
Surgical timing of endoluminal repair of Stanford type B aortic coarctation and relationship to prognosis: a single-center retrospective cohort study
Highlight box
Key findings
• Endoluminal repair for TBAD in the acute phase may contribute to aortic remodeling and shorten the length of hospital stay.
What is known, and what is new?
• Endoluminal repair is the preferred option for TBAD, but the timing of TBAD treatment is controversial;
• Endoluminal repair for TBAD in the acute phase is effective in reducing the length of hospital stay and helps to reduce the diameter of the false lumen and promote complete thrombosis of the false lumen, and coronary artery disease, pleural effusion, non-acute phase surgery, and involvement of the abdominal aorta are all independent risk factors affecting the prognosis of TBAD treated with endoluminal repair.
What is the implication, and what should change now?
• The clinical choice of endoluminal repair for TBAD in the acute phase contributes to aortic remodeling and patient recovery.
Introduction
Stanford type B aortic dissection (TBAD) is a rare cardiovascular emergency that accounts for 25% to 40% of all aortic dissections. Clinical studies have found that TBAD is characterized by rapid onset and high risk, and some patients can die within hours to days after the onset of the disease (1). It is estimated that the annual number of cases of aortic coarctation in China is about 50,000–100,000, and its prognosis is poor, with an overall hospital mortality rate for TBAD of 13% (2,3). Currently, endoluminal repair is the preferred option for TBAD treatment in complex cases with symptoms such as reperfusion injury, persistent chest pain, progression of the entrapment, or a high risk of rupture (4). This procedure is mainly performed by placing a laminated stent in the true lumen to seal the proximal rupture of the entrapment, thus achieving a reduction in the arterial pressure of the entrapment and reducing aortic re-rupture. The timing of surgery is the key and difficult point in the treatment of TBAD. Clinically, the period from rupture to treatment or treatment is usually called acute phase within 14 days. At present, the timing of surgery for TBAD patients is controversial. Previous studies have pointed out that acute TBAD is prone to its own displacement and loosening after endoluminal repair due to aortic wall edema and poor support, and thus the clinical recommendation is to perform the procedure after 2 weeks for relatively stable patients (5). However, a recent study noted a significant reduction in pseudoluminal thrombosis with endoluminal repair for chronic-phase TBAD and no significant benefit in preventing aortic dissection (6). Based on this, this study compares the prognosis of TBAD treated with intracavitary repair at different surgical timing and analyzes the factors influencing the prognosis, aiming to provide a reference for the diagnosis and treatment of these patients, which are reported below. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1736/rc).
Methods
General data
One hundred and ten patients with TBAD admitted to the Second Hospital of Hebei Medical University from June 2014 to June 2022 were retrospectively selected as the study subjects. They were divided into an acute group (onset time ≤14 days, 63 cases) and a non-acute group (onset time >14 days, 47 cases) according to the time to surgical treatment. The inclusion criteria were as follows: meeting the diagnostic criteria for TBAD (7) and undergoing computed tomography (CT) angiography or magnetic resonance angiography (MRA) detection to confirm the diagnosis; treated with endoluminal repair in our hospital, as shown in Figure 1; complete clinical data; and age between 18 and 85 years. Patients were excluded according to the following criteria: contraindications to surgery; combined aortic aneurysm; atypical aortic coarctation; traumatic, medically induced coarctation; combined serious infectious diseases; or combined hereditary diseases.
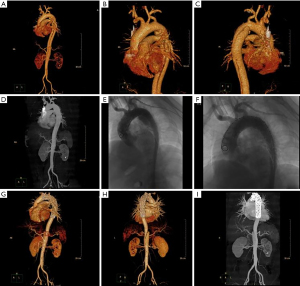
Study methods
Clinical data collection
The clinical case data of all patients were collected through the case management system, including age, sex, comorbidities, smoking, alcohol consumption, involvement of abdominal aorta, multiple ruptures in the coarctation, pleural effusion, maximum hematoma thickness, admission systolic blood pressure, admission diastolic blood pressure, admission heart rate, technical success rate, overlapping stent length, overlapping stent diameter, immediate postoperative contrast type I endoleaks, and length of hospital stay. All clinical case data were checked for completeness and validity by professional data inspectors, and incomplete data were excluded. According to the timing of surgery, the patients were divided into acute group (onset time ≤14 days, 63 cases) and non-acute group (onset time >14 days, 47 cases). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of The Second Hospital of Hebei Medical University (No. 2023-R004). Individual consent for this retrospective analysis was waived.
