Intrathoracic vacuum therapy for the therapy of pleural empyema—a systematic review and analysis of the literature
Highlight box
Key findings
• Intrathoracic vacuum therapy is a safe treatment option for patients with pleural empyema.
• Vacuum sponge therapy adds a treatment option to otherwise inoperable, critically ill patients.
• Vacuum therapy has the potential to become a primary treatment alternative for pleural empyema stage II and III.
What is known and what is new?
• Intrathoracic vacuum therapy is currently used mostly for volume reduction in patients with OWT.
• Recently, vacuum therapy has been technically adapted to allow minimally invasive approaches to apply this treatment to pleural empyema cavities without the need for OWT creation and rib resection.
What is the implication, and what should change now?
• More data and larger scaled analyses are needed to further validate the potential of thoracic vacuum therapy in pleural empyema.
• Randomised prospective trials are needed to evaluate vacuum therapy as a primary treatment option for empyema.
Introduction
In the last 20 years, the incidence of pleural empyema as well as mortality rates have been increasing (1-4). Treatment ranges—depending on the stage of the disease—from non-surgical interventions such as antibiotic therapy and chest tube placement to thoracoscopic or open surgery. Treatment of pleural empyema, especially chronic empyema remains a challenge with mortality rates of up to 15% having been reported (5). This explains the endeavor to explore new treatment options for this condition.
Both open surgery and video-assisted thoracoscopy (VATS) have been shown in meta-analysis to significantly reduce length of hospital stay (LOHS) in empyema patients compared to thoracostomy drainage alone (6). The goal of standard surgical therapy is the complete unleashing and decortication of the visceral pleura, enabling a re-expansion of the lung. An exception are patients with post-pneumonectomy empyema, where no lung tissue remains to fill the empyema cavity. The surgical approach is dictated by the condition of the pleural space as well as the condition of the patient. If single-lung ventilation is tolerated, VATS should be attempted according to the American Association of Thoracic Surgery consensus guidelines for stage II and II/III pleural empyema (7). It has proven benefits compared to thoracotomy in postoperative outcomes. These include improved postoperative pain control, shorter LOHS and reduction in 30-day overall mortality (8,9). VATS has now become the standard of care worldwide for the initial treatment of pleural empyema and should be attempted when possible. In reality a large number of patients need to be converted to open surgery, in patients with advanced empyema this amounts up to 46% (8).
In cases of incomplete postoperative lung re-expansion, tissue flaps may be warranted to fill the pleural cavity to prevent reformation of fluid collections. Open window thoracostomy (OWT) treatment is indicated as a rescue procedure only in patients which are unfit to undergo decortication (7), but OWT significantly impairs quality of life and leads to prolonged hospital stays (10,11). In post pneumonectomy patients, OWT may also impair the re-expansion of the contralateral side and lead to worsening right heart failure.
Vacuum sponge therapy was introduced over 30 years ago, and there is hardly any chronic or difficult wound where application has not at least been tried. It reduces edema, promotes bacterial clearance and increases perfusion to the wound bed (12). Intrathoracic vacuum therapy is a relatively new treatment option for pleural empyema. Vacuum sponge treatment of pleural empyema might have potential benefits: (I) the suction therapy induces a softening of the empyema membrane and thereby enables a collapse of the cavity. Hence, complete decortication may not be necessary. Due to the negative pressure established in the thoracic cavity, the diaphragm is elevated and may facilitate closure of relevant tissue defects. (II) The healthy parts of the lung could potentially be left untouched. With less aggressive decortication and spreading of the infectious material, surgically induced aggravation of septic conditions or even a septic shock might be reduced. (III) Additionally, bleeding and air-leak complications often seen in patients having received surgical treatment might be reduced by this approach (D). Due to the pre-existing empyema-sac, a single lung ventilation might not be necessary, reducing patient burden and simplifying the anaesthesiologic procedure.
The aim of the current manuscript is a systematic review of the current literature of intrathoracic vacuum therapy for pleural empyema and to discuss its potential drawbacks and benefits in comparison with the conventional surgical approach. The review also aims to highlight where additional research needs to be carried out and what benefits this may have for patients being treated for pleural empyema. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1188/rc).
Methods
Inclusion criteria
We searched for prospective and retrospective studies describing vacuum therapy for pleural empyema, regardless of country of origin.
Exclusion criteria
Single case reports were excluded from the analysis.
