
Real-world evidence of safety and influence for lung cancer surgery under COVID-19 pandemic in Japan
Highlight box
Key findings
• Even during the COVID-19 pandemic period, lung cancer surgery was performed safely in Japan.
What is known and what is new?
• Pathological findings of lung cancer tended to be progressive under the COVID-19 pandemic.
• When the newly diagnosed COVID-19 cases increased, the number of surgeries for lung cancer decreased in a delayed fashion.
What is the implication, and what should change now?
• The hospitals should remain open even during future COVID waves and continue to use efficient screening methods and conduct proper vaccination.
Introduction
The COVID-19 pandemic is anticipated to continue causing damage on society and economies around the world by imposing substantial burdens on morbidity and mortality (1). As a result of the ensuing lockdown, most non-COVID-19 health services have decreased, and there is growing concern about the impact on other patient groups, especially cancer patients, for whom prompt diagnosis and treatment initiation are essential for achieving optimal results (2-4). The COVID-19 pandemic requires immediate staff and capacity relocation, and nonemergency clinical services, such as elective specialized surgery for cancer, must be postponed because of the unrecognized pressure on hospital wards and the intensive care unit (ICU) (5). Some studies have revealed a substantial increase in the number of avoidable cancer deaths estimated as a result of diagnostic and therapeutic delays due to the COVID-19 pandemic (6,7). Although recent studies showed non-inferiority of surgical outcome during the COVID-19 pandemic, one particular subset of cancer patients who might be at high risk includes those who require surgery for lung cancer, as many of these cases occur in older people who have respiratory comorbidities (8,9). The risk-to-benefit ratio for lung cancer treatment must be carefully weighed in the pandemic era, but only few studies have demonstrated the real situation of surgical practice for lung cancer in Japan.
In this study, we collected data from patients with primary lung cancer who underwent surgery from 2018 to 2021. To investigate the impact of the COVID-19 pandemic on lung cancer surgery, we compared real-world data from our hospital including patient background, surgical procedure and approach, histology, pathological stage, and short-term outcome after lung cancer resection between 2018–2019 and 2020–2021. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1289/rc).
Methods
Patients
The inclusion criterion for this study was all pulmonary resections for primary lung cancer performed between 2018 and 2021 in our institution. Lung metastases and benign tumors were excluded. We retrospectively collected patient demographic, clinical, and pathological data for those who fulfilled the inclusion criteria from a review of the medical records. Consequently, 936 patients with lung cancer were retrospectively analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Nagoya University Hospital approved this retrospective study (approval No. 2016-0507), and individual consent for this retrospective analysis was waived.
Design and assessments
Patients were divided into two groups: the prepandemic group, which included patients who underwent surgery between 2018 and 2019, and the pandemic group, which included patients who underwent surgery between 2020 and 2021. Patient and tumor characteristics, including gender, age, surgical procedure and approach, TNM stage, histological type, postoperative complications by Clavien-Dindo classification, 30-day mortality, 90-day mortality, first-visit date to our department, and operation date, were collected from medical records. The number of patients diagnosed each day with COVID-19 in Japan was also collected from the website of the Ministry of Health, Labour and Welfare (10).
Patient screening for COVID-19 infection
During 2020–2021, the practice of preoperative COVID-19 screening and isolation was implemented in line with the patient admission protocol at Nagoya University. The admission date was 24 hours preoperatively both before and after the outbreak. During the COVID-19 pandemic, patients were advised to be isolated for 14 days and record their symptoms and body temperature for 20 days before admission. In addition, a nasopharyngeal COVID-19 antigen test was performed on admission. Surgical activity was maintained, and the surgical department was not shut down during the pandemic period. As a postoperative screening protocol, a follow-up was performed once in 3 months during the first 2 years and once in 6 months during 2–5 years. COVID-19 screening test was only conducted for patients who displayed any infectious diseases symptoms postoperatively.
