Surgical treatment of cardiac tumors: a 5-year experience from a single cardiac center
Introduction
Tumors of the heart are rare compared with other cardiac diseases and tumors from other organs, with the incidence of approximately 0.3% of patients underwent cardiac surgery (1,2). Most cardiac tumors are benign, of which cardiac myxomas are the most encountered in surgery, with the incidence reported to be ranged from 70–80% in cardiac tumors (3-5). Because of their various presentations, symptoms of cardiac tumors vary from absent to nonspecific, and they usually present themselves insidiously. Symptoms generated from cardiac tumors include arrhythmias, blood flow obstruction, embolization and other individual findings. Once the blood flow tract is obstructed by cardiac tumor, patients may be manifested by dyspnea, dizziness, syncope, palpitations and heart failure (6-8).
Non-invasive procedures, such as echocardiography, computed tomography (CT), and magnetic resonance imaging (MRI) of the heart, are commonly used in helping diagnose cardiac tumors (9,10). Surgical treatment is advisable for patients to remove the tumor to prevent life-threatening events occurring. Complete resection is often performed to remove benign tumors in cardiac chambers. As for malignant tumors of the heart, combined surgical resection and chemotherapy are utilized to prolong survival for a small percentage of patients (4,11-13).
In this study, we retrospectively reviewed patients underwent open heart surgery who was diagnosed of cardiac tumors in our cardiac center from January 2008 to December 2013, data related to clinical presentation, surgery, perioperative information, type of tumor and long-term follow-up were analyzed. We sought to investigate the effect and efficacy outcomes of surgical treatment for cardiac tumors.
Methods
Patients
Permission of medical data extraction was obtained by verbal informed consent, which was recorded by telephone for each patient included in our study, the consent procedure of our study and conduction of this study were approved by Ethics Committee of Shanghai Changzheng Hospital. All information of each patient was anonymized and de-identified prior to analysis. All patients with the diagnosis of cardiac tumor involving benign and malignant ones treated with open heart surgery at the cardiac center of the Second Military Medical University from January 2008 to December 2013 have been included in this retrospective study. The patients included belonged to a consecutive series who were carried out cardiac surgery to treat cardiac tumors with or without valve surgery and other concomitant cardiac surgery.
Complete medical records and follow-up data of patients included in our study were collected using the patient database of our center, and follow-up of patients were conducted by contacting patients using telephone or by outpatient clinics. Pathological diagnosis of cardiac tumors was obtained from clinical pathological institute of our center. All patients presented with chest pain or older than 45 yrs were carried out coronary angiography to rule out coronary heart disease (CHD) (14,15).
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software. Quantitative data were expressed as mean ± SD and were compared with 2-sample Wilcoxon rank sum tests or 3-sample Kruskal-Wallis H tests for independent samples, whereas dichotomous variables were reported as absolute values and proportions. Differences in proportion were compared with a χ2 test or Fisher exact test as appropriate. Univariate analysis was performed with the Cox regression analysis. For each variable, the odds ratio (OR), 95% confidence interval (CI), and P value were provided. The long term survival curves of patients included in our study were constructed according to the Kaplan-Meier method (16,17).
Results
Patients
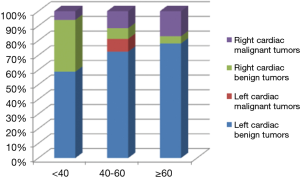
We included a total of 131 patients diagnosed of cardiac tumors in this study, of these 50 were male patients and 81 were females, with a mean age of 51.39±16.37. We stratified patients’ age to three groups, ≤40, 40–60 and ≥60 yrs. The result showed that benign tumors in the left heart and malignant tumors in the right heart demonstrated an ascendant tendency with the increased stratification of age, while the contrary trend was seen on benign tumors in the right heart. The analysis also indicated that malignant tumors in the left heart only occurred at the age between 40 and 60 in our study (Table 1) (Figure 1).
