density Cell Sheet Culture Induced Mesenchymal Stromal Cell Aging by Targeting Cell Cycle Inhibitor p27. Front Bioeng Biotechnol 2020;8:946. [Crossref] [PubMed]
- Sang B, Zhang YY, Guo ST, et al. Dual functions for OVAAL in initiation of RAF/MEK/ERK prosurvival signals and evasion of p27-mediated cellular senescence. Proc Natl Acad Sci U S A 2018;115:E11661-70. [Crossref] [PubMed]
- Seluanov A, Danek J, Hause N, et al. Changes in the level and distribution of Ku proteins during cellular senescence. DNA Repair (Amst) 2007;6:1740-8. [Crossref] [PubMed]
- Marthandan S, Menzel U, Priebe S, et al. Conserved genes and pathways in primary human fibroblast strains undergoing replicative and radiation induced senescence. Biol Res 2016;49:34. [Crossref] [PubMed]
- Bikkavilli RK, Avasarala S, Van Scoyk M, et al. Wnt7a is a novel inducer of β-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene 2015;34:5317-28. [Crossref] [PubMed]
- Gong X, Carmon KS, Lin Q, et al. LGR6 is a high affinity receptor of R-spondins and potentially functions as a tumor suppressor. PLoS One 2012;7:e37137. [Crossref] [PubMed]
- Cortesi EE, Meeusen B, Vanstapel A, et al. Increased LGR6 Expression Sustains Long-Term Wnt Activation and Acquisition of Senescence in Epithelial Progenitors in Chronic Lung Diseases. Cells 2021;10:3437. [Crossref] [PubMed]
- Velasco R, Licciardello C. A genealogy of the citrus family. Nat Biotechnol 2014;32:640-2. [Crossref] [PubMed]
- Wang Y, Chen Y, Zhang H, et al. Polymethoxyflavones from citrus inhibited gastric cancer cell proliferation through inducing apoptosis by upregulating RARβ, both in vitro and in vivo. Food Chem Toxicol 2020;146:111811. [Crossref] [PubMed]
- Xu JJ, Liu Z, Tang W, et al. Tangeretin from Citrus reticulate Inhibits Respiratory Syncytial Virus Replication and Associated Inflammation in Vivo. J Agric Food Chem 2015;63:9520-7. [Crossref] [PubMed]
- Wang Y, Qian J, Cao J, et al. Antioxidant Capacity, Anticancer Ability and Flavonoids Composition of 35 Citrus (Citrus reticulata Blanco) Varieties. Molecules 2017.
- Li RF, Chen XY, Xu Y, et al. Inhibitory effects of alkaline extract from the pericarp of Citrus reticulata Blanco on collagen behavior in bleomycin-induced pulmonary fibrosis. J Ethnopharmacol 2021;269:113761. [Crossref] [PubMed]
- Zhou XM, Wen GY, Zhao Y, et al. Inhibitory effects of alkaline extract of Citrus reticulata on pulmonary fibrosis. J Ethnopharmacol 2013;146:372-8. [Crossref] [PubMed]
- Feng F, Wang Z, Li R, et al. Citrus alkaline extracts prevent fibroblast senescence to ameliorate pulmonary fibrosis via activation of COX-2. Biomed Pharmacother 2019;112:108669. [Crossref] [PubMed]
- Han D, Xu Y, Peng WP, et al. Citrus Alkaline Extracts Inhibit Senescence of A549 Cells to Alleviate Pulmonary Fibrosis via the β-Catenin/P53 Pathway. Med Sci Monit 2021;27:e928547. [Crossref] [PubMed]
- Zhang M, Xie Y, Zhou Y, et al. Exendin-4 enhances proliferation of senescent osteoblasts through activation of the IGF-1/IGF-1R signaling pathway. Biochem Biophys Res Commun 2019;516:300-6. [Crossref] [PubMed]
- Wang Z, Li W, Guo Q, et al. Insulin-Like Growth Factor-1 Signaling in Lung Development and Inflammatory Lung Diseases. Biomed Res Int 2018;2018:6057589. [Crossref] [PubMed]
- Zhao LD, Bie LY, Hu L, et al. IGF-1 induces cellular senescence in rat articular chondrocytes via Akt pathway activation. Exp Ther Med 2020;20:49. [Crossref] [PubMed]
- Nguyen XX, Muhammad L, Nietert PJ, et al. IGFBP-5 Promotes Fibrosis via Increasing Its Own Expression and That of Other Pro-fibrotic Mediators. Front Endocrinol (Lausanne) 2018;9:601. [Crossref] [PubMed]