Quality of included studies
We performed ROBINS-I scoring to evaluate the risk of bias in the included retrospective studies (13). The overall risk of bias of the retrospective studies was low to moderate in twelve studies, but two studies showed a serious risk of bias, mainly due to missing data. No testing for publication bias for the primary outcome was performed as recommended by the Cochrane Collaboration due to the small number of studies and patients.
Outcomes
The primary analysed outcome was the rate of successful healing of pleural empyema. This outcome was heterogeneously defined in the studies analysed, ranging from reduced systemic signs of infection and negative cultures (14) to 50% reduction in empyema cavity and reduced infectious signs (15), to clean cavity and ability to perform thoracic closure (16,17). The other studies did not have clearly defined success surrogates. Secondary outcomes were mortality, hospitalisation time (in days), complications, median duration of therapy (in days), and number of interventions.
Search strategy
We performed a systematic review according the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist (18) searching for published and unpublished trials in German and English using the Cochrane central register of controlled trials and MEDLINE (1 January 2008 to 1 August 2022). Searches were carried out using medical subject headings and free-text words.
A MEDLINE (Ovid interface) was performed during August 2022. The search terms applied are listed in Table 1. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE. In addition, we searched the reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. We also searched relevant journals and conference abstracts to address the issue of publication bias.
Table 1
| Search set | Terms | Results |
|---|---|---|
| #1 | Vacuum assisted closure (MeSH Terms) AND Pleural empyema (MeSH Terms) | 47 |
| #2 | Negative pressure wound therapy (MeSH Terms) AND Pleural empyema (MeSH Terms) | 47 |
| #3 | Intrathoracic vacuum therapy (MeSH Terms) AND Pleural empyema (MeSH Terms) | 13 |
MeSH, medical subject headings.
Data collection and analysis
The titles and abstracts of the manuscripts were independently assessed by two investigators (MT and BOS). Studies that clearly did not meet the inclusion criteria were excluded. The full texts of all possibly relevant articles were evaluated to determine eligibility. Disagreements were resolved by consultation with a third investigator (KB).
Independently, the following data were retrieved: authors, year of publication, country, inclusion and exclusion criteria, study methodology, number of treated patients, age and sex of patients, underlying cause of empyema, method of vacuum sponge application (thoracotomy/VATS, OWT), duration of treatment, number of interventions, outcomes including treatment-related complications, healing rate, overall LOHS, length of postoperative stay (LOPS), in-hospital mortality and possible ambulatory treatment and time to ambulatory treatment.
Study quality
Risk of bias was evaluated by the two investigators based on the ROBINS-I score, validating each grade of confounding, selection, classification of and deviation from intervention, missing data, outcome measurement, and selection of reported results (13). Disagreements were objectively discussed by the two investigators until an agreement was reached.
Quality of evidence
The quality of the evidence was poor overall. The main limitations were few included studies for analysis, inconsistencies among studies and only retrospective case series with poor standardisation and limited patient numbers. The statistical methods used in all case series were not sufficient to make statements about significant treatment differences.
Results
Description of studies
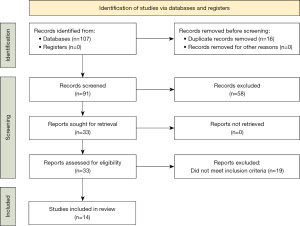
A total of 33 publications were found using the search strategy (Table 1). We screened the titles and abstracts of 33 records and discarded 19 records, as they did not meet the inclusion criteria. We obtained the full text of 14 articles for in-depth review to be included in this review. The PRISMA flowchart is shown in Figure 1.
Eight case series from Germany, one case series from Turkey, two from the USA, and one from the Netherlands, Switzerland and Japan were analysed.
Three case series reported on the use of the Instill-vacuum system (19,20), eight on conventional vacuum sponge therapy (15,16,21-25) and 2 on the mini-vacuum therapy (26). One series combined the mini-vacuum therapy with instill-vacuum technology (27), while one study compared all three vacuum types with each other (28).
Studies and patient characteristics
The 14 studies included assessed 165 patients. The median age was 64 years, ranging from 23 to 94 years. There was a predominance of male patients (78.7% vs. 21.4%) (Table 2).