Statistical analysis
Variables were represented as the median and interquartile range, and categorical data were described as counts and percentages. We performed the Mann-Whitney test and Fisher’s exact test to compare the distributions of the continuous and categorical variables between the prepandemic and pandemic groups, respectively. To understand the relationship between pandemic waves and surgery volume, Spearman analysis was applied for correlation analysis between the increased ratio of COVID-19 patients in Japan and the volume of operation in our hospital. An increase in the ratio of COVID-19 patients was calculated as follows: in a given month, the anterior and posterior half-month cumulative total daily number of patients diagnosed with COVID-19 in Japan was calculated, and the present half-month cumulative total was divided by the previous value and multiplied by 100. To calculate the correlation, this increase ratio was compared with the number of surgeries conducted in the current month or the following month (Figure S1). Values of P<0.05 were considered to be statistically significant. Statistical analysis was performed using EZR, which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (11).
Results
Patient characteristics
We included in this study 936 patients with primary lung cancer who underwent surgical intervention in our institute from 2018 through 2021. Of the patients, 62.2% were male. Most patients had a smoking history, and 19.1% of patients suffered from chronic obstructive pulmonary disease. Lobectomy or bilobectomy were the most common surgical procedures in this period. In terms of pathological characteristics, a large majority of patients were diagnosed with stage I lung adenocarcinoma.
Comparison of clinicopathological features before and after the COVID-19 outbreak
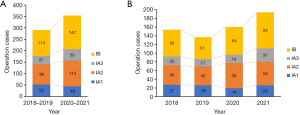
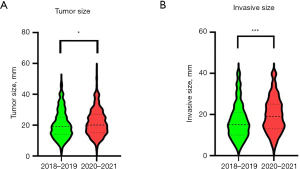
Table 1 compares the characteristics of patients in 2018–2019 (prepandemic) and in 2020–2021 (pandemic). The number of patients did not decrease in the pandemic group (n=443) in comparison with the prepandemic group (n=493). Patient background characteristics, including age, body mass index, sex, smoking, and comorbidities, were not different between the two groups. In the prepandemic group, the proportion of patients who received video-assisted thoracic surgery (VATS) and robot-assisted thoracic surgery (RATS) approaches was significantly higher compared with the pandemic group (P<0.001). With respect to pathological findings, stage I cases showed the highest increase in the pandemic period compared with the prepandemic period, although other stages were similar between two groups. The distribution of stage I diseases indicated that only stage IA1 was decreased and that IB had the greatest increase after the pandemic (Figure 1A). In particular, stage IA3 and IB tumors had a higher increase in 2021 compared with the other years (Figure 1B). When tumor and invasive size in stage I were compared between the two groups, the pandemic group showed significantly larger sizes compared with the prepandemic group (tumor size: P=0.031, invasive size: P<0.001, Figure 2).
Table 1
| Variables | Year 2018–2019 (n=443) | Year 2020–2021 (n=493) | P value |
|---|---|---|---|
| Age, years, median [IQR] | 71 [66–75] | 72 [65–76] | 0.444 |
| BMI, kg/m2, median [IQR] | 22.5 [20.3–24.8] | 22.7 [20.7–24.95] | 0.192 |
| Sex (male), n (%) | 272 (61.4) | 314 (63.7) | 0.499 |
| Smoking, n (%) | 0.071 | ||
| Non-smoker | 155 (35.0) | 143 (29.0) | |
| Ex-smoker | 227 (51.2) | 289 (58.6) | |