- Rana T, Jiang C, Liu G, et al. PAI-1 Regulation of TGF-β1-induced Alveolar Type II Cell Senescence, SASP Secretion, and SASP-mediated Activation of Alveolar Macrophages. Am J Respir Cell Mol Biol 2020;62:319-30. [Crossref] [PubMed]
- Hernandez DM, Kang JH, Choudhury M, et al. IPF pathogenesis is dependent upon TGFβ induction of IGF-1. FASEB J 2020;34:5363-88. [Crossref] [PubMed]
- Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997;275:1943-7. [Crossref] [PubMed]
- Gao Q, Ye F, Xia X, et al. Correlation between PTEN expression and PI3K/Akt signal pathway in endometrial carcinoma. J Huazhong Univ Sci Technolog Med Sci 2009;29:59-63. [Crossref] [PubMed]
- Ding J, Ning B, Gong W, et al. Cyclin D1 induction by benzo[a]pyrene-7,8-diol-9,10-epoxide via the phosphatidylinositol 3-kinase/Akt/MAPK- and p70s6k-dependent pathway promotes cell transformation and tumorigenesis. J Biol Chem 2009;284:33311-9.
- Lu Y, Azad N, Wang L, et al. Phosphatidylinositol-3-kinase/akt regulates bleomycin-induced fibroblast proliferation and collagen production. Am J Respir Cell Mol Biol 2010;42:432-41. [Crossref] [PubMed]
- Sai X, Qin C, Wu Y, et al. Downregulation of PTEN mediates bleomycin-induced premature senescence in lung cancer cells by suppressing autophagy. J Int Med Res 2020;48:300060520923522. [Crossref] [PubMed]
- Hsu HS, Liu CC, Lin JH, et al. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci Rep 2017;7:14272. [Crossref] [PubMed]
- Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal 2011;23:1515-27. [Crossref] [PubMed]
- Qiu T, Tian Y, Gao Y, et al. PTEN loss regulates alveolar epithelial cell senescence in pulmonary fibrosis depending on Akt activation. Aging (Albany NY) 2019;11:7492-509. [Crossref] [PubMed]
- Nie Y, Yu K, Li B, et al. S-allyl-l-cysteine attenuates bleomycin-induced pulmonary fibrosis and inflammation via AKT/NF-κB signaling pathway in mice. J Pharmacol Sci 2019;139:377-84. [Crossref] [PubMed]
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008;585:325-37. [Crossref] [PubMed]
- Veith C, Drent M, Bast A, et al. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol Appl Pharmacol 2017;336:40-8. [Crossref] [PubMed]
- Boots AW, Drent M, de Boer VC, et al. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr 2011;30:506-12. [Crossref] [PubMed]
- Boots AW, Veith C, Albrecht C, et al. The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulm Med 2020;20:112. [Crossref] [PubMed]
- Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun 1992;186:624-31. [Crossref] [PubMed]
- Spencer JP, Rice-Evans C, Williams RJ. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem 2003;278:34783-93. [Crossref] [PubMed]
- Hohmann MS, Habiel DM, Coelho AL, et al. Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo. Am J Respir Cell Mol Biol 2019;60:28-40. [Crossref] [PubMed]
- Schafer MJ, White TA, Iijima K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 2017;8:14532. [Crossref] [PubMed]
- Lehmann M, Korfei M, Mutze K, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J 2017;50:1602367. [Crossref] [PubMed]
- Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019;40:554-63. [Crossref] [PubMed]
- Scaiola A, Mangia F, Imseng S, et al. The 3.2-Å resolution structure of human mTORC2. Sci Adv 2020;6:eabc1251. [Crossref] [PubMed]
- Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 2020;21:183-203. [Crossref] [PubMed]
- Fu W, Hall MN. Regulation of mTORC2 Signaling. Genes (Basel) 2020;11:1045. [Crossref] [PubMed]
- Festuccia WT. Regulation of Adipocyte and Macrophage Functions by mTORC1 and 2 in Metabolic Diseases. Mol Nutr Food Res 2021;65:e1900768. [Crossref] [PubMed]
- Astle MV, Hannan KM, Ng PY, et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene 2012;31:1949-62. [Crossref] [PubMed]
