Standardization of intra- and peri-operative management to reduce postoperative drainage time after major thoracoscopic pulmonary resections
Highlight box
Key findings
• The standardized management in thoracoscopic major pulmonary resections significantly reduced postoperative drainage time by multivariate analysis.
What is known and what is new?
• Standardized intra- and peri-operative management significantly reduced the postoperative drainage time after thoracoscopic major pulmonary resections.
What is the implication, and what should change now?
• Surgeons must master a fissureless or a unidirectional dissection technique and apply standardized perioperative drainage management.
Introduction
After major thoracoscopic pulmonary resections, early removal of the chest drainage tube is important to relieve postoperative pain and facilitate patient mobilization. Most previous enhanced recovery after surgery (ERAS) reports on the lung emphasized that this was important, although such management remains uncommon after thoracic surgery (1-3). Two key factors (one intraoperative and one postoperative factor) affect early removal of the chest drainage tube. The first is intraoperative reduction of postoperative pulmonary air leaks; usually, longer postoperative drainage is attributable to prolonged air leakage. The second is standardization of postoperative drain management; the tube is often removed only when the surgeon decides; surgeons tend to hesitate due to excessive caution, which prolongs the drainage time. However, with excessively early removal, there may be a need for tube reinsertion or postoperative readmission; great care is thus required.
Based on those findings, we introduced standardized intra- and peri-operative management to reduce the postoperative drainage time, beginning in February 2019. In this study, we investigated whether this standardization reduced the postoperative drainage time. Moreover, we examined how such management affected re-admission within 30 days after operation (because of pleural complications). We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1377/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of the Japanese Red Cross Maebashi Hospital (approval No. 2022–11). The need for individual patient consent was waived given the retrospective nature of the work. The standardized management was as follows (Figure 1):
- Intraoperatively, any dense fissures were left untreated to avoid postoperative air leakage. A fissureless or unidirectional dissection technique served as an alternative; pulmonary vessels and bronchi were divided at the hilum in patients with dense fissures (4-8), classified as fissural grade III or IV (Craig, 1997), or inflammation rendering it difficult to expose the pulmonary artery using counter-traction (9).
- The chest drain was removed when air leakage stopped, regardless of the fluid volume or the surgeon’s preference.

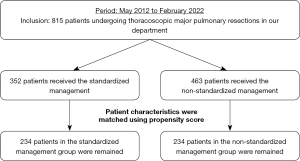
From May 2012 to February 2022, 815 patients with malignant or benign disease undergoing major thoracoscopic pulmonary resection via a uniportal or multiportal approach were enrolled (Figure 2). The patients were divided into standardized management (n=352) and non-standardized management (n=463) groups. We compared the characteristics and perioperative results of the two groups before and after (n=234 in each group) propensity-score matching by univariate analysis. The clinical data analyzed included age, sex, American Society of Anesthesiologists (ASA) score, smoking index (pack-year), forced expiratory volume in 1 s (FEV1), %FEV1, tumor location, diseases, surgical procedure and approach, operative time, intraoperative blood loss, rates of significant vessel injury and conversion to thoracotomy, duration of postoperative drainage, postoperative hospitalization time, morbidity (Clavien-Dindo grade ≥ III), rate of readmission within 30 days after operation, and 30-day postoperative mortality. Factors contributing to postoperative drainage time and re-admission within 30 days (because of pleural complications) were identified by multivariate analysis. Our department has introduced a uniportal approach for anatomical pulmonary resections since 2019. In the introduction period, the rate of a uniportal approach was small. However, the rate gradually increased in line with gaining the experience. Therefore, the standardized management group included both including uniportal and multiportal approaches.