Follow-up
Outpatient and telephone follow-ups were used for all patients to record postoperative aortic remodeling, postoperative complications, and death at 5 months postoperatively, with a follow-up period ending in November 2022.
Observation indexes
Comparisons between the two groups were analyzed for the following variables: (I) baseline data; (II) surgery and hospitalization data; (III) aortic remodeling; (IV) postoperative follow-up results; (V) statistical analysis of the clinical case data of TBAD patients with different prognoses, with poor prognosis defined as severe complications, such as in-hospital/30 days postoperative renal failure, ischemic disease, endoleaks, aortic dilatation, retrograde type A aortic coarctation, and death; (VI) multifactorial regression analysis of factors influencing the prognosis of TBAD treated with endoluminal repair.
Statistical analysis
SPSS 20.0 (SPSS Inc., Chicago, USA) was used to analyze the data, with count data expressed as n (%) and measurement data expressed as mean ± standard deviation (SD). The baseline data of the two groups were compared by χ2 test, and the surgery, hospitalization, and aortic remodeling of the two groups were compared by t-test. The risk factors affecting the prognosis of TBAD treated by endoluminal repair were analyzed by univariate and multivariate logistic regression. Test level α=0.05, two-sided test.
Results
Comparison of baseline information between the two groups
Among the 110 TBAD patients, 57 were male and 53 were female; age ranged from 37 to 78 years, with a mean of 56.79±5.98 years; history of smoking in 35 cases, history of alcohol consumption in 21 cases; hypertension in 56 cases, diabetes mellitus in 37 cases, coronary artery disease in 11 cases; involvement of the abdominal aorta in 16 cases, multiple rupture of the entrapment in 15 cases, and pleural effusion in 18 cases. There were no statistically significant differences between the two groups when comparing the baseline data of gender, age, smoking history, drinking history, comorbidities, cumulative abdominal aorta, multiple ruptures in the entrapment, maximum hematoma thickness, diastolic blood pressure, and systolic blood pressure (P=0.364, 0.415, 0.693, 0.614, 0.130, 0.315, 0.429, 0.250, 0.310, 0.634). The percentage of pleural effusion and heart rate were higher in the acute group than in the non-acute group, and the differences were statistically significant (P=0.015, <0.001), as shown in Table 1.
Table 1
| Variables | Acute group (n=63) | Non-acute group (n=47) | χ2/t | P |
|---|---|---|---|---|
| Gender | 0.825 | 0.364 | ||
| Male | 35 (55.56) | 22 (46.81) | ||
| Female | 28 (44.44) | 25 (53.19) | ||
| Age (years) | 57.19±5.83 | 56.25±6.14 | 0.818 | 0.415 |
| Smoking history | 21 (33.33) | 14 (29.79) | 0.156 | 0.693 |
| Drinking history | 11 (17.46) | 10 (21.28) | 0.254 | 0.614 |
| Comorbidities | 0.130 | |||
| High blood pressure | 36 (57.14) | 20 (42.55) | 2.293 | – |
| Diabetes | 23 (36.51) | 14 (29.79) | 0.545 | 0.461 |
| Coronary heart disease | 7 (11.11) | 4 (8.51) | 0.202 | 0.653 |
| Involvement of the abdominal aorta | 11 (17.46) | 5 (10.64) | 1.008 | 0.315 |
| Interlayer multi-breakage | 10 (15.87) | 5 (10.64) | 0.626 | 0.429 |
| Pleural effusion | 15 (23.81) | 3 (6.38) | 5.973 | 0.015 |
| Maximum hematoma thickness (mm) | 16.78±3.62 | 16.02±3.11 | 1.156 | 0.250 |
| Admission diastolic blood pressure (mmHg) | 71.36±11.65 | 73.68±12.01 | 1.020 | 0.310 |
| Admission systolic blood pressure (mmHg) | 129.42±14.16 | 128.06±15.58 | 0.477 | 0.634 |
| Admission heart rate (beats/min) | 85.86±12.35 | 74.22±11.4 | 5.052 | <0.001 |
Data are presented as mean ± SD or n (%). SD, standard deviation.