Table 2
| Author | Year | Country | Patients (n) | Male (n) | Female (n) | Age [years, median (range)] |
|---|---|---|---|---|---|---|
| Al-Mufarrej et al. (21) | 2010 | USA | 6 | 5 | 1 | 55.5 [23–72] |
| Aru et al. (22) | 2010 | USA | 5 | 3 | 2 | 51 [41–61] |
| Ditterich et al. (23) | 2006 | Germany | 3 | 1 | 2 | 75 [41–95] |
| Hofmann et al. (19) | 2015 | Germany | 3 | 2 | 1 | 75 [56–87] |
| Karapinar et al. (15) | 2016 | Turkey | 8 | 8 | 0 | 59.5 [49–86] |
| Nishii et al. (16) | 2021 | Japan | 10 | 7 | 3 | 73.5 [53–77] |
| Palmen et al. (24) | 2009 | The Netherlands | 11 | 8 | 3 | 51 [23–73] |
| Schreiner et al. (20) | 2013 | Germany | 7 | 5 | 2 | 50 [32–74] |
| Sziklavari et al. (25) | 2011 | Germany | 8 | 8 | 0 | 68 [53–76] |
| Sziklavari et al. (26) | 2013 | Germany | 6 | 5 | 1 | 53.5 [41–72] |
| Sziklavari et al. (28) | 2016 | Germany | 43 | 39 | 4 | 64 [25–91] |
| Sziklavari et al. (27) | 2015 | Germany | 15 | 13 | 2 | 71 [25–91] |
| Groetzner et al. (14) | 2009 | Germany | 13 | 11 | 2 | 60 [41–82] |
| Saadi et al. (17) | 2011 | Switzerland | 27 | 15 | 12 | 64 [37–77] |
Even though in most of the studies patients were judged as highly morbid, only very few detailed data for comorbidities were available (not shown).
All patients in the reported trials received concomitant antibiotic therapy. Success rates ranged from 66.7–100%, with 4 trials reporting a success rate of 100%. Recurrences with the need for revisional surgery were reported in 4 case series.
All but 3 series included patients with bronchopulmonary fistulas (BPF) following pulmonary resection, with BPF rates ranging from 0–80%.
Cause of empyema
Overall, 105 of 165 patients (63.6%) had empyema following surgical procedures. The most common procedures were lobectomy (29 patients) and pneumonectomy (27 patients). However, there was a large variety of procedures including Pancoast resection, oesophagus resection, sternotomy for aortic rupture and vertebral spine fusion.
Among the patients without prior surgical procedures (60 patients, 36.4%) the leading causes of pleural empyema were primary empyema (35 patients) and pneumonia. Other causes like pneumothorax or liver abscess were rare (Table 3).
Table 3
| Author | Year | Patients (n) | Cause of pleural empyema [n] |
|---|---|---|---|
| Al-Mufarrej et al. (21) | 2010 | 6 | Lobectomy [4]; pneumonectomy [2] |
| Aru et al. (22) | 2010 | 5 | Pneumonia [4]; Pneumonectomy [1] |
| Ditterich et al. (23) | 2006 | 3 | Pneumonia [1]; lobectomy [1]; penetrating thoracic wall abscess [1] |
| Hofmann et al. (19) | 2015 | 3 | Pneumonia [2]; thoracotomy [1] |
| Karapinar et al. (15) | 2016 | 8 | Pneumonectomy [8] |
| Nishii et al. (16) | 2021 | 10 | Pneumonia [5]; lung resection [2]; secondary pneumothorax [1]; chest drain related infection [1]; liver abscess [1] |
| Palmen et al. (24) | 2009 | 11 | Lobectomy [4]; pneumonia [2]; traumatic pneumothorax [2]; chronic tuberculosis [1]; recurrent pneumothorax [1]; trapped lung after benign pleural effusion [1] |
| Schreiner et al. (20) | 2013 | 7 | Lobectomy [2]; Pneumonectomy [2]; Primary empyema [2]; Chronic empyema [1] |
| Sziklavari et al. (25) | 2011 | 8 | Lobectomy [3]; Primary empyema [2]; Decortication [2]; Pneumonectomy [1]; Chest wall reconstruction [1]; Lung volume reduction [1] |
| Sziklavari et al. (26) | 2013 | 6 | Lobectomy [2]; Wedge-resection [1]; Sternotomy for aortic rupture [1]; Decortication for Boerhaave-syndrome [1]; Primary empyema [1] |
| Sziklavari et al. (28) | 2016 | 43 | Primary empyema [17]; Other thoracic procedures [15]; Pneumonectomy [6]; Lobectomy [5] |
| Sziklavari et al. (27) | 2015 | 15 | Primary empyema [8]; Lobectomy [3]; Pneumonectomy [2]; Vertebral spine fusion [1]; Oesophagus resection [1] |
| Groetzner et al. (14) | 2009 | 13 | Primary empyema [5]; Lobectomy [4]; Pneumonectomy [3]; Pancoast resection [1] |
| Saadi et al. (17) | 2011 | 27 | Intrathoracic gastrointestinal leaks [12]; Pneumonia [7]; Lobectomy [4]; Bilobectomy [2]; Pneumonectomy [2] |
Outcomes
We compared the reviewed studies with existing meta-analyses (6) or large cohort studies (10,11) considering either drainage or surgical treatment of pleural empyema.