Thoracoscopic procedure
A fissureless or unidirectional dissection technique was used for patients in the standardized management group with a dense fissure. The use of this technique in the non-standardized management group was at the discretion of the surgeon, even if the fissure was fused. The uniportal approach involved the creation of a 3.5–4-cm incision in the fourth or fifth intercostal space from the anterior axillary line; the multiportal approach used three or four ports, including a 3–4-cm access port and 1.5-cm ports. All major thoracoscopic pulmonary resections were performed with patients under general anesthesia on one-lung ventilation in the lateral decubitus position. A 5- or 10-mm 30° thoracoscope (flexible or inflexible) was employed. The large vessels and bronchi were divided with a stapler. Small-caliber vessels were divided using an energy device after proximal ligation. Lung parenchyma including interlobar fissure or intersegmental plane was divided by staplers in thick parts while proximal ligation with string or electrocautery cutting was applied in thin parts. Specimens were placed in endovascular bags and retrieved via the incision after pulmonary resection. The incision was lengthened as required and we did not use a rib-spreader. Systemic lymphadenectomy (to ≥ ND2a-1) was performed in all patients undergoing lobectomy to treat primary lung cancer. ND2a-1 surgery involved lymphadenectomy with selective mediastinal dissection; for ND2a-2 surgery, radical mediastinal dissection was employed (10). In our institution, an anatomical segmentectomy was usually performed which was different from a wide wedge resection. Therefore, dominant pulmonary vessels and bronchi were divided among any patients undergoing a segmentectomy in this study. The mediastinal lymph nodes were sampled in patients undergoing segmentectomy for primary lung cancer. However, for patients with pulmonary metastases or benign disease, neither lymph node dissection nor sampling was performed during lobectomy or segmentectomy. When an air leak was evident on the sealing test performed at the end of surgery, we applied a polyglycolic acid felt (a Neoveil sheet; Igaki Medical Planning Co. Ltd., Kyoto, Japan) using fibrin glue (Beriplast P; CSL Behring, King of Prussia, PA, USA) with or without 3-0 absorbable monofilament sutures. Finally, a 19-Fr Blake chest drain (Ethicon, Paramus, NJ, USA) or 24-Fr double-lumen chest tube was positioned in the thorax.
Postoperative management
Our surgical team including senior and resident surgeons went the rounds of our patients in the morning every day. We argued whether air leakage was found or not at the rounds. The chest drain was removed when air leakage stopped in the standardized management group, regardless of the fluid volume or the surgeon’s preference on postoperative day 1 or later. However, from July 2021, we started early removal of chest drain on postoperative day 0 for a patient undergoing thoracoscopic segmentectomy when air leakage was not detected in the sealing test at the end of operation and postoperatively for 2–4 hours, The patients receiving early removal of chest drain on postoperative day 0 were included in the standardized management group. In the non-standardized management group, the tube was usually removed when air leakage stopped or the daily pleural effusion amount fell below 500 mL (on postoperative day 1 or later); however, tube removal was at the surgeon’s discretion. After discharge, postoperative follow-up using chest X-ray in outpatient ward was performed for any patient on around postoperative day 10 and 30.