Comparison of surgery and hospitalization between the two groups
Among the 110 patients with TBAD, 1 case failed in endovascular repair and 15 cases had type I endoleak immediately after operation. The length of hospitalization was shorter in the acute group than in the non-acute group, and the difference was statistically significant (t=3.292, P=0.001). The technical success rate, overlying stent length, overlying stent diameter, and immediate postoperative contrast type I endoleaks were compared between the two groups, and the differences were not statistically significant (P=0.386, 0.551, 0.093, 0.176), as shown in Table 2.
Table 2
| Variables | Acute group (n=63) | Non-acute group (n=47) | χ2/t | P |
|---|---|---|---|---|
| Technical success rate | 56 (88.89) | 33 (70.21) | 0.753 | 0.386 |
| Length of laminating bracket | 16.08±2.02 | 16.31±1.96 | 0.598 | 0.551 |
| Diameter of laminating support | 3.32±0.41 | 3.45±0.38 | 1.697 | 0.093 |
| Immediate postoperative contrast type I endoleaks | 11 (17.46) | 4 (8.51) | 1.831 | 0.176 |
| Length of hospitalization (days) | 13.32±3.44 | 15.69±4.1 | 3.292 | 0.001 |
Data are presented as mean ± SD or n (%). SD, standard deviation.
Comparison of aortic remodeling between the two groups
Of the 110 TBAD patients, 69 had complete thrombosis of the false lumen. The rate of complete thrombosis of the false lumen and the difference in the maximum diameter of the false lumen were higher in the acute group than in the non-acute group, and the maximum postoperative diameter of the false lumen was lower than in the non-acute group, with statistically significant differences (P=0.029, <0.001, 0.004), as shown in Table 3.
Table 3
| Variables | Acute group (n=63) | Non-acute group (n=47) | χ2/t | P |
|---|---|---|---|---|
| Complete thrombosis of the false lumen | 45 (71.43) | 24 (51.06) | 4.775 | 0.029 |
| Maximum diameter of preoperative false cavity | 32.64±6.83 | 33.71±7.42 | 0.783 | 0.435 |
| Maximum diameter of postoperative false cavity | 14.75±3.74 | 17.16±4.85 | 2.943 | 0.004 |
| Difference in the maximum diameter of the false cavity | 17.52±4.76 | 14.11±4.42 | 3.831 | <0.001 |
Data are presented as mean ± SD or n (%). SD, standard deviation.
Comparison of postoperative follow-up results between the two groups
After 5 months of follow-up, 20 patients had adverse events, including 6 cases of renal failure, 3 cases of ischemic disease, 5 cases of endoleak, 2 cases of aortic dilatation, 3 cases of retrograde type A aortic dissection and 1 case of death. There was no statistically significant difference in the incidence of renal failure, ischemic disease, endoleaks, aortic dilatation, retrograde type A aortic coarctation, and death between the two groups (P=0.223, 0.739, 0.085, 0.098, 0.395, 0.386), as shown in Table 4.