Vacuum therapy, duration and interval of changes
In 71.5% of the cases (118 patients) vacuum therapy was applicated following OWT. Some authors applied vacuum sponge systems at the same time as OWT creation (17,25,28), while others applied the vacuum sponge several days after initial OWT creation (16,24). The mini-vacuum therapy was used in 14 patients (8.5%) and instill-vacuum therapy in 33 patients (20.0%).
The sub-atmospheric pressure applied to the vacuum sponge dressing ranged from 25–125 mmHg, with most authors using 75–125 mmHg. The interval of changes of the vacuum sponge systems varied from 2 to 7 days. The median number of vacuum sponge changes was between 1 and 13.
The overall duration of treatment ranged from medians of 6–120 days in the existing studies for patients treated by vacuum sponge (Table 4). While the treatment duration does appear long in these studies, it should be mentioned that 10 studies included in the review used the vacuum sponge system for patients with an OWT (Table 5). Taking into account the reports on OWT-outcomes by Reyes et al. (11) and Regnard et al. (10) with median treatment times of 454 and 182.5 days respectively (Table 6), there does appear to be some benefit to the addition of vacuum sponge therapy in these cases.
Table 4
| Author | Year | Therapy | Changes of vacuum system (n), median [range] | Interval of changes (d) | Duration of therapy (d), median [range] |
|---|---|---|---|---|---|
| Al-Mufarrej et al. (21) | 2010 | OWT/VAC | 6 [5–8] | 2–4 | 64 [40–79] |
| Aru et al. (22) | 2010 | OWT/VAC | 11 [3–20] | 2–3 | NA |
| Ditterich et al. (23) | 2006 | OWT/VAC | 7 [4–40] | 3 | 120 [12–122] |
| Hofmann et al. (19) | 2015 | Instill-VAC | 1 [1–3] | 3–4 | 7 [5–12] |
| Karapinar et al. (15) | 2016 | OWT/VAC | 6 [6–6] | 3 | 18 [18–18] |
| Nishii et al. (16) | 2021 | OWT/VAC | NA | 3–4 | 59 [4–190] |
| Palmen et al. (24) | 2009 | Instill-VAC | NA | 3–5 | 31 [12–50] |
| Schreiner et al. (20) | 2013 | OWT/VAC | NA | NA | 6 [6–10] |
| Sziklavari et al. (25) | 2011 | OWT/VAC | 2 [1–4] | 3–7 | NA |
| Sziklavari et al. (26) | 2013 | Mini-VAC | 2 [1–4] | 3–5 | 11.5 [4–18] |
| Sziklavari et al. (28) | 2016 | Instill-VACMini-VACOWT/VAC | 2 [1–6] | 3–4 | 14 [5–48] |
| Sziklavari et al. (27) | 2015 | Instill-VAC | 1 [1–5] | 3–4 | 9 [5–25] |
| Groetzner et al. (14) | 2009 | OWT/VAC | 13 [3–32] | 2–4 | 64 [10–134] |
| Saadi et al. (17) | 2011 | OWT/VAC | 6 [2–16] | 2–4 | 22 [5–66] |
OWT, open window thoracostomy; VAC, vacuum therapy; NA, not applicable.