- Nogueira V, Park Y, Chen CC, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 2008;14:458-70. [Crossref] [PubMed]
- Calhoun C, Shivshankar P, Saker M, et al. Senescent Cells Contribute to the Physiological Remodeling of Aged Lungs. J Gerontol A Biol Sci Med Sci 2016;71:153-60. [Crossref] [PubMed]
- Laberge RM, Sun Y, Orjalo AV, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 2015;17:1049-61. [Crossref] [PubMed]
- Houssaini A, Breau M, Kebe K, et al. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight 2018;3:e93203. [Crossref] [PubMed]
- Jung SH, Hwang HJ, Kang D, et al. mTOR kinase leads to PTEN-loss-induced cellular senescence by phosphorylating p53. Oncogene 2019;38:1639-50. [Crossref] [PubMed]
- Kim YY, Jee HJ, Um JH, et al. Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell 2017;16:1094-103. [Crossref] [PubMed]
- Bernard M, Yang B, Migneault F, et al. Autophagy drives fibroblast senescence through MTORC2 regulation. Autophagy 2020;16:2004-16. [Crossref] [PubMed]
- Yates DH. mTOR treatment in lymphangioleiomyomatosis: the role of everolimus. Expert Rev Respir Med 2016;10:249-60. [Crossref] [PubMed]
- Johnson SC, Yanos ME, Bitto A, et al. Dose-dependent effects of mTOR inhibition on weight and mitochondrial disease in mice. Front Genet 2015;6:247. [Crossref] [PubMed]
- Chen X, Xu H, Hou J, et al. Epithelial cell senescence induces pulmonary fibrosis through Nanog-mediated fibroblast activation. Aging (Albany NY) 2019;12:242-59. [Crossref] [PubMed]
- Bridle KR, Popa C, Morgan ML, et al. Rapamycin inhibits hepatic fibrosis in rats by attenuating multiple profibrogenic pathways. Liver Transpl 2009;15:1315-24. [Crossref] [PubMed]
- Swaminathan S, Arbiser JL, Hiatt KM, et al. Rapid improvement of nephrogenic systemic fibrosis with rapamycin therapy: possible role of phospho-70-ribosomal-S6 kinase. J Am Acad Dermatol 2010;62:343-5. [Crossref] [PubMed]
- Gao Y, Xu X, Ding K, et al. Rapamycin inhibits transforming growth factor β1-induced fibrogenesis in primary human lung fibroblasts. Yonsei Med J 2013;54:437-44. [Crossref] [PubMed]
- Han Q, Lin L, Zhao B, et al. Inhibition of mTOR ameliorates bleomycin-induced pulmonary fibrosis by regulating epithelial-mesenchymal transition. Biochem Biophys Res Commun 2018;500:839-45. [Crossref] [PubMed]
- Molina-Molina M, Machahua-Huamani C, Vicens-Zygmunt V, et al. Anti-fibrotic effects of pirfenidone and rapamycin in primary IPF fibroblasts and human alveolar epithelial cells. BMC Pulm Med 2018;18:63. [Crossref] [PubMed]
- Chung EJ, Sowers A, Thetford A, et al. Mammalian Target of Rapamycin Inhibition With Rapamycin Mitigates Radiation-Induced Pulmonary Fibrosis in a Murine Model. Int J Radiat Oncol Biol Phys 2016;96:857-66. [Crossref] [PubMed]
- Herranz N, Gallage S, Mellone M, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 2015;17:1205-17. [Crossref] [PubMed]
- Infante B, Bellanti F, Correale M, et al. mTOR inhibition improves mitochondria function/biogenesis and delays cardiovascular aging in kidney transplant recipients with chronic graft dysfunction. Aging (Albany NY) 2021;13:8026-39. [Crossref] [PubMed]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225-60. [Crossref] [PubMed]
- Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 2012;26:203-34. [Crossref] [PubMed]
- Urban MB, Schreck R, Baeuerle PA. NF-kappa B contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J 1991;10:1817-25. [Crossref] [PubMed]
- Lopes-Paciencia S, Saint-Germain E, Rowell MC, et al. The senescence-associated secretory phenotype and its regulation. Cytokine 2019;117:15-22. [Crossref] [PubMed]