| Current smoker | 61 (13.8) | 61 (12.4) | |
| Comorbidity, n (%) | |||
| Hypertension | 128 (28.9) | 192 (38.9) | 0.001 |
| Diabetes mellitus | 61 (13.8) | 87 (17.6) | 0.107 |
| COPD (FEV1.0% <70) | 178 (40.2) | 209 (42.4) | 0.507 |
| Interstitial pneumonia | 30 (6.8) | 39 (7.9) | 0.533 |
| Ischemic heart disease | 10 (2.3) | 6 (1.2) | 0.313 |
| Cerebrovascular disease | 4 (0.9) | 7 (1.4) | 0.553 |
| Other malignant disease | 30 (6.8) | 42 (8.5) | 0.329 |
| Procedure, n (%) | 0.632 | ||
| Lobectomy/bilobectomy | 330 (74.5) | 370 (75.1) | |
| Segmentectomy/sublobar | 88 (19.9) | 105 (21.3) | |
| Pneumonectomy | 2 (0.5) | 2 (0.4) | |
| Lung resection + chest wall | 16 (3.6) | 12 (2.4) | |
| Others | 7 (1.5) | 4 (0.8) | |
| Approach, n (%) | <0.001 | ||
| Open | 247 (55.8) | 143 (29.0) | |
| VATS | 126 (28.4) | 207 (42.0) | |
| RATS | 70 (15.8) | 143 (29.0) | |
| Pathology, n (%) | 0.411 | ||
| LUAD | 318 (71.8) | 361 (73.2) | |
| LUSQ | 81 (18.3) | 95 (19.3) | |
| Others | 44 (9.9) | 37 (7.5) | |
| Pathological stage, n (%) | 0.334 | ||
| 0 | 11 (2.5) | 12 (2.4) | |
| I | 291 (65.7) | 354 (71.8) | |
| II | 72 (16.3) | 65 (13.2) | |
| III | 60 (13.5) | 55 (11.2) | |
| IV | 9 (2.0) | 7 (1.4) |
IQR, interquartile range; BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1.0%, forced expiratory volume % in one second; VATS, video-assisted thoracic surgery; RATS, robot-assisted thoracic surgery; LUAD, lung adenocarcinoma; LUSQ, lung squamous cell carcinoma.
Short-term outcome after surgery
Table 2 displays the outcomes after surgery. The median hospital stay after surgery was 6 days, and the 30- and 90-day mortality rates were similar in both groups. Complications were seen in 92 of 443 (20.8%) patients in the prepandemic group and 108 of 493 (21.9%) patients in the pandemic group. A total of 42 of 443 (9.4%) patients in the prepandemic group and 54 of 493 (10.9%) patients in the pandemic group were classified as Clavien-Dindo grades 3–4, which comprises prolonged air leakage requiring chemical pleurodesis or surgical treatment, bronchopleural fistula, atelectasis, empyema, bleeding, and cerebral infarction; there was no significant difference in the proportion of these complications between the two groups. As a severe complication, only one patient had COVID-19 infection after lung cancer resection. The patient developed COVID-19 pneumonia and bronchopleural fistula 5 months after right upper lobectomy for lung cancer. Pleural drainage was performed and antibiotic treatment was administered in the ICU. Two weeks after treatment initiation, we identified negative conversion of SARS-CoV-2 antigen and spontaneous closure of the bronchopleural fistula.
Table 2
| Variables | Year 2018–2019 (n=443) | Year 2020–2021 (n=493) | P value |
|---|---|---|---|
| Length of stay after operation, median [IQR] | 6 [5–7] | 6 [5–8] | 0.426 |
| 30-day mortality, n (%) | 2 (0.45) | 0 (0) | 0.244 |
| 90-day mortality, n (%) | 2 (0.45) | 3 (0.81) | 1 |
| Complication, n (%) | 0.762 | ||
| Clavien-Dindo grade 1–2 | 50 (11.2) | 54 (10.9) | |
| Clavien-Dindo grade 3–4 | 42 (9.4) | 54 (10.9) | |
| Detail of Clavien-Dindo grade 3–4, n (%) | |||
| Prolonged air leakage | 29 (6.5) | 37 (7.5) | 0.554 |
| Bronchopleural fistula | 2 (0.45) | 2 (0.40) | 1 |
| Pneumonia | 10 (2.3) | 9 (1.8) | 0.652 |
| Atelectasis | 6 (1.4) | 1 (0.20) | 0.057 |
| Chylothorax | 5 (1.1) | 2 (0.40) | 0.265 |
| Empyema | 5 (1.1) | 7 (1.4) | 0.777 |
| Bleeding | 3 (0.68) | 4 (0.81) | 1 |
| Cerebral infarction | 0 (0) | 2 (0.40) | 0.501 |
IQR, interquartile range.