Table 5
| Author | Year | Length of stay, mean (SD) or [range] | Success rate | Vacuum-related morbidity | In-hospital mortality |
|---|---|---|---|---|---|
| Al-Mufarrej et al. (21) | 2010 | NA | 66.7% | 0% | 0% |
| Aru et al. (22) | 2010 | 46 (26.3) | 100% | 0% | 0% |
| Ditterich et al. (23) | 2006 | 97 (59.6) | 100% | 0% | 33.3% |
| Hofmann et al. (19) | 2015 | NA | 100% | 0% | 0% |
| Karapinar et al. (15) | 2016 | NA | 75% | 0% | 0% |
| Nishii et al. (16) | 2021 | 216.7 (168.6) | 90% | 20% | 10% |
| Palmen et al. (24) | 2009 | NA | 90.1% | 0% | 0% |
| Schreiner et al. (20) | 2013 | NA | 85.7% | 0% | 14.3% |
| Sziklavari et al. (25) | 2011 | NA | 87.5% | 0% | 12.5% |
| Sziklavari et al. (26) | 2013 | NA | 100% | 0% | 0 |
| Sziklavari et al. (28) | 2016 | NA | 74.4% | 0% | 9.3% |
| Sziklavari et al. (27) | 2015 | NA | 80% | 0% | 6.6% |
| Groetzner et al. (14) | 2009 | NA | 84.6% | 0% | 0 |
| Saadi et al. (17) | 2011 | 44.5 [20–114] | 81.5% | 3.7% | 18.5% |
SD, standard deviation; NA, not applicable.
Only 4 studies applied the vacuum sponge system without the use of an OWT (19,26-28). In these cases, the vacuum dressings (black polyurethane ester dressing) were applied using Alexis wound retractors without the need for an OWT or rib resection. In these reports, median treatment time ranged from 7–14 days. This appears to be in line with the report by Redden et al. which analysed VATS and open thoracotomy with drainage thoracostomy in a meta-analysis (6).
Length of stay, morbidity and mortality
The mean length of stay ranges from 44.5 to 216.7 days. Overall, in-hospital mortality was 5.5%. Four studies with a total of 13 patients performed vacuum therapy in ambulatory settings (14,21,22,25).
Generally, vacuum sponge-related complications were rare. Treatment-related complications were reported in 2 studies; One series showed one case of bleeding from the internal mammary vein (17), while another series showed two cases of increased visible pulmonary air leakage and two cases of empyema related sepsis (16).
Discussion
Our systematic review of the existing literature of pleural vacuum therapy for empyema identified a total of 14 case series and retrospective patient groups. Most authors presented either single case reports (which were excluded in this review) or retrospective case series. Low case numbers, lack of randomisation as well as inadequate or no statistical evaluation in these studies suggest low quality of the data published regarding this therapy so far. Although the current evidence is poor, this comprehensive literature review gives an overall impression of the potential that thoracic vacuum therapy holds for the treatment of pleural empyema. A total of 165 patients were included in this review, of which 101 (61.2%) were patients with an empyema secondary to thoracic surgery. While outcomes were defined heterogeneously or sometimes not defined clearly at all in the studies analysed, a successful treatment outcome was defined by the authors of this review as discharge from hospital with a closed thoracic wound without the need for antibiotic therapy, re-intervention or renewed drain placement.
Three variations of the vacuum technique are described in the current literature: (I) conventional vacuum sponge treatment for pleural empyema is usually performed through an existing OWT. (II) The mini-vacuum technique described by Hofmann and colleagues uses an Alexis-Wound protector to insert the vacuum dressing without the need for OWT (29). (III) The Instill-vacuum therapy combines the traditional vacuum therapy with an automated, controlled antiseptic fluid delivery option, with the idea of flushing the cavity and reducing the bacterial load (19).
The technique is mostly used for the closure of OTW wounds or at least a volume reduction of these (30). Most patients with post-resection empyema received an OWT as treatment for the empyema, with vacuum treatment then applied to aid in the closure of these wounds. OWT is a salvage operation used for debilitated patients and patients who have failed either thoracostomy drainage or surgical therapy. These patients have significantly prolonged treatment, as shown in past reports on these patient populations (10,11).
Patients undergoing OWT treatment are usually poor surgical candidates either with complicated empyema not treatable with other treatment options, such as chest tube placement or surgery, or patients in whom these options have failed and where OWT is the last line of therapy. In the current literature there appears to be a selection bias of the most morbid patients with empyema who then receive vacuum sponge therapy to aid in the treatment of OWT and not as a first line treatment option for empyema.