- Kawahara TL, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 2009;136:62-74. [Crossref] [PubMed]
- Osorio FG, Bárcena C, Soria-Valles C, et al. Nuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory response. Genes Dev 2012;26:2311-24. [Crossref] [PubMed]
- Tilstra JS, Robinson AR, Wang J, et al. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest 2012;122:2601-12. [Crossref] [PubMed]
- Fafián-Labora JA, O'Loghlen A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol 2020;30:628-39. [Crossref] [PubMed]
- Tian Y, Li H, Qiu T, et al. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-κB activation. Aging Cell 2019;18:e12858. [Crossref] [PubMed]
- Iannetti A, Ledoux AC, Tudhope SJ, et al. Regulation of p53 and Rb links the alternative NF-κB pathway to EZH2 expression and cell senescence. PLoS Genet 2014;10:e1004642. [Crossref] [PubMed]
- Jing X, Sun W, Yang X, et al. CCAAT/enhancer-binding protein (C/EBP) homologous protein promotes alveolar epithelial cell senescence via the nuclear factor-kappa B pathway in pulmonary fibrosis. Int J Biochem Cell Biol 2022;143:106142. [Crossref] [PubMed]
- Chen C, Zhou M, Ge Y, et al. SIRT1 and aging related signaling pathways. Mech Ageing Dev 2020;187:111215. [Crossref] [PubMed]
- Sim H, Choo S, Kim J, et al. Fisetin Suppresses Pulmonary Inflammatory Responses Through Heme Oxygenase-1 Mediated Downregulation of Inducible Nitric Oxide Synthase. J Med Food 2020;23:1163-8. [Crossref] [PubMed]
- Hussain T, Al-Attas OS, Alamery S, et al. The plant flavonoid, fisetin alleviates cigarette smoke-induced oxidative stress, and inflammation in Wistar rat lungs. J Food Biochem 2019;43:e12962. [Crossref] [PubMed]
- Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018;36:18-28. [Crossref] [PubMed]
- Zhang L, Tong X, Huang J, et al. Fisetin Alleviated Bleomycin-Induced Pulmonary Fibrosis Partly by Rescuing Alveolar Epithelial Cells From Senescence. Front Pharmacol 2020;11:553690. [Crossref] [PubMed]
- Choi MS, Choi JY, Kwon EY. Fisetin Alleviates Hepatic and Adipocyte Fibrosis and Insulin Resistance in Diet-Induced Obese Mice. J Med Food 2020;23:1019-32. [Crossref] [PubMed]
- Hu LF, Feng J, Dai X, et al. Oral flavonoid fisetin treatment protects against prolonged high-fat-diet-induced cardiac dysfunction by regulation of multicombined signaling. J Nutr Biochem 2020;77:108253. [Crossref] [PubMed]
- Yazicioglu T, Mühlfeld C, Autilio C, et al. Aging impairs alveolar epithelial type II cell function in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2020;319:L755-69. [Crossref] [PubMed]
- Kadota T, Fujita Y, Araya J, et al. Human bronchial epithelial cell-derived extracellular vesicle therapy for pulmonary fibrosis via inhibition of TGF-β-WNT crosstalk. J Extracell Vesicles 2021;10:e12124. [Crossref] [PubMed]
- Pang B, Wang Y, Chang X. A Novel Tumor Suppressor Gene, ZNF24, Inhibits the Development of NSCLC by Inhibiting the WNT Signaling Pathway to Induce Cell Senescence. Front Oncol 2021;11:664369. [Crossref] [PubMed]
- Miao J, Huang J, Luo C, et al. Klotho retards renal fibrosis through targeting mitochondrial dysfunction and cellular senescence in renal tubular cells. Physiol Rep 2021;9:e14696. [Crossref] [PubMed]
- Huang Q, Chen Y, Shen S, et al. Klotho antagonizes pulmonary fibrosis through suppressing pulmonary fibroblasts activation, migration, and extracellular matrix production: a therapeutic implication for idiopathic pulmonary fibrosis. Aging (Albany NY) 2020;12:5812-31. [Crossref] [PubMed]
- Xiang Y, Wang SH, Wang L, et al. Effects of Ginsenoside Rg1 Regulating Wnt/β-Catenin Signaling on Neural Stem Cells to Delay Brain Senescence. Stem Cells Int 2019;2019:5010184. [Crossref] [PubMed]