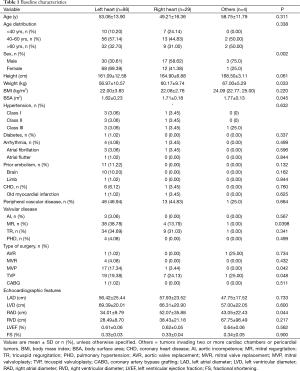
Full table
Benign and malignant tumors
In all patients analyzed in this study, pathological analyses of cardiac tumors resected from the heart were performed to make accurate clinical diagnosis. The result showed that 79.47% of the primary intracardiac tumors were benign, with the predominant percentage of 70.99% myxomas, which occurred in 93 patients. Hemangiomas and fibroma were the second mostly seen begin tumors, which occurred in 6 (4.58%) and 2 (1.52%) patients respectively, followed by intermediate endothelia tumor in one case and lipoma in one case. Primitive neuroectodermal tumor, a kind of unusual begin cardiac tumor were also encountered in our study. Primary malignant neoplasms accounted for 16.03% of all the cardiac tumors in our study. Undifferentiated sarcoma and angiosarcoma were mostly seen in primary malignant cardiac tumors, which occurred in 8 (6.10%) and 4 (3.05%) cases respectively. Lymphoma and rhabdomyosarcoma was diagnosed in 3 (2.29%) and 2 (1.52%) patients respectively. Inflammatory myofibroblastic tumor, synovial sarcoma, liposarcoma and pericardial mesothelioma were encountered in one case respectively. The remainder malignant neoplasms were secondary cardiac tumors transferred from bones (n=2), liver (n=2) and kidney (n=2).
Site distribution of tumors in the cardiac chambers
According to the result of analysis, 75.57% of all the cardiac tumors occurred in the left cardiac chambers, with 68.70% in the left atrium (LA) and 6.87% in the left ventricle (LV). For tumors in left cardiac chambers, most were begin (94.8%) with myxomas occupying the predominance, while the malignant tumors contributed to only 5.2% of all the patients, and among which, 85.7% were primary cardiac tumors. As for tumors in the right cardiac chambers, most were malignant ones, statistics revealed that half of the right cardiac tumors were primary malignant tumors (50%), while benign tumors affected only 35.7% of the patients, and the metastatic cardiac tumors took the residual (14.3%). Whereas, biatrial cardiac tumors occurred in 2 patients, all were begin ones. There was one case of myxoma with the tumor invading both LV and RV, and pericardial mesothelioma was encountered in one case in our study (Table 2) (Figure 2).
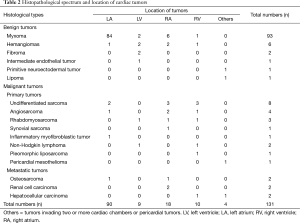
Full table

Surgical treatment
Tumor resection under extracorporeal circulation which was installed using aortic and bicaval venous cannulation was performed in all patients included in our study with or without other concomitant cardiac surgeries. En bloc excision of the tumor mass was aimed in surgery or otherwise accordingly if it was not possible. Concomitant mitral valvuloplasty (MVP) and mitral valve replacement (MVR) were performed in 18 (13.74%) and 4 (3.05%) cases respectively, while tricuspid valvuloplasty (TVP) was conducted in 27 (20.61%) of the included patients. Only one patient received coronary artery bypass grafting (CABG) and 2 (1.52%) patients were performed aortic valve replacement (AVR). The tumor mass ranged from 1 cm × 1 cm to 10 cm × 11 cm in size, with a mean size of 4.2 cm × 5.6 cm.
In-hospital mortality and long-term follow up
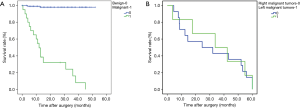
Among 131 patients diagnosed of cardiac tumors in our study who were underwent surgical treatment at our institution, there were 2 in-hospital deaths (death occurring within 30 days of operation or before discharge). Complications related to surgery postoperatively were recorded in 15 (11.45%) patients, and there were 6 (4.58%) patients had episodes of arrhythmias and 4 (1.73%) had major bleeding in the chest, renal dysfunction and wound infection were both observed in 1 case. The survival curves of patients included in our study were constructed according to the Kaplan-Meier method, the result showed that patients with cardiac tumors in the left heart chambers had a significant higher survival rate than that in the right heart chambers (P<0.001), additionally, patients with malignant cardiac tumors had significant lower survival rate than those with benign tumors (P<0.001) (Table 3).