The reporting of patient performance status is poor in the studies available for review. Only 4 studies cited the Karnofsky-Index as a marker for patient debilitation, with the mean Karnofsky-Index in these series <50%, demonstrating that these series performed vacuum therapy on extremely poor surgical candidates. It seems likely that the other series contained similarly ill patient populations, even though this can only be assumed due to insufficient reporting.
The patients with OWT treated with vacuum sponge therapy in the reviewed studies showed mortality rates of 0–33%, compared to 4.3% and 6.4% respectively in two large series for OWT (10,11). Other studies have reported mortality rates for OWT of up to 13.3% (31). The length of stay was only stated in 4 of 10 studies where vacuum therapy was used after OWT. The length of stay (days, mean) ranged from 44.5–216.7 days. This appears slightly longer than the range in other published articles on OWT, where the LOHS ranged from 4–150 days (10,32-34).
Intrathoracic vacuum treatment as a stand-alone therapy has a large potential in the therapy of empyema. The system is well established in other wounds and the dressing material can easily be adapted for intrathoracic use. In addition to the well-known black vacuum sponges, several materials such as open pore film and small pore sponges (white sponge) were developed to tailor an optimal therapy for every purpose and to influence the ingrowth rate of the material and the changing interval (35,36). The open pore film can be easily placed in a small room such as the thoracic cavity and to wrap the lung to affect a granulation stimulus on the complete empyema cavity.
Minimally invasive application techniques have been developed and refined to allow application without the need for single lung ventilation and could in some cases be carried out in analgo-sedation with propofol or midazolam, even in an outpatient setting. In the absence of the large tissue defect of an OWT for accessing the pleural cavity, minimally invasive procedures for example utilise the Alexis wound retractor to place or remove the vacuum foams (29). This means that a rib resection is not necessary, possibly leading to pain relief and higher levels of patient comfort. Even completely minimally invasive techniques have been developed, as shown in flexible endoscopic studies (37). Modifications including the vacuum-instill therapy combining negative pressure treatment with intermittent irrigation of the wound showed promising results for achieving a sterile wound cavity (19). The possibility of ambulatory treatment promises a cost effectiveness by reducing the LOHS.
Vacuum sponge therapy also promises reduced postoperative morbidity and decreased risk of possibly life-threatening complications associated with empyema surgery. These include persistent air leaks, hemorrhage, injury to vital structures, severe postoperative pain, and sepsis. The latter in particular, is associated with high lethality in the case of septic shock due to resulting bacteremia, especially for elderly and multimorbid patients (38).
Vacuum therapy on the other hand can be applied solely on the parts of the lung which are affected and may thereby reduce the postoperative morbidity. Treatment related complications were rare in the patients reviewed in this paper, with only 2 patients showing prolonged air leakage and no revisional surgeries for postoperative bleeding being necessary.
The review of the current literature showed 4 studies that applied the vacuum system without the use of an OWT (19,26-28). Mortality rates of 0–9.3% are reported for these patients. While these are still higher than the published results in a large meta-analysis comparing thoracostomy drainage to open surgery and vacuum therapy (6), it seems likely that these higher mortality rates are due to a selection bias leading to patients which are poor surgical candidates due to debilitation being selected for vacuum treatment as a less invasive procedure. Additionally, the small sample size (67 patients across 4 studies) in the studies for vacuum therapy without OWT makes the comparability of mortality rates to a meta-analysis (6) (8 RCTs, 391 patients) difficult. LOHS could not be compared for vacuum therapy without OWT, as these data were not provided in the papers available for review. The median treatment time ranged from 7–14 days. This appears to be in line with the report by Redden et al. (6). Another drawback of the vacuum therapy is the need for several re-interventions associated with additional anesthesia exposure, potential complications, and stress for patients.
Overall, the standard of reporting complications, comorbidities and other treatment parameters was poor in all studies. The RAPID score could not be assessed for any patients, neither were intensive care unit treatment times documented. The authors of this paper believe that the condition of patients with pleural empyema should be clearly analysed and documented using a validated scoring system such as the Karnofsky Performance Index and/or the RAPID score. The RAPID score has been shown to accurately predict treatment outcomes in patients with pleural empyema (39). Improvements in the RAPID score and/or Karnofsky Performance Index should also be used to evaluate the treatment outcomes.
Follow-up ranged widely across studies, from no follow-up to a maximum of 96 months. Some studies reported LOS, while others only reported length of stay after first vacuum sponge application. These parameters could therefore not be assessed with any large degree of confidence.