Table 4
| Variables | Acute group (n=63), postoperative complications | Non-acute group (n=47), postoperative complications | χ2 | P |
|---|---|---|---|---|
| Renal failure | 2 (3.17) | 4 (8.51) | 1.486 | 0.223 |
| Ischemic disease | 2 (3.17) | 1 (2.13) | 0.111 | 0.739 |
| Internal leakage | 1 (1.59) | 4 (8.51) | 2.974 | 0.085 |
| Aortic dilation | 0 (0.00) | 2 (4.26) | 2.730 | 0.098 |
| Retrograde type A aortic coarctation | 1 (1.59) | 2 (4.26) | 0.722 | 0.395 |
| Death rate | 1 (1.59) | 0 (0.00) | 0.753 | 0.386 |
Data are shown as n (%).
Comparison of clinical case data of TBAD patients with different prognoses
The differences between the good prognosis group and the poor prognosis group were statistically significant in diabetes, coronary artery disease, pleural effusion, involvement of abdominal aorta, and maximum diameter of false lumen (P<0.05). There were no statistically significant differences in gender, age, smoking, alcohol consumption, hypertension, timing of surgery, multiple ruptures of the entrapment, systolic blood pressure, diastolic blood pressure, and heart rate (P>0.05), as shown in Table 5.
Table 5
| Variables | Poor prognosis group (n=20) | Good prognosis group (n=90) | χ2/t | P |
|---|---|---|---|---|
| Gender | 1.701 | 0.192 | ||
| Male | 13 (65.00) | 44 (48.89) | ||
| Female | 7 (35.00) | 46 (51.11) | ||
| Age (years) | 58.12±5.34 | 56.40±6.10 | 1.273 | 0.206 |
| Smoking | 9 (45.00) | 26 (28.89) | 1.958 | 0.162 |
| Drinking | 4 (20.00) | 17 (18.89) | 0.013 | 0.909 |
| Comorbidities | ||||
| High blood pressure | 13 (65.00) | 43 (47.78) | 1.942 | 0.163 |
| Diabetes | 11 (55.00) | 26 (28.89) | 4.998 | 0.025 |
| Coronary heart disease | 5 (25.00) | 6 (6.67) | 6.111 | 0.013 |
| Timing of surgery | 4.955 | 0.026 | ||
| Acute phase | 7 (35.00) | 56 (62.22) | ||
| Non-acute phase | 13 (65.00) | 34 (37.78) | ||
| Involvement of the abdominal aorta | 6 (30.00) | 10 (11.11) | 4.697 | 0.030 |
| Interlayer multi-breakage | 4 (20.00) | 11 (12.22) | 0.841 | 0.359 |
| Pleural effusion | 7 (35.00) | 11 (12.22) | 6.203 | 0.013 |
| Maximum diameter of the false cavity | 4.053 | 0.044 | ||
| >22 mm | 19 (95.00) | 67 (74.44) | ||
| ≤22 mm | 1 (5.00) | 23 (25.56) | ||
| Admission diastolic blood pressure | 3.438 | 0.064 | ||
| >90 mmHg | 7 (35.00) | 15 (16.67) | ||
| ≤90 mmHg | 13 (65.00) | 75 (83.33) | ||
| Admission systolic blood pressure | 1.443 | 0.230 | ||
| >140 mmHg | 7 (35.00) | 20 (22.22) | ||
| ≤140 mmHg | 13 (65.00) | 70 (77.78) | ||
| Admission heart rate | 0.736 | 0.391 | ||
| >100 times/min | 6 (30.00) | 19 (21.11) | ||
| ≤100 times/min | 14 (70.00) | 71 (78.89) |
Data are shown as mean ± standard deviation or n (%). TBAD, type B aortic dissection.