Statistical analysis
We used Fisher’s exact test to compare categorical variables, and the t-test and Mann-Whitney U test to compare continuous variables. A P value <0.05 was considered statistically significant. Multivariate analysis of categorical variables was performed using logistic regression; continuous variables were subjected to multiple linear regression. The two groups were propensity score-matched. Propensity scores were calculated by logistic regression including the following variables: age, sex, ASA score, smoking index, FEV1, %FEV1, tumor location, disease, and surgical procedure. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which has a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Table 1 compares the characteristics and perioperative outcomes of patients receiving standardized and non-standardized management before propensity score-matching. In the former group, the patients were significantly older and the %FEV1 was lower. A uniportal approach or segmentectomy was more common in the standardized management group. Moreover, complex type of segmentectomy was more frequently performed in the standardized management group. In eight patients undergoing segmentectomy, chest drain was removed on postoperative day 0. Most perioperative outcomes (operative time, rate of significant vessel injury, duration of postoperative drainage, postoperative hospitalization time, and morbidity) were better in the standardized management group. Table 2 compares the characteristics and perioperative outcomes of the standardized and non-standardized management groups after propensity score-matching. Given the more frequent use of the uniportal approach in the former group, the operative time (standardized management group: 152±47 min; non-standardized management group: 193±46 min, P<0.001) and duration of postoperative drainage (standardized management group: 1.8±1.4 days; non-standardized management group: 2.7±1.9 days, P<0.001) were superior in the standardized management group. The median hospitalization time for the standardized management group was significantly shorter than for the non-standardized management group (standardized management group: 3 days; non-standardized management group: 4 days, P<0.0001). There was no other significant difference between the two groups. Table 3 lists the factors affecting postoperative drainage time, as revealed by multivariate analyses. Standardized management [estimated regression coefficient: −0.47; 95% confidence interval (CI): −0.78 to −0.16; P=0.003] significantly reduced the postoperative drainage time; but the smoking index (estimated regression coefficient: 0.0064; 95% CI: 0.00049 to 0.012; P=0.034), operative time (estimated regression coefficient: 0.01; 95% CI: 0.0068 to 0.013; P<0.001), and blood loss (estimated regression coefficient: 0.0016; 95% CI: 0.00069 to 0.0025; P<0.001) also contributed. Table 4 lists the factors affecting re-admission within 30 days after the operation (because of pleural complications), as revealed by multivariate analyses. Standardized management did not significantly increase re-admission [odds ratio (OR) =1.76; 95% CI: 0.557 to 5.58; P=0.34]. Table 5 lists the pleural complications of both groups after propensity score-matching.
Table 1
| Variables | Standardized management (n=352) | Non-standardized management (n=463) | P value |
|---|---|---|---|
| Age (years) | 71±11 | 70±9.8 | 0.016 |
| Sex | 0.22 | ||
| Female, n (%) | 196 (55.7) | 278 (60.0) | |
| Male, n (%) | 156 (44.3) | 185 (40.0) | |
| ASA score, median (IQR) | 2 (1–3) | 2 (1–3) | 0.37 |
| Smoking index (pack-years) | 26±30 | 30±32 | 0.12 |
| FEV1 (mL) | 2,168±665 | 2,183±586 | 0.73 |
| %FEV1 (%) | 94±19 | 101±22 | <0.001 |
| Tumor location, n (%) | 0.16 | ||
| RUL | 103 (29.3) | 140 (30.2) | |
| RML | 25 (7.1) | 32 (6.9) | |
| RLL | 100 (28.4) | 98 (22.2) | |
| LUL | 67 (19.0) | 101 (21.8) | |
| LLL | 57 (16.2) | 92 (19.9) | |
| Disease, n (%) | 0.29 | ||
| Primary lung cancer | 292 (83.0) | 375 (81.0) | |
| Metastatic lung cancer | 26 (7.4) | 46 (9.9) | |
| Other malignancy | 0 (0.0) | 3 (0.6) | |
| Benign conditions | 34 (9.7) | 39 (8.4) | |
| Surgical procedure, n (%) | <0.001 | ||
| Bilobectomy | 1 (0.3) | 2 (0.4) | |
| Lobectomy | 228 (64.8) | 375 (81.0) | |
| Segmentectomy | 123 (34.9) | 86 (18.6) | 0.036 |
| Simple | 56 (45.5) | 52 (60.5) | |
| Complex | 67 (54.5) | 34 (39.5) | |
| Surgical approach, n (%) | <0.001 | ||
| Multiport | 82 (23.3) | 450 (97.2) | |
| Uniport | 270 (76.7) | 13 (2.8) | |
| Operative time (min) | 149±45 | 206±56 | <0.001 |
| Blood loss (g) | 44±83 | 100±648 | 0.11 |
| Significant vessel injury, n (%) | 18 (5.1) | 47 (10.2) | 0.009 |
| Conversion to thoracotomy, n (%) | 19 (5.4) | 32 (6.9) | 0.47 |
| Duration of postoperative drainage (days) | 1.7±1.6 | 2.9±2.2 | <0.001 |
| Postoperative hospitalization time (days), median (IQR) | 3 (2–4) | 6 (4–7) | <0.001 |
| Morbidity (C-D classification grade ≥3), n (%) | 40 (11.4) | 112 (24.2) | <0.001 |
| Readmission within 30 days after operation, n (%) | 18 (5.1) | 22 (4.8) | 0.87 |
| 30-day postoperative mortality, n (%) | 2 (0.6) | 1 (0.2) | 0.58 |
ASA, American Society of Anesthesiologists; IQR, interquartile range; FEV, forced expiratory volume; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; C-D classification, Clavien-Dindo classification.