The heterogenous patient cohorts across all analysed studies also makes it difficult to assess the quality of treatment for different causes of empyema. For example, intersitial pneumonia may lead to contractile changes in the lungs, inhibiting their re-expansion needed for reduction of the empyema cavity. Further studies should focus on patients with just one cause of empyema to allow an analysis of more homogenous patient groups. However, the tissue softening of the empyema capsule through vacuum therapy may lead to a reduction of the empyema cavity independent of interstitial lung changes.
Conclusions
In summary, it must be stated that the current level of evidence is poor, but the reports included in this review point in the direction of safe use of intrathoracic vacuum therapy for pleural empyema, with the potential of outpatient treatment to reduce LOHS. Vacuum sponge therapy does not only add a treatment option to otherwise inoperable, critically ill patients but might be a potential treatment alternative to the ‘normal’ pleural empyema stage II and III candidate. More data and larger scaled analyses are needed to further validate the potential of thoracic vacuum therapy in pleural empyema, optimally prospective or randomised control trials comparing minimally invasive vacuum sponge therapy with VATS decortication for patients with pleural empyema.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1188/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1188/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1188/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Finley C, Clifton J, Fitzgerald JM, et al. Empyema: an increasing concern in Canada. Can Respir J 2008;15:85-9. [Crossref] [PubMed]
- Farjah F, Symons RG, Krishnadasan B, et al. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 2007;133:346-51. [Crossref] [PubMed]
- Grijalva CG, Zhu Y, Nuorti JP, et al. Emergence of parapneumonic empyema in the USA. Thorax 2011;66:663-8. [Crossref] [PubMed]
- Søgaard M, Nielsen RB, Nørgaard M, et al. Incidence, length of stay, and prognosis of hospitalized patients with pleural empyema: a 15-year Danish nationwide cohort study. Chest 2014;145:189-92. [Crossref] [PubMed]
- Maskell NA, Batt S, Hedley EL, et al. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med 2006;174:817-23. [Crossref] [PubMed]
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017;3:CD010651. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Chambers A, Routledge T, Dunning J, et al. Is video-assisted thoracoscopic surgical decortication superior to open surgery in the management of adults with primary empyema? Interact Cardiovasc Thorac Surg 2010;11:171-7. [Crossref] [PubMed]
- Majeed FA, Chatha SS, Zafar U, et al. Surgical Management of Paediatric Empyema: Open Thoracotomy versus Video-assisted Thoracic Surgery. J Coll Physicians Surg Pak 2020;30:309-12. [Crossref] [PubMed]
- Regnard JF, Alifano M, Puyo P, et al. Open window thoracostomy followed by intrathoracic flap transposition in the treatment of empyema complicating pulmonary resection. J Thorac Cardiovasc Surg 2000;120:270-5. [Crossref] [PubMed]
- Reyes KG, Mason DP, Murthy SC, et al. Open window thoracostomy: modern update of an ancient operation. Thorac Cardiovasc Surg 2010;58:220-4. [Crossref] [PubMed]
- Wood BC, Molnar JA. Subatmospheric pressure therapy: basic science review. J Surg Orthop Adv 2011;20:168-75.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Groetzner J, Holzer M, Stockhausen D, et al. Intrathoracic application of vacuum wound therapy following thoracic surgery. Thorac Cardiovasc Surg 2009;57:417-20. [Crossref] [PubMed]
- Karapinar K, Saydam Ö, Metin M, et al. Experience with Vacuum-Assisted Closure in the Management of Postpneumonectomy Empyema: An Analysis of Eight Cases. Thorac Cardiovasc Surg 2016;64:258-62. [Crossref] [PubMed]
- Nishii K, Nakajima T, Yamamoto T, et al. Management of thoracic empyema with broncho-pulmonary fistula in combination with negative-pressure wound therapy. Gen Thorac Cardiovasc Surg 2021;69:843-9. [Crossref] [PubMed]