Multi-factor regression analysis of factors influencing the prognosis of TBAD treated with endoluminal repair
Variables with statistical significance in Table 5 were assigned with the variables of poor prognosis group =1, good prognosis group =0; male =1, female =0; age >60 years =1, ≤60 years =0; with smoking =1, without smoking =0; with alcohol =1, without alcohol =0; with hypertension =1, without hypertension =0; with diabetes =1, without diabetes =0; with coronary artery disease =1, without coronary artery disease =0; acute phase =0, non-acute phase =1; involvement of the abdominal aorta =1, no involvement of the abdominal aorta =0; presence of multiple ruptures of entrapment =1, no multiple ruptures of entrapment =0; presence of pleural effusion =1, no pleural effusion =0; maximum diameter of pseudocavity >22 mm =1, ≤22 mm =0; admission diastolic blood pressure >90 mmHg =1, ≤22 mm =0; admission systolic blood pressure >140 mmHg =1, ≤140 mmHg =0; admission heart rate >100 beats/min =1, ≤100 beats/min =0. One-way logistic regression analysis showed that diabetes mellitus [odds ratio (OR) =3.009, P=0.030], coronary artery disease (OR =4.667, P=0.021), timing of surgery (OR =0.327, P=0.031), involvement of abdominal aorta (OR =3.429, P =0.037), pleural effusion (OR =3.867, P=0.017), and maximum diameter of the false lumen (OR =8.143, P=0.046) were all factors influencing the prognosis of TBAD treated with endoluminal repair (P<0.05), as shown in Table 6.
Table 6
| Indicators | β | SE | Wald χ2 | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Gender (male vs. female) | 0.663 | 0.514 | 1.666 | 1.942 | 0.709–5.318 | 0.197 |
| Age (>60 vs. ≤60 years) | 0.455 | 0.562 | 0.656 | 1.576 | 0.524–4.743 | 0.418 |
| Smoking (with vs. without) | 0.700 | 0.506 | 1.914 | 2.014 | 0.747–5.430 | 0.167 |
| Drinking (with vs. without) | 0.071 | 0.621 | 0.013 | 1.074 | 0.318–3.622 | 0.909 |
| High blood pressure (with vs. without) | 0.708 | 0.514 | 1.896 | 2.030 | 0.741–5.560 | 0.168 |
| Diabetes (with vs. without) | 1.101 | 0.506 | 4.737 | 3.009 | 1.116–8.112 | 0.030 |
| Coronary heart disease (yes vs. no) | 1.540 | 0.667 | 5.330 | 4.667 | 1.262–17.258 | 0.021 |
| Timing of surgery (acute vs. non-acute phase) | 1.118 | 0.517 | 4.681 | 0.327 | 0.119–0.900 | 0.031 |
| Involvement of the abdominal aorta (yes vs. no) | 1.232 | 0.592 | 4.330 | 3.429 | 1.074–10.943 | 0.037 |
| Interlayer multi-breakage (with vs. without) | 0.585 | 0.645 | 0.823 | 1.795 | 0.507–6.357 | 0.364 |
| Pleural effusion (with vs. without) | 1.353 | 0.569 | 5.657 | 3.867 | 1.269–11.787 | 0.017 |
| Maximum diameter of the pseudocavity (>22 vs. ≤22 mm) | 2.097 | 1.051 | 3.978 | 8.143 | 1.037–63.940 | 0.046 |
| Admission diastolic blood pressure (>90 vs. ≤90 mmHg) | 0.990 | 0.548 | 3.272 | 2.692 | 0.921–7.874 | 0.070 |
| Admission systolic blood pressure (>140 vs. ≤140 mmHg) | 0.634 | 0.533 | 1.414 | 1.885 | 0.663–5.357 | 0.234 |
| Admission heart rate (>100 vs. ≤100 times/min) | 0.471 | 0.552 | 0.728 | 1.602 | 0.543–4.726 | 0.394 |
TBAD, type B aortic dissection; SE, standard error; OR, odds ratio; CI, confidence interval.
Discussion
With the change in people’s diet and lifestyle, the incidence of aortic coarctation is on the rise. Endoluminal repair is an important tool for TBAD treatment and is conducted by placing a stent into the breach through a minimally invasive incision, significantly reducing the risk factor of surgery and allowing patients to recover quickly (8). However, to date, the surgical indications for endoluminal repair for TBAD remain controversial. A domestic study has shown that early endoluminal repair treatment has a lower rate of postoperative complications and mortality in TBAD than conservative treatment (9).