Impact of COVID-19 pandemic on the volume of lung cancer operations
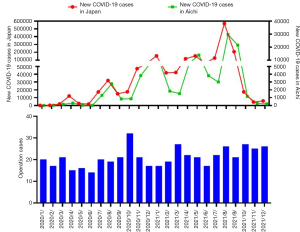
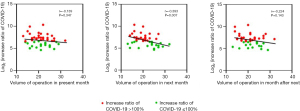
We investigated the effect of the COVID-19 pandemic on the volume of lung cancer operations performed in our department (Figure 3). The first case of Japanese SARS-CoV-2 infection was detected in January 2020, and the first wave of the pandemic occurred in April of the same year. The number of newly diagnosed COVID-19 cases in Aichi prefecture was highly correlated with that in Japan. Operation cases decreased around April. Under the third and fourth waves, operation cases decreased again in a delayed fashion. These distributions of operation cases contained no correlation with those of 2018–2019 (r=−0.010, Figure S2). When the increase ratio of new COVID-19 cases in Japan was compared with the volume of lung cancer operations, a negative correlation was detected with the number of surgeries performed in the next month, although no correlation was found in the present month and the month after next (present month: r=−0.139, P=0.347, next month: r=−0.393, P=0.007, month after next: r=−0.224, P=0.143, Figure 4). The same relationship was investigated using 2022 data and we realized that no negative correlation was observed for this dataset (Figure S3). Additionally, number of surgeries around the COVID waves gradually increased from the first to the fifth wave (Figure S4). On the other hand, the median time from the first visit to surgery (TFS) was 32 days for the prepandemic group and 31 days for the pandemic group, and no significant difference in TFS was found between the two groups (P=0.150). This finding indicated that in our institution, surgical activity was maintained and no shutdown of the surgical department occurred during the pandemic period.
Discussion
During the COVID-19 pandemic, secondary effects such as limited health care service availability should be considered. In recent years, estimates of the impact of cancer treatment delays on survival have been reported (5,12,13). Several guidelines and recommendations for cancer diagnosis and therapy under a pandemic situation have been provided based on expert opinions (14). However, evidence regarding the safety and influence of surgical treatment on lung cancer remain limited in this field under the COVID-19 pandemic. In this study, we evaluated the volume of surgery, clinicopathological features, and short-term outcomes of patients with lung cancer by comparing the real-world data in our department between prepandemic and pandemic periods.
As shown in Figures 3,4, new COVID-19 cases belatedly affected the volume of lung cancer surgery. Indeed, when the World Health Organization declared COVID-19 to be a global pandemic in March 2020, the problem of delayed surgical therapy worsened (15,16). The effects of COVID-19 also have had an impact on cancer diagnoses around the world. In the United Kingdom, referrals for cancer-suspected cases decreased by about 80% (7,17). The potential causes of these delays in diagnosis and treatment are the limitations in hospital resources and the hesitancy of patients to receive necessary health care services during the COVID-19 pandemic (18,19). According to a study of lung cancer patients who participated in clinical trials during the SARS outbreak, 64% of patients were hesitant to visit a hospital due to fear of infection, and 4% of patients decided to cease all treatment because of infection issues. In another study using the United States National Cancer Database, when the interval between the cancer diagnosis and surgical therapy was greater than 12 weeks, patients with clinical stage I non–small cell lung cancer were found to have a higher risk of upstaging and a worse rate of overall survival (20). In our study, the pathological outcome of patients with stage I cancer tended to be larger in tumor and invasive size during the pandemic period (Figures 1,2). This is probably due to the delay in surgical treatment and the selection of surgical indication for lung cancer of higher malignancy. However, there was no significant delay in TFS during the pandemic period compared with the prepandemic period. No shutdown or limitation in the surgical department occurred in our hospital after the COVID-19 outbreak, although many institutions stopped performing elective surgery for non-cancer cases in other countries (21). Even if hospital activity was maintained, the waves of COVID-19 affected the volume of diagnosis and surgery for lung cancer in a delayed fashion, likely because of patient hesitancy to receive necessary health care services. Recently, patient hesitancy seemed to have decreased because the number of surgeries was minimally almost affected by the latest COVID wave as shown in Figures S3,S4. One of the reasons for this change is prevalence of vaccines and other therapies including Paxlovid (22). Therefore, the hospitals should remain open even during future COVID waves and continue to use efficient screening methods and conduct proper vaccination.