- Saadi A, Perentes JY, Gonzalez M, et al. Vacuum-assisted closure device: a useful tool in the management of severe intrathoracic infections. Ann Thorac Surg 2011;91:1582-9. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Hofmann HS, Neu R, Potzger T, et al. Minimally Invasive Vacuum-Assisted Closure Therapy With Instillation (Mini-VAC-Instill) for Pleural Empyema. Surg Innov 2015;22:235-9. [Crossref] [PubMed]
- Schreiner W, Oster O, Stapel P, et al. V. A. C. INSTILL® therapy - new option in septic thoracic surgery. Zentralbl Chir 2013;138:117-20. [Crossref] [PubMed]
- Al-Mufarrej F, Margolis M, Tempesta B, et al. Outpatient management of post-pneumonectomy and post-lobectomy empyema using the vacuum-assisted closure system. Surg Today 2010;40:711-8. [Crossref] [PubMed]
- Aru GM, Jew NB, Tribble CG, et al. Intrathoracic vacuum-assisted management of persistent and infected pleural spaces. Ann Thorac Surg 2010;90:266-70. [Crossref] [PubMed]
- Ditterich D, Rexer M, Rupprecht H. Vacuum assisted closure in the treatment of pleural empyema -- first experiences with intra-thoracal application. Zentralbl Chir 2006;131:S133-8. [Crossref] [PubMed]
- Palmen M, van Breugel HN, Geskes GG, et al. Open window thoracostomy treatment of empyema is accelerated by vacuum-assisted closure. Ann Thorac Surg 2009;88:1131-6. [Crossref] [PubMed]
- Sziklavari Z, Grosser C, Neu R, et al. Complex pleural empyema can be safely treated with vacuum-assisted closure. J Cardiothorac Surg 2011;6:130. [Crossref] [PubMed]
- Sziklavari Z, Grosser C, Neu R, et al. Minimally invasive vacuum-assisted closure therapy in the management of complex pleural empyema. Interact Cardiovasc Thorac Surg 2013;17:49-53. [Crossref] [PubMed]
- Sziklavari Z, Ried M, Neu R, et al. Mini-open vacuum-assisted closure therapy with instillation for debilitated and septic patients with pleural empyema. Eur J Cardiothorac Surg 2015;48:e9-16. [Crossref] [PubMed]
- Sziklavari Z, Ried M, Zeman F, et al. Short-term and long-term outcomes of intrathoracic vacuum therapy of empyema in debilitated patients. J Cardiothorac Surg 2016;11:148. [Crossref] [PubMed]
- Hofmann HS, Schemm R, Grosser C, et al. Vacuum-assisted closure of pleural empyema without classic open-window thoracostomy. Ann Thorac Surg 2012;93:1741-2. [Crossref] [PubMed]
- Ried M, Graml J, Großer C, et al. Para- and Postpneumonic Pleural Empyema: Current Treatment Strategies in Children and Adults. Zentralbl Chir 2015;140:S22-8. [Crossref] [PubMed]
- Pairolero PC, Arnold PG, Trastek VF, et al. Postpneumonectomy empyema. The role of intrathoracic muscle transposition. J Thorac Cardiovasc Surg 1990;99:958-66; discussion 966-8.
- Jadczuk E. Posptneumonectomy empyema. Eur J Cardiothorac Surg 1998;14:123-6. [Crossref] [PubMed]
- Krassas A, Grima R, Bagan P, et al. Current indications and results for thoracoplasty and intrathoracic muscle transposition. Eur J Cardiothorac Surg 2010;37:1215-20. [Crossref] [PubMed]
- Stefani A, Jouni R, Alifano M, et al. Thoracoplasty in the current practice of thoracic surgery: a single-institution 10-year experience. Ann Thorac Surg 2011;91:263-8. [Crossref] [PubMed]
- Loske G, Schorsch T, Rucktaeschel F, et al. Open-pore film drainage (OFD): a new multipurpose tool for endoscopic negative pressure therapy (ENPT). Endosc Int Open 2018;6:E865-71. [Crossref] [PubMed]
- Kantowski M, Karstens KF. Endoskopische Vakuumtherapie der Anastomoseninsuffizienz – Schritt für Schritt. Gastroenterologie up2date. 2021;17:322-35.
- Kantowski M, Kunze A. New strategies and materials in endoscopic vacuum therapy in the lower gastrointestinal tract. Chirurg 2018;89:960-8. [Crossref] [PubMed]
- Kumar A, Anand S. Lung Decortication. StatPearls. Treasure Island (FL): StatPearls Publishing, Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Rahman NM, Kahan BC, Miller RF, et al. A clinical score (RAPID) to identify those at risk for poor outcome at presentation in patients with pleural infection. Chest 2014;145:848-55. [Crossref] [PubMed]