In this study, by grouping patients according to the timing of surgery for treatment and counting the surgery and hospitalization in both groups, we found that the hospitalization time was shorter in the acute group than in the non-acute group, suggesting that early endoluminal repair can effectively shorten the hospitalization time, which is consistent with the findings of Cheng et al. (10). Patients with TBAD in the subacute and chronic stages have a significantly reduced remodeling capacity due to the continuous enlargement of the false lumen as the disease continues to progress, and thus these patients have a slower postoperative recovery.
Studies have noted a correlation between pseudolumen patency and aortic dilatation, localized pseudolumen aneurysmal expansion, and arterial dissection (11,12). In this study, we found that the rate of complete thrombosis of the false lumen and the difference in the maximum diameter of the false lumen were higher in the acute group than in the non-acute group, and the maximum diameter of the false lumen after surgery was lower than in the non-acute group, suggesting that early surgery helps to reduce the diameter of the false lumen and promotes complete thrombosis of the false lumen. Fanelli et al. also pointed out that the false lumen is more likely to form thrombosis and contract more significantly in patients with TBAD in the acute phase (13). The reason for this may be because the aorta in patients with acute and subacute TBAD is not fibrotic after surgery and thus has better intimal compliance, which contributes to pseudoluminal thrombosis. In contrast, the aortic wall of patients with TBAD in the chronic phase is less adaptable to the overlapping stent, and thrombosis is less likely to form within the residual false lumen (14).
Although endoluminal repair has achieved good early and mid-term outcomes in treating TBAD, its postoperative complications, such as renal impairment, ischemic disease, endoleaks, and retrograde entrapment, remain unavoidable (15,16). In the present study, there was no significant difference in the incidence of serious postoperative complications and death between the two groups. The findings of Torrent et al. that the timing of endoluminal repair treatment for simple TBAD did not predict postoperative complications or mortality coincide with the present study’s findings (17). Endoleaks and retrograde entrapment are the more common complications after endoluminal repair, which are mainly related to the inability of the overlapping stent to fit the aortic wall adequately. Studies have pointed out that patients with TBAD have some degree of renal injury after surgery due to the use of large amounts of contrast agent during endoluminal repair and the effect of postoperative stent placement on renal artery hemodynamics (18,19). The causes of ischemic diseases (mainly stroke) are mostly inadequate proximal anchoring distance and cerebrovascular hypoperfusion.
In this study, coronary artery disease was found to be an independent risk factor affecting the prognosis of TBAD treated with endoluminal repair. Patients with combined coronary artery disease are more prone to cardiovascular disease and have a poorer prognosis after surgery due to stenosis and myocardial ischemia. In 2021, the Chinese Medical Association recommended that different coronary surgical strategies be selected for coronary lesions and entrapment involvement to reduce aortic shear stress and prevent aortic dissection (20). Previous studies have considered diabetes the number one risk factor for cardiovascular disease (21). However, in the present study, diabetes was not a high-risk factor affecting the prognosis of TBAD treated with endoluminal repair, a result similar to the findings of Chen et al. (22). Another study found that diabetic patients had a lower risk of aortic coarctation (23). This variability in results may be associated with the study population or the use of antiglycemic and antihypertensive drugs. In this study, age was not a risk factor affecting the prognosis of TBAD patients, and the reason for this may be related to the small sample size of this study and the inclusion of a population with no patients under 40 years of age (24). Pleural effusion is mainly caused by ruptured aortic bleeding and its surrounding inflammatory response, which can lead to altered aortic flow status and, consequently, to poor prognosis. The non-acute phase of surgery is a risk factor for the prognosis of TBAD treated with endoluminal repair, and the reason for this may be related to the greater susceptibility to thrombosis of the false lumen when endoluminal repair is performed in the acute phase. In addition, patients undergoing early surgery are less likely to develop ischemic disease because of the relatively low degree of damage to organs and tissues of the body due to the shorter time of vascular inflammatory infiltration (25,26). Studies have shown that when TBAD involves the abdominal aorta and its branches, severe digestive symptoms can trigger serious complications, such as acute intestinal ischemic syndrome (27,28). In general, the diameter of the false lumen is larger than the true lumen, and it increases with disease progression, which in turn affects aortic valve closure and systemic circulation status (29). In addition, progressive expansion of the TBAD false lumen correlates with systemic coagulation (30). In the present study, the maximum diameter of the false lumen >22 mm was not a risk factor affecting the prognosis of TBAD patients, and the reason for this may be related to the insufficient sample size in this study.