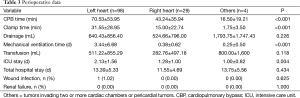
Full table
Comparison of tumors between left and right cardiac chambers
Data on distribution of tumors between left and right cardiac chambers were shown in Table 2. Patients with tumors in the left cardiac chambers had similar basic characteristics to patients with right heart tumors except that patients with tumors in the left cardiac chambers had significantly higher female constitution (P=0.002), lower BSA (P=0.045) and lower weight (P=0.033). On types of surgery, MVP was more performed in the left heart tumors (P=0.042) while TVP was more often carried out in right heart tumors. Compared with patients with tumors in the right cardiac chambers, patients with tumors in the left cardiac chambers had significant higher CPB time (P<0.001), cross clamp time (P<0.001) and time of mechanical ventilation (P<0.001), furthermore, they also had longer ICU stay (P<0.001) but not with total hospital stay (P=0.434). Additionally, begin tumors were more common in left cardiac chambers while malignant neoplasms were more frequently seen in right cardiac chambers. In terms of survival, patients with left heart tumors had a significant higher survival rate at 1-year, 3-year, and 5-year follow-up (P<0.001), and the survival rate in patients with left heart tumors was 92.78%, 88.73% and 82.70% at 1-year, 3-year, and 5-year follow-up, while the relative survival rate in patients with right heart tumors was 59.61%, 49.23% and 19.70% respectively. But our analysis showed that there was no significant difference between patients with left sided malignant tumors and those with right sided malignant tumors (P=0.807) (Figure 3).
Discussion
In this report, we presented a large series of cardiac tumors involving reviewed comprehensive clinical data of these consecutively diagnosed patients with cardiac tumors who received surgical treatment in our center. Compared with most of previous reports, we uniquely collected data on both benign and malignant cardiac tumors to provide a comprehensive clinical data on such issue.
In our data analysis, 79.47% of all the cardiac tumors were benign, which was similar to previous studies (4,18,19). The majority of benign tumors of the heart were located in the LA, while the RA came the second. Furthermore, most of the benign tumors were myxomas, effecting women mostly with a median age of 51.39±16.37. Primary malignant cardiac tumors are scarcely occurred in heart disease. In our studies, we found a percentage of 20.61% with all the malignant tumors, involving 21 primary tumors and 6 metastatic ones. Although significantly uncommon, lymphoma and rhabdomyosarcoma are the second most common malignant tumors of the heart in our series, which was not consistent with previous studies in whose report papillary fibroelastoma (PFE) was the second most common ones. The remainder of the tumors was generally represented by significant rare inflammatory myofibroblastic tumor, synovial sarcoma, liposarcoma and pericardial mesothelioma. Metastatic neoplasms of the heart constituted to 4.6% of all cardiac tumors including metastatic hepatic carcinoma, renal carcinoma and osteosarcoma in our report. Extremely unusual tumors of the heart involving primitive neuroectodermal tumor, rhabdomyosarcoma, primary lymphoma and inflammatory myofibroblastoma were also recorded in this report.
Because myocytes never divide and the heart is less exposed to external irritation, primary cardiac neoplasm is significantly less common found in autopsies, comparing with other organs (20,21). According to the available literature, primary cardiac tumors comprised 95–97% of all cardiac neoplasms, while metastatic tumors of the heart comprised 3–5%, which is consistent with the result of our report (6,18,20). Myxomas, most common primary cardiac tumors, are usually located in the LA and RA, and are rarely seen in left and right ventricle. As to non-myxomas cardiac tumors, ventricles are the most susceptible chambers, our patients also confirm to the location distribution of relative tumor occurrence as evident in other series (22-24).
For most patients diagnosed with cardiac tumors, intracardiac obstruction and peripheral embolism are the two main constitutional symptoms. In early reports, some authors demonstrated a frequency of 30–40% of embolization in their reports (20,25,26). Recent reports of the sign of embolism associated with cardiac tumors showed a downward trend with the frequency of 20–25% (6,27-29), and in this retrospective study we reported the frequency of 21.5%. Symptoms of intracardiac and flow tract obstruction induced by cardiac tumors remain the foremost in all clinical signs with the incidence of 60–70%, which is also the main reason why patients get examined.