- Zhan H, Huang F, Ma W, et al. Protective Effect of Ginsenoside Rg1 on Bleomycin-Induced Pulmonary Fibrosis in Rats: Involvement of Caveolin-1 and TGF-β1 Signal Pathway. Biol Pharm Bull 2016;39:1284-92. [Crossref] [PubMed]
- Li X, Liu X, Deng R, et al. Betulinic acid attenuated bleomycin-induced pulmonary fibrosis by effectively intervening Wnt/β-catenin signaling. Phytomedicine 2021;81:153428. [Crossref] [PubMed]
- Okazaki H, Sato S, Koyama K, et al. The novel inhibitor PRI-724 for Wnt/β-catenin/CBP signaling ameliorates bleomycin-induced pulmonary fibrosis in mice. Exp Lung Res 2019;45:188-99. [Crossref] [PubMed]
- Miao J, Liu J, Niu J, et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 2019;18:e13004. [Crossref] [PubMed]
- Pfaff EM, Becker S, Günther A, et al. Dickkopf proteins influence lung epithelial cell proliferation in idiopathic pulmonary fibrosis. Eur Respir J 2011;37:79-87. [Crossref] [PubMed]
- Cao L, Lee SG, Park SH, et al. Sargahydroquinoic acid (SHQA) suppresses cellular senescence through Akt/mTOR signaling pathway. Exp Gerontol 2021;151:111406. [Crossref] [PubMed]
- Wang W, Li P, Xu J, et al. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Biosci Rep 2018;38:BSR20171454. [Crossref] [PubMed]
- Liu YL, Chen BY, Nie J, et al. Polydatin prevents bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β/Smad/ERK signaling pathway. Exp Ther Med 2020;20:62. [Crossref] [PubMed]
- Xu C, Huang X, Tong Y, et al. Icariin modulates the sirtuin/NF κB pathway and exerts anti aging effects in human lung fibroblasts. Mol Med Rep 2020;22:3833-9. [Crossref] [PubMed]
- Soydas T, Yaprak Sarac E, Cinar S, et al. The protective effects of metformin in an in vitro model of aging 3T3 fibroblast under the high glucose conditions. J Physiol Biochem 2018;74:273-81. [Crossref] [PubMed]
- Lim JS, Lee DY, Kim HS, et al. Identification of a novel senomorphic agent, avenanthramide C, via the suppression of the senescence-associated secretory phenotype. Mech Ageing Dev 2020;192:111355. [Crossref] [PubMed]
- Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004;23:2369-80. [Crossref] [PubMed]
- Zhang X, Dong Y, Li WC, et al. Roxithromycin attenuates bleomycin-induced pulmonary fibrosis by targeting senescent cells. Acta Pharmacol Sin 2021;42:2058-68. [Crossref] [PubMed]
- Zhang CY, Duan JX, Yang HH, et al. COX-2/sEH dual inhibitor PTUPB alleviates bleomycin-induced pulmonary fibrosis in mice via inhibiting senescence. FEBS J 2020;287:1666-80. [Crossref] [PubMed]
- Waters DW, Blokland KEC, Pathinayake PS, et al. STAT3 Regulates the Onset of Oxidant-induced Senescence in Lung Fibroblasts. Am J Respir Cell Mol Biol 2019;61:61-73. [Crossref] [PubMed]
- Baek AR, Hong J, Song KS, et al. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp Mol Med 2020;52:2034-45. [Crossref] [PubMed]
- Zhang LM, Zhang J, Zhang Y, et al. Interleukin-18 promotes fibroblast senescence in pulmonary fibrosis through down-regulating Klotho expression. Biomed Pharmacother 2019;113:108756. [Crossref] [PubMed]
- Pan J, Li D, Xu Y, et al. Inhibition of Bcl-2/xl With ABT-263 Selectively Kills Senescent Type II Pneumocytes and Reverses Persistent Pulmonary Fibrosis Induced by Ionizing Radiation in Mice. Int J Radiat Oncol Biol Phys 2017;99:353-61. [Crossref] [PubMed]
- Zhu Y, Doornebal EJ, Pirtskhalava T, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955-63. [Crossref] [PubMed]
- Chen P, Zhang Q, Zhang H, et al. Carnitine palmitoyltransferase 1C reverses cellular senescence of MRC-5 fibroblasts via regulating lipid accumulation and mitochondrial function. J Cell Physiol 2021;236:958-70. [Crossref] [PubMed]