Conclusions
In conclusion, endoluminal repair for TBAD in the acute phase may contribute to aortic remodeling and pseudoluminal complete thrombosis and shorten the hospital stay. The prognosis of TBAD patients can be assessed clinically in combination with high-risk factors such as coronary artery disease, pleural effusion, and involvement of the abdominal aorta, and risk stratification and early intervention can be performed to reduce the associated morbidity and mortality. In addition, local branch artery flow is preserved by optimizing true-lumen intraluminal repair of the thoracic aorta, such as through windowing, chimneying, and multiple stent overlays to isolate the entrapment breach. Pseudoluminal flow blocking techniques, such as spring coils and bioprotein gel embolization, can also be used to better address the problem of pseudoluminal patency in aortic coarctation with smaller pseudolumina.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1736/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1736/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1736/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of The Second Hospital of Hebei Medical University (No. 2023-R004). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800-11. [Crossref] [PubMed]
- Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol 2019;16:203-12. [Crossref] [PubMed]
- Milewicz DM, Ramirez F. Therapies for Thoracic Aortic Aneurysms and Acute Aortic Dissections. Arterioscler Thromb Vasc Biol 2019;39:126-36. [Crossref] [PubMed]
- Harky A, Chan JSK, Wong CHM, et al. Systematic review and meta-analysis of acute type B thoracic aortic dissection, open, or endovascular repair. J Vasc Surg 2019;69:1599-1609.e2. [Crossref] [PubMed]
- Xie E, Yang F, Liu Y, et al. Timing and Outcome of Endovascular Repair for Uncomplicated Type B Aortic Dissection. Eur J Vasc Endovasc Surg 2021;61:788-97. [Crossref] [PubMed]
- Cangussú LR, Lopes MR, Barbosa RHA. The importance of the early diagnosis of aorta coarctation. Rev Assoc Med Bras (1992) 2019;65:240-5. [Crossref] [PubMed]
- Dijkema EJ, Leiner T, Grotenhuis HB. Diagnosis, imaging and clinical management of aortic coarctation. Heart 2017;103:1148-55. [Crossref] [PubMed]
- Wilson-Smith AR, Muston B, Kamalanathan H, et al. Endovascular repair of acute complicated type B aortic dissection-systematic review and meta-analysis of long-term survival and reintervention. Ann Cardiothorac Surg 2021;10:723-30. [Crossref] [PubMed]
- Xiang DQ, Zheng CS, Liang HM, et al. Endovascular Repair of Stanford Type B Aortic Dissection: analysis of Single-Center Long-Term Effect. Journal of Clinical Radiology 2020;39:161-4.
- Cheng QJ, He YY, Gao PC, et al. Selection of operative time for endovascular repair of Stanford type B aortic intramural hematoma. China Journal of Modern Medicine 2022;32:81-4.
- Sayed A, Munir M, Bahbah EI. Aortic Dissection: A Review of the Pathophysiology, Management and Prospective Advances. Curr Cardiol Rev 2021;17:e230421186875. [Crossref] [PubMed]
- Kato Y, Tatsuishi W, Konishi Y, et al. Case of stanford type B aortic dissection treated with thoracic endovascular aortic repair and retrograde abdominal artery embolization. Annals of Vascular Surgery-Brief Reports and Innovations 2022;2:100108.