Retrospective studies reported COVID-19 infection mortality rates of upward of 50% in patients with lung cancer even without lung resection (23). In terms of patients who received lung cancer surgery, the mortality rate was extremely high among those positive for COVID-19 within 30 days postoperatively (24). This vulnerable group might be particularly susceptible to subsequent pulmonary complications due to immunosuppressive responses to surgery as well as immune-suppressive mediators from the tumor (25,26). Although there is little information regarding the perioperative safety of patients with lung cancer, a recent study conducted in the United States showed that the postoperative COVID-19 infection rate was 7.3%, and there was no difference in major complications after surgery between the prepandemic and pandemic groups (8). In our study, only one patient developed COVID-19 five months after lung cancer resection, with no difference observed in the postoperative complication rate between the two groups (Table 2). To maintain the safety of surgery, we should continue to preoperatively screen all patients for SARS-CoV-2 infection by nasopharyngeal swab and provide careful postdischarge follow-up and isolation from the risk of COVID-19 infection, especially within the first month after surgery.
Numerous studies have already reported the benefits of both VATS and RATS compared with open surgery in terms of less invasiveness for patients (27-30), and recently RATS was suggested to provide safe and equivalent surgical efficacy compared with VATS (31,32). From the trends in surgical approach, the application of VATS and RATS could successfully be extended without an increase of major complications during this coronavirus pandemic era. Specifically, frequency of RATS recently increased in our department. In our opinion, the reason for the increase in minimally invasive surgery is that it can be safely performed even during the COVID-19 pandemic. However, the number of minimally invasive surgeries have gradually increased from 2019. We speculate that the reason for this is probably institutional, and not an effect of the pandemic.
Our study has several potential limitations. This was a retrospective study conducted at a single institution on a rather small number of patients. To assess a patient’s prognosis, particularly during a pandemic, long-term follow-up is necessary. Systematic COVID-19 screening was performed only for preoperative patients. Postoperative information regarding COVID-19 was obtained from patient declaration or screening at the time of hospital readmission.
Conclusions
We were able to safely perform lung cancer surgery in a sustainable manner during the COVID-19 pandemic. However, among patients who received surgical treatment, we observed enlargement of the tumor in early-stage lung cancer during the COVID-19 pandemic period.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1289/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1289/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1289/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1289/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Institutional Review Board of Nagoya University Hospital (approval No. 2016-0507) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19:141-54. [Crossref] [PubMed]
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112:S92-107. [Crossref] [PubMed]
- Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin Cancer Inform 2020;4:1059-71. [Crossref] [PubMed]
- Kaufman HW, Chen Z, Niles J, et al. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw Open 2020;3:e2017267. [Crossref] [PubMed]
- Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol 2020;31:1065-74. [Crossref] [PubMed]
- Park JY, Lee YJ, Kim T, et al. Collateral effects of the coronavirus disease 2019 pandemic on lung cancer diagnosis in Korea. BMC Cancer 2020;20:1040. [Crossref] [PubMed]
- Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023-34. [Crossref] [PubMed]
- Villena-Vargas J, Lutton EM, Mynard N, et al. Safety of lung cancer surgery during COVID-19 in a pandemic epicenter. J Thorac Cardiovasc Surg 2022;164:378-85. [Crossref] [PubMed]
- Caballero-Milán M, Colomina MJ, Marin-Carcey LA, et al. Impact of the SARS-CoV-2 (COVID19) pandemic on the morbidity and mortality of high risk patients undergoing surgery: a non-inferiority retrospective observational study. BMC Anesthesiol 2021;21:295. [Crossref] [PubMed]
- COVID-19 trends in Japan from Ministry of Health, Labour and Welfare database. Ministry of Health, Labour and Welfare. Available online: https://covid19.mhlw.go.jp/extensions/public/en/index.html. Accessed April 23 2022 2022.