Table 2
| Variables | Standardized management (n=234) | Non-standardized management (n=234) | P value |
|---|---|---|---|
| Age (years) | 70±11 | 70±8.4 | 0.32 |
| Sex, n (%) | 0.78 | ||
| Female | 104 (44.4) | 108 (46.2) | |
| Male | 130 (55.6) | 126 (53.8) | |
| ASA score, median (IQR) | 2 (2–2) | 2 (2–2) | 0.96 |
| Smoking index (pack-years) | 28±32 | 26±29 | 0.53 |
| FEV1 (mL) | 2,198±600 | 2,214±640 | 0.78 |
| %FEV1 (%) | 96±19 | 96±20 | 0.88 |
| Tumor location, n (%) | 0.99 | ||
| RUL | 74 (31.6) | 77 (32.9) | |
| RML | 16 (6.8) | 18 (7.7) | |
| RLL | 60 (25.6) | 57 (24.4) | |
| LUL | 45 (19.2) | 42 (17.9) | |
| LLL | 39 (16.7) | 40 (17.1) | |
| Diseases, n (%) | 0.93 | ||
| Primary LC | 197 (84.2) | 200 (85.5) | |
| Metastatic LC | 17 (7.3) | 16 (6.8) | |
| Other | 20 (8.5) | 18 (7.7) | |
| Surgical procedure, n (%) | 0.8 | ||
| Bilobectomy | 1 (0.4) | 1 (0.4) | |
| Lobectomy | 171 (73.1) | 177 (75.6) | |
| Segmentectomy | 62 (26.5) | 56 (23.9) | 0.71 |
| Simple | 32 (51.6) | 32 (57.1) | |
| Complex | 30 (48.4) | 24 (42.9) | |
| Surgical approach, n (%) | <0.001 | ||
| Multiport | 76 (32.5) | 231 (98.7) | |
| Uniport | 158 (67.5) | 3 (1.3) | |
| Operative time (min) | 152±47 | 193±46 | <0.001 |
| Blood loss (g) | 47±90 | 66±243 | 0.27 |
| Significant vessel injury, n (%) | 13 (5.6) | 17 (7.3) | 0.57 |
| Conversion to thoracotomy, n (%) | 16 (6.8) | 14 (6.0) | 0.85 |
| Duration of postoperative drainage (days) | 1.8±1.4 | 2.7±1.9 | <0.001 |
| Postoperative hospitalization time (days), median (IQR) | 3 (2–4) | 4 (3–6) | <0.001 |
| Morbidity (C-D classification grade ≥3) | 29 (12.4) | 45 (19.2) | 0.057 |
| Readmission within 30 days of operation, n (%) | 11 (4.8) | 15 (6.4) | 0.55 |
| 30-day postoperative mortality, n (%) | 0 (0.0) | 1 (0.4) | 1 |
ASA, American Society of Anesthesiologists; IQR, interquartile range; FEV, forced expiratory volume; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; C-D classification, Clavien-Dindo classification.