- Fanelli F, Cannavale A, O'Sullivan GJ, et al. Endovascular Repair of Acute and Chronic Aortic Type B Dissections: Main Factors Affecting Aortic Remodeling and Clinical Outcome. JACC Cardiovasc Interv 2016;9:183-91. [Crossref] [PubMed]
- Li D, Peng L, Wang Y, et al. Predictor of false lumen thrombosis after thoracic endovascular aortic repair for type B dissection. J Thorac Cardiovasc Surg 2020;160:360-7. [Crossref] [PubMed]
- Howard C, Sheridan J, Picca L, et al. TEVAR for complicated and uncomplicated type B aortic dissection-Systematic review and meta-analysis. J Card Surg 2021;36:3820-30. [Crossref] [PubMed]
- Alvarez-Fuente M, Ayala A, Garrido-Lestache E, et al. Long-Term Complications After Aortic Coarctation Stenting. J Am Coll Cardiol 2021;77:2448-50. [Crossref] [PubMed]
- Torrent DJ, McFarland GE, Wang G, et al. Timing of thoracic endovascular aortic repair for uncomplicated acute type B aortic dissection and the association with complications. J Vasc Surg 2021;73:826-35. [Crossref] [PubMed]
- Tossas-Betancourt C, van Bakel TMJ, Arthurs CJ, et al. Computational analysis of renal artery flow characteristics by modeling aortoplasty and aortic bypass interventions for abdominal aortic coarctation. J Vasc Surg 2020;71:505-516.e4. [Crossref] [PubMed]
- Mukaiyama Y, Okada A, Kawakatsu Y, et al. Complete post-operative resolution of "temporary" end-stage kidney disease secondary to aortic dissection without static renal artery obstruction: a case study. BMC Nephrol 2019;20:368. [Crossref] [PubMed]
- Group MChinese Medical Association, Editorial Board of Chinese Journal of Cardiology. Chinese expert consensus on management of patients with acute aortic dissection complicating with coronary artery disease. Chinese Journal of Cardiology 2021;49:1074-81. [Crossref] [PubMed]
- Fedchenko M, Mandalenakis Z, Dellborg H, et al. Cardiovascular risk factors in adults with coarctation of the aorta. Congenit Heart Dis 2019;14:549-58. [Crossref] [PubMed]
- Chen YF, Huang B, Li XF, et al. Clinical Features and Outcomes of Acute Aortic Dissection Patients Complicating with Diabetes. Chinese Circulation Journal 2021;36:1107-13.
- Liu H, Shi L, Zeng T, et al. Type 2 diabetes mellitus reduces clinical complications and mortality in Stanford type B aortic dissection after thoracic endovascular aortic repair: A 3-year follow-up study. Life Sci 2019;230:104-10. [Crossref] [PubMed]
- Dou L, Gao W, Wu C, et al. The effect of hypertension on the prognosis of acute aortic dissection. Chinese Journal of Emergency Medicine 2019;28:614-8.
- Evangelista A, Isselbacher EM, Bossone E, et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018;137:1846-60. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Eleshra A, Kölbel T, Panuccio G, et al. Endovascular Therapy for Nonischemic vs Ischemic Complicated Acute Type B Aortic Dissection. J Endovasc Ther 2020;27:145-52. [Crossref] [PubMed]
- Thakkar D, Dake MD. Management of Type B Aortic Dissections: Treatment of Acute Dissections and Acute Complications from Chronic Dissections. Tech Vasc Interv Radiol 2018;21:124-30. [Crossref] [PubMed]
- He X, Peng L, Cheng C, et al. To Explore the Effect of Preoperative Hemodynamic Factors on The Outcome of Pseudolumen After Stanford Type B Aortic Dissection TEVAR Based on Computer Fluid Dynamics. Heart Surg Forum 2022;25:E483-8. [Crossref] [PubMed]
- Wang D, Chen J, Sun J, et al. The diagnostic and prognostic value of D-dimer in different types of aortic dissection. J Cardiothorac Surg 2022;17:194. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