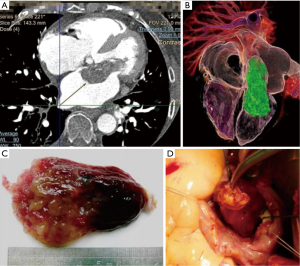
Recent progress of medical imaging technology makes CT and cardiac MRI more often utilized in diagnosis of cardiac neoplasms. Using 3-D reconstructing technology with CT or MRI, surgeons can assess the location, size and pedicle of the tumor intuitively before surgery, which was greatly helpful in guiding the approach of the resection of the tumor in surgery (9,10). Several cases of our series with giant mass in the heart showed great difficulties before surgery, by simulating steps of surgery outside the body using 3-D reconstructing technology, we successfully complete the giant mass resection. In a word, modern medical imaging technology provided wonderful scenery of the intracardiac tumor and helped minimize perioperative risk (Figure 4).
Surgery is advisable to improve prognosis in patients with resectable cardiac tumors to minimize the risk of embolic events and their associated morbidity. The in-hospital death and perioperative surgery-associated complications in our series were consistent with previous reports. The most frequent complication following surgery of cardiac tumor resection was arrhythmia, with 4.6% in our reports, comparing with 7–10% in other studies (3,18). The overall long-term survival of patients included in our studies at a median follow-up of 5 years was satisfactory, which confirm the effect of surgery in treatment of cardiac tumors. The survival rate was 66.41% at 5 years follow-up in our reports, slightly higher than reports from other centers. For patients treated with surgery resection with benign tumors, they can achieve a significant higher survival of 82.70% at follow-up, much higher than those with malignant tumors. Incidence of recurrence of the series in our studies was rare (4.76%) in benign cardiac tumors, while more common in malignant ones (31.27%) which is also the leading factor influencing the mortality of patients with malignant cardiac tumors (4,20,30,31).
In summary, cardiac tumors comprise a small percentage of cardiac diseases which needed surgery treatment. Once diagnosed with cardiac tumors, whether benign or malignant, surgical resection is an effective way in improving survival and long-term life quality with low rate of morbidity and mortality and the prognosis is excellent.
Acknowledgements
Funding: This work was supported by the National Nature Science Foundation of China (No. 81170232, 81200181, 81270419 and 81300102). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Amano J, Kono T, Wada Y, et al. Cardiac myxoma: its origin and tumor characteristics. Ann Thorac Cardiovasc Surg 2003;9:215-21. [PubMed]
- Abad C. Cardiac tumors (II). Malignant primary tumors. Metastatic tumors. Carcinoid tumor. Rev Esp Cardiol 1998;51:103-14. [Crossref] [PubMed]
- Agarwal V, Agarwal SK, Srivastava AK, et al. Primary cardiac tumors: surgical experience and follow-up. Indian Heart J 2003;55:632-6. [PubMed]
- Barreiro M, Renilla A, Jimenez JM, et al. Primary cardiac tumors: 32 years of experience from a Spanish tertiary surgical center. Cardiovasc Pathol 2013;22:424-7. [Crossref] [PubMed]
- Beghetti M, Gow RM, Haney I, et al. Pediatric primary benign cardiac tumors: a 15-year review. Am Heart J 1997;134:1107-14. [Crossref] [PubMed]
- Cresti A, Chiavarelli M, Glauber M, et al. Incidence rate of primary cardiac tumors: a 14-year population study. J Cardiovasc Med (Hagerstown) 2016;17:37-43. [Crossref] [PubMed]
- Dioszeghy C, Kamaras G, Frigyik A. Primary cardiac tumor identified as the cause of seizure. West J Emerg Med 2011;12:84-6. [PubMed]
- Georghiou GP, Vidne BA, Sahar G, et al. Primary cardiac valve tumors. Asian Cardiovasc Thorac Ann 2010;18:226-8. [Crossref] [PubMed]