Table 3
| Factors | Estimated regression coefficient | 95% CI | P value |
|---|---|---|---|
| Age (continuous) | 0.0012 | −0.025 to 0.027 | 0.93 |
| Sex: male (vs. female) | 0.27 | −0.4 to 0.95 | 0.42 |
| ASA score: 3 (vs. 1–2) | −0.1 | −0.61 to 0.4 | 0.69 |
| Smoking index (continuous) | 0.0064 | 0.00049 to 0.012 | 0.034 |
| FEV1 (continuous) | −0.00014 | −0.00077 to 0.00049 | 0.66 |
| %FEV1 (continuous) | 0.0064 | −0.0096 to 0.022 | 0.43 |
| Tumor location: upper lobes (vs. lower lobes) | 0.19 | −0.093 to 0.48 | 0.19 |
| Disease: metastatic LC and benign conditions (vs. primary LC) | −0.054 | −0.47 to 0.36 | 0.8 |
| Surgical procedure: segmentectomy (vs. lobectomy) | −0.099 | −0.44 to 0.24 | 0.57 |
| Operative time (continuous) | 0.01 | 0.0068 to 0.013 | <0.001 |
| Blood loss (continuous) | 0.0016 | 0.00069 to 0.0025 | <0.001 |
| Conversion to thoracotomy: performed (vs. not performed) | 0.35 | −4.39 to 5.09 | 0.51 |
| Standardized management (vs. non-standardized) | −0.47 | −0.78 to −0.16 | 0.003 |
CI, confidence interval; ASA, American Society of Anesthesiologists; FEV, forced expiratory volume; LC, lung cancer.
Table 4
| Factors | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age (continuous) | 1.02 | 0.92–1.13 | 0.76 |
| Sex: male (vs. female) | 0.65 | 0.046–9.25 | 0.75 |
| ASA score: 3 (vs. 1–2) | 1.17 | 0.2–6.92 | 0.86 |
| Smoking index (continuous) | 0.99 | 0.96–1.01 | 0.3 |
| FEV1.0 (continuous) | 1 | 0.997–1 | 0.81 |
| %FEV1.0 (continuous) | 0.98 | 0.92–1.04 | 0.55 |
| Tumor location: upper lobes (vs. lower lobes) | 0.6 | 0.21–1.72 | 0.34 |
| Disease: metastatic LC and benign conditions (vs. primary LC) | 1.81 | 0.49–6.75 | 0.38 |
| Surgical procedure: segmentectomy (vs. lobectomy) | 0.49 | 0.12–1.96 | 0.31 |
| Operative time (continuous) | 1 | 0.993–1.01 | 0.53 |
| Blood loss (continuous) | 1 | 0.999–1 | 0.21 |
| Conversion to thoracotomy: performed (vs. not performed) | 0.41 | 0.021–8.13 | 0.56 |
| Standardized management (vs. not standardized) | 1.76 | 0.56–5.58 | 0.34 |
CI, confidence interval; ASA, American Society of Anesthesiologists; FEV, forced expiratory volume; LC, lung cancer.
Table 5
| Variables | Standardized management (n=234), n (%) | Non-standardized management (n=234), n (%) |
|---|---|---|
| Excessive pleural effusion | 3 (1.3) | 2 (0.9) |
| Pleuritis | 1 (0.4) | 1 (0.4) |
| Delayed pulmonary fistula | 5 (2.1) | 2 (0.9) |
| Chylothorax | 0 (0.0) | 2 (0.9) |
Discussion
Standardized intra- and peri-operative management significantly reduced the postoperative drainage time after major thoracoscopic pulmonary resections. Moreover, the management did not increase postoperative readmission within 30 days of the operation; inadequate drainage can cause lung and/or pleural morbidity. Our protocol has proven safe since its introduction. A delayed pulmonary fistula is the most common morbidity associated with early drain removal; the rate was 2.1% in our standardized management group, which is acceptable.