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Malagón T, Yong JHE, Tope P, et al. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer 2022;150:1244-54. [Crossref] [PubMed]
- Shipe ME, Haddad DN, Deppen SA, et al. Modeling the Impact of Delaying the Diagnosis of Non-Small Cell Lung Cancer During COVID-19. Ann Thorac Surg 2021;112:248-54. [Crossref] [PubMed]
- Mazzone PJ, Gould MK, Arenberg DA, et al. Management of Lung Nodules and Lung Cancer Screening During the COVID-19 Pandemic: CHEST Expert Panel Report. Chest 2020;158:406-15. [Crossref] [PubMed]
- Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed 2020;91:157-60. [Crossref] [PubMed]
- Thoracic Surgery Outcomes Research Network, Inc. COVID-19 Guidance for Triage of Operations for Thoracic Malignancies: A Consensus Statement From Thoracic Surgery Outcomes Research Network. Ann Thorac Surg 2020;110:692-6. [Crossref] [PubMed]
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol 2020;21:1035-44. [Crossref] [PubMed]
- Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N Engl J Med 2020;382:2049-55. [Crossref] [PubMed]
- Zhang Y, Li J, Li ZK, et al. Impact of Coronavirus Disease 2019 on Clinical Characteristics in Patients With Lung Cancer: A Large Single-Centre Retrospective Study. Front Oncol 2021;11:693002. [Crossref] [PubMed]
- Heiden BT, Eaton DB Jr, Engelhardt KE, et al. Analysis of Delayed Surgical Treatment and Oncologic Outcomes in Clinical Stage I Non-Small Cell Lung Cancer. JAMA Netw Open 2021;4:e2111613. [Crossref] [PubMed]
- Mynard N, Saxena A, Mavracick A, et al. Lung Cancer Stage Shift as a Result of COVID-19 Lockdowns in New York City, a Brief Report. Clin Lung Cancer 2022;23:e238-42. [Crossref] [PubMed]
- Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. Clin Infect Dis 2022;ciac443. [Crossref] [PubMed]
- Rogado J, Pangua C, Serrano-Montero G, et al. Covid-19 and lung cancer: A greater fatality rate? Lung Cancer 2020;146:19-22. [Crossref] [PubMed]
- Cai Y, Hao Z, Gao Y, et al. Coronavirus Disease 2019 in the Perioperative Period of Lung Resection: A Brief Report From a Single Thoracic Surgery Department in Wuhan, People's Republic of China. J Thorac Oncol 2020;15:1065-72. [Crossref] [PubMed]
- Dai M, Liu D, Liu M, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov 2020;10:783-91. [Crossref] [PubMed]
- Budisan L, Zanoaga O, Braicu C, et al. Links between Infections, Lung Cancer, and the Immune System. Int J Mol Sci 2021;22:9394. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Casiraghi M, Sedda G, Diotti C, et al. Postoperative outcomes of robotic-assisted lobectomy in obese patients with non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2020;30:359-65. [Crossref] [PubMed]
- Sheetz KH, Claflin J, Dimick JB. Trends in the Adoption of Robotic Surgery for Common Surgical Procedures. JAMA Netw Open 2020;3:e1918911. [Crossref] [PubMed]
- Guo F, Ma D, Li S. Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer: A Meta-analysis. Medicine (Baltimore) 2019;98:e17089. [Crossref] [PubMed]