- Esposito A, De Cobelli F, Ironi G, et al. CMR in assessment of cardiac masses: primary benign tumors. JACC Cardiovasc Imaging 2014;7:733-6. [Crossref] [PubMed]
- Randhawa K, Ganeshan A, Hoey ET. Magnetic resonance imaging of cardiac tumors: part 2, malignant tumors and tumor-like conditions. Curr Probl Diagn Radiol 2011;40:169-79. [Crossref] [PubMed]
- Awamleh P, Alberca MT, Gamallo C, et al. Left atrium myxosarcoma: an exceptional cardiac malignant primary tumor. Clin Cardiol 2007;30:306-8. [Crossref] [PubMed]
- Dell'amore A, Albertini A, Lamarra M. Twenty years experience in oncologic surgery for primary cardiac tumors. G Chir 2013;34:106-11. [PubMed]
- Fuchida S, Yamada N, Uchida R, et al. Malignant lymphoma presenting as a cardiac tumor and superior vena caval syndrome successfully treated by haploidentical stem cell transplantation. Leuk Lymphoma 2005;46:1517-21. [Crossref] [PubMed]
- Nield LE, Mendelson M, Ahmad N, et al. Clinical review of obstructive primary cardiac tumors in childhood. Congenit Heart Dis 2014;9:244-51. [Crossref] [PubMed]
- Perchinsky MJ, Lichtenstein SV, Tyers GF. Primary cardiac tumors: forty years' experience with 71 patients. Cancer 1997;79:1809-15. [Crossref] [PubMed]
- Alferes VR, Kenny DA. SPSS programs for the measurement of nonindependence in standard dyadic designs. Behav Res Methods 2009;41:47-54. [Crossref] [PubMed]
- Weaver B, Wuensch KL. SPSS and SAS programs for comparing Pearson correlations and OLS regression coefficients. Behav Res Methods 2013;45:880-95. [Crossref] [PubMed]
- Barnes H, Conaglen P, Russell P, et al. Clinicopathological and surgical experience with primary cardiac tumors. Asian Cardiovasc Thorac Ann 2014;22:1054-8. [Crossref] [PubMed]
- Blondeau P. Primary cardiac tumors--French studies of 533 cases. Thorac Cardiovasc Surg 1990;38 Suppl 2:192-5. [Crossref] [PubMed]
- Basso C, Valente M, Poletti A, et al. Surgical pathology of primary cardiac and pericardial tumors. Eur J Cardiothorac Surg 1997;12:730-7; discussion 737-8. [Crossref] [PubMed]
- Grande AM, Ragni T, Viganò M. Primary cardiac tumors. A clinical experience of 12 years. Tex Heart Inst J 1993;20:223-30. [PubMed]
- Bossert T, Gummert JF, Battellini R, et al. Surgical experience with 77 primary cardiac tumors. Interact Cardiovasc Thorac Surg 2005;4:311-5. [Crossref] [PubMed]
- Cianciulli TF, Saccheri MC, Lax JA, et al. Left ventricular thrombus mimicking primary cardiac tumor in a patient with primary antiphospholipid syndrome and recurrent systemic embolism. Cardiol J 2009;16:560-3. [PubMed]
- Milgalter E, Lotan H, Schuger L, et al. Cardiac myxomas--surgical experience with a multi-faceted tumor. Thorac Cardiovasc Surg 1987;35:115-8. [Crossref] [PubMed]
- Centofanti P, Di Rosa E, Deorsola L, et al. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg 1999;68:1236-41. [Crossref] [PubMed]
- Edwards FH, Hale D, Cohen A, et al. Primary cardiac valve tumors. Ann Thorac Surg 1991;52:1127-31. [Crossref] [PubMed]
- Elbardissi AW, Dearani JA, Daly RC, et al. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation 2008;118:S7-15. [Crossref] [PubMed]
- Qiu LS, Sun YJ, Ding WX, et al. Treatment strategies for pediatric patients with primary cardiac tumors. Zhonghua Wai Ke Za Zhi 2011;49:227-31. [PubMed]
- Takano T, Amano J. Cardiac tumor, constrictive pericarditis and pulmonary thromboembolism. Kyobu Geka 2011;64:658-65. [PubMed]
- Beranek JT. Factor VIII-related antigen in the cardiac myxoma: its relevance to tumor histogenesis. Arch Pathol Lab Med 1989;113:1324. [PubMed]
- Padalino MA, Vida VL, Boccuzzo G, et al. Surgery for primary cardiac tumors in children: early and late results in a multicenter European Congenital Heart Surgeons Association study. Circulation 2012;126:22-30. [Crossref] [PubMed]