The main intraoperative strategy to reduce postoperative air leakage is to avoid exposing the pulmonary artery at a fused fissure. We then use a fissureless technique to perform pulmonary lobectomy; this is commonly used in patients with dense fissures (4-6). We previously described our pulmonary segmentectomy technique in a case report (7,8). During both lobectomy and segmentectomy, it is essential that dense fissures are not dissected to avoid postoperative air leakage. Several retrospective studies reported that the postoperative drainage time was reduced after fissureless lobectomy; the only prospective study, by Stamenovic et al., revealed that thoracoscopic fissureless lobectomy was significantly superior to conventional lobectomy in terms of prolonged air leakage, the duration of chest tube drainage, and the length of hospital stay; the operative times were similar (4-6).
In this study, we focused on early removal of postoperative chest drain. Early drain removal is usually emphasized in ERAS programs which were initially developed by colorectal surgeons but are gradually being applied to thoracic surgery (1-3,11,12). According to the ERAS Society/European Society of Thoracic Surgeons (ESTS) guidelines, early chest drain removal is one of 45 factors that enhances recovery (2). Rogers et al. found that early chest drain removal facilitated patient mobilization (3). They removed the drain when no leakage was evident for 6 h and <500 mL of fluid had been collected in the previous 24 h. Several authors have reported successful “no-drain management” (13-17). Although most patients managed in this way underwent wedge resection or bullectomy, a few required major pulmonary resections. Murakami et al. reported that chest drains were successfully removed in the operating room in 63% of patients undergoing thoracoscopic anatomical pulmonary resections, and no patient required re-drainage (13). Postoperative pain was significantly reduced in the no-drainage group. Pfeuty et al. reported that the success rate of early (postoperative day 0) removal of a digital drainage device was 45%, although drainage was involved (17). Postoperative pain was significantly reduced, as also found by Murakami et al. Although both studies reported excellent results, the authors cautioned that active drain management should be applied only in carefully selected patients. Also, most articles on early drain removal reported reduced postoperative hospitalization times (13-17). We found that the median postoperative time was significantly shorter in the standardized than non-standardized management group, although the mean times did not differ because several patients in the latter group were hospitalized for a long time (hence the large standard deviation). The shorter median postoperative time in the standardized management group indicates that the standardized management shortened postoperative hospitalization, i.e., promoted rapid recovery.
In the standardized management group, the chest drain was removed when air leakage stopped regardless of the fluid volume. In spite of this active postoperative drain management, the group did not reveal significant increase of postoperative complications related with pleural disease, which indicated that chest drain can be safely removed when we appropriately confirmed no air leakage and tendency of postoperative bleeding without taking care about the pleural effusion. Only 1.3% of the patients in the standardized management group required re-drainage for excessive pleural effusion after removal of the postoperative initial drainage tube, which was considered acceptable rate.
This study had several limitations, including a retrospective single-institution design. First, the number of included patients were different between the two groups before propensity score matching. In addition, the new protocol favors a uniportal thoracoscopic approach, which is more difficult than a multiportal approach, because a uniportal approach was introduced in our institution in 2019 and the rate of adoption of it gradually increased. Also, the impact of surgical skill including the learning curve and the surgical instruments used (including staplers) was not considered. Except for adoption of fissureless technique, sealants and staple-line buttress materials have been used to avoid postoperative air leakage. On finding an air leak during the sealing test performed at the end of surgery, we applied polyglycolic acid using fibrin glue, with or without 3-0 absorbable monofilament sutures, in both groups. The number of patients treated in this manner was not recorded. This might affect the postoperative drainage times although these approaches did not provide clear benefits and are not universally applicable (18,19). Additionally, the number of patients with pleural adhesion or dense fissure was unclear, which might affect the postoperative drainage time. Moreover, the medical costs were not compared between the two groups due to the lack of data. And then, early discharge might be facilitated due to the recent tendency of desiring early social reintegration. Finally, chest drain was removed on postoperative day 0 in eight patients, which might affect the shorter postoperative drainage time in the standardized management group, although the number was small.
Conclusions
Standardized intra- and peri-operative management significantly reduced the postoperative drainage time after major thoracoscopic major pulmonary resections, without increasing re-admissions within 30 days among patients with pleural complications caused by insufficient drainage. Surgeons must master a fissureless or a unidirectional dissection technique, avoid dissection of fused fissures, and apply standardized perioperative drainage management.
Acknowledgments
The authors thank all of the surgeons and coworkers who contributed to this study, as well as the editors and reviewers for their assistance with the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1377/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1377/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1377/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1377/coif). HI served as unpaid editorial board member of Journal of Thoracic Disease from August 2022 to July 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Japanese Red Cross Maebashi Hospital (Approval No. 2022–11) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haro GJ, Sheu B, Marcus SG, et al. Perioperative Lung Resection Outcomes After Implementation of a Multidisciplinary, Evidence-based Thoracic ERAS Program. Ann Surg 2021;274:e1008-13. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- Ng T, Ryder BA, Machan JT, et al. Decreasing the incidence of prolonged air leak after right upper lobectomy with the anterior fissureless technique. J Thorac Cardiovasc Surg 2010;139:1007-11. [Crossref] [PubMed]
- Gómez-Caro A, Calvo MJ, Lanzas JT, et al. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg 2007;31:203-8. [Crossref] [PubMed]
- Stamenovic D, Bostanci K, Messerschmidt A, et al. Fissureless fissure-last video-assisted thoracoscopic lobectomy for all lung lobes: a better alternative to decrease the incidence of prolonged air leak? Eur J Cardiothorac Surg 2016;50:118-23. [Crossref] [PubMed]
- Igai H, Matsuura N, Kamiyoshihara M. Uniportal thoracoscopic right anterior basal (S8) segmentectomy using unidirectional dissection. Multimed Man Cardiothorac Surg 2021;2021: [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Matsuura N. Uniportal thoracoscopic lateral and posterior basal (S9+10) segmentectomy. Multimed Man Cardiothorac Surg 2020;2020: [Crossref] [PubMed]
- Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb 1997;42:233-4.
- Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg 2006;81:1028-32. [Crossref] [PubMed]
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. [Crossref] [PubMed]
- Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466-77. [Crossref] [PubMed]
- Murakami J, Ueda K, Tanaka T, et al. The Validation of a No-Drain Policy After Thoracoscopic Major Lung Resection. Ann Thorac Surg 2017;104:1005-11. [Crossref] [PubMed]
- Liu CY, Hsu PK, Leong KI, et al. Is tubeless uniportal video-assisted thoracic surgery for pulmonary wedge resection a safe procedure? Eur J Cardiothorac Surg 2020;58:i70-6. [Crossref] [PubMed]
- Li S, Jiang L, Ang KL, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017;51:689-93. [Crossref] [PubMed]
- Yang SM, Wang ML, Hung MH, et al. Tubeless Uniportal Thoracoscopic Wedge Resection for Peripheral Lung Nodules. Ann Thorac Surg 2017;103:462-8. [Crossref] [PubMed]
- Pfeuty K, Lenot B. Early postoperative day 0 chest tube removal using a digital drainage protocol after thoracoscopic major pulmonary resection. Interact Cardiovasc Thorac Surg 2020;31:657-63. [Crossref] [PubMed]
- Miller JI Jr, Landreneau RJ, Wright CE, et al. A comparative study of buttressed versus nonbuttressed staple line in pulmonary resections. Ann Thorac Surg 2001;71:319-22; discussion 323. [Crossref] [PubMed]
- Belda-Sanchís J, Serra-Mitjans M, Iglesias Sentis M, et al. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2010;2010:CD003051. [Crossref] [PubMed]