
A retrospective analysis of treatment selection and risk factors of treatment failure and recurrence in patients with spontaneous pneumothorax
Highlight box
Key findings
• Observation was superior to tube drainage in terms of success rate to cease air leak and recurrence rate, although the differences were not statistically significant in multivariate analyses.
What is known and what is new?
• Current guidelines recommend pleural intervention for patients with large pneumothorax or underlying lung disease.
• Large pneumothorax and presence of underlying lung disease is not an independent risk factor for persistent air leak or recurrence.
What is the implication, and what should change now?
• Owing to treatment invasiveness, observation would be recommended first in patients without risk factors. Further confirmation is required to clarify these findings in a prospective study.
Introduction
Patients with spontaneous pneumothorax generally have a favorable prognosis and rarely develop fatal respiratory distress (1,2). Treatment options in initial management include conservative observation with or without oxygen supplementation, needle aspiration, and tube drainage. The indications for each of these differ among the published guidelines (3,4). Previous reports detailing initial management mainly focused on comparing needle aspiration and tube drainage (5,6). Furthermore, current guidelines still focus on interventional therapies based on pneumothorax size (3,4), despite the fact that pneumothorax size does not always correlate well with symptoms or clinical derangement of vital signs. Recent reports have referred to the efficacy of conservative observation even for large pneumothorax that is recommended as pleural intervention in current guidelines (7,8).
Treatment options in initial management are evaluated by the objectives of the treatments: recovery from life-threatening conditions, cessation of air leak, and prevention of recurrence (9). Since initial treatment is primarily to manage air leakage, evaluation of treatment efficacy cannot be carried out in terms of recurrence alone. A recent randomized trial comparing observation and pleural intervention showed that success rate of observation to manage air leak was non-inferior to that of pleural intervention, and the recurrence rate of observation was superior to that of pleural intervention (7). However, the relationship between treatment selection and lung collapse, which is usually considered to be an indication in initial management, has not been well studied.
In this study, we retrospectively reviewed initial management for spontaneous pneumothorax and examined the risk factors of prolonged air leak and recurrence, considering the degree of lung collapse. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1486/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Kansai Medical University (approval date: May 28, 2018; approval No. 2017320). The requirement for informed consent was waived because of the retrospective nature of the study.
Study design and population
Spontaneous pneumothorax in patients who underwent initial management in the outpatient clinic or admitted to our hospital between January 2006 and December 2015 were included and analyzed in this retrospective, single-institutional study. Patients with primary spontaneous pneumothorax (PSP) and secondary spontaneous pneumothorax (SSP) were included in this study. Patients up to 50 years of age without underlying lung disease were defined as PSP. SSP was defined as pneumothorax in patients with apparent underlying lung disease or those older than 50. Patients of first and previous episodes of pneumothorax were included in this study. We excluded patients who had previously undergone pleurodesis or surgery in the analysis of initial management.
Classification of lung collapse
The degree of lung collapse was categorized according to the chest radiographic appearance as low, middle, or high as per the definition proposed by The Japan Society for Pneumothorax and Cystic Lung Disease (JSPCLD) as follows: (I) low, apex is at the same level or higher than the clavicle; (II) middle, the degree between low and high and (III) high, total or almost total collapse. High and middle degrees of lung collapse and low degree of pneumothorax defined by JSPCLD are almost equivalent to large pneumothorax and small pneumothorax, respectively, as defined by the American College of Chest Physicians (ACCP) guidelines (3).
Treatments and evaluations
Initial treatment denoted as treatment first performed for pneumothorax was selected at the discretion of the attending physician. Three methods were evaluated for the initial treatment: observation, needle aspiration, and tube drainage.
To evaluate efficacy of the initial treatment, “success” and “failure” were defined as follows (shown in Figure 1). “Success” indicates stoppage of air leak which did not require further treatment, while “failure” means ongoing air leak which required further treatment. When an observation finally achieved lung re-expansion by chest radiograph, the treatment was evaluated as a “success”. When conservative observation could not achieve lung re-expansion and further treatments were needed, the treatment was assessed as a “failure”. When a chest radiograph confirmed lung re-expansion after needle aspiration and did not show lung re-collapse in the follow-up, the treatment was evaluated as a “success”. Patients who required multiple needle aspirations and did not need further treatment modality were also defined as “success”. When patients treated with needle aspiration required further modalities to achieve stable lung expansion, the treatment was evaluated as a “failure”. When tube drainage achieved lung re-expansion and air leak cessation, the treatment was evaluated as a “success”. Cessation of air leak was confirmed when the underwater drainage no longer bubbled. When a patient did not achieve stable lung re-expansion or showed persistent air leak after initial treatment, the treatment was considered a “failure”. If minor leak was suspected, clamping chest tube was attempted, and patients underwent observation symptomatically and radiographically. In case of failure of tube drainage, further treatments were performed to cease air leakage. The durations required for each treatment were not specified in this retrospective study.
In some successful cases, additional treatments were performed to prevent recurrence even after cessation of air leak. Additional treatments included surgery, chemical pleurodesis, and the three initial treatments. Surgical interventions in this study included resection, ligation, and suture of the air leak point or bulla, depending on the case. Concurrently, a polyglycolic acid sheet was used for coverage in most cases to reinforce the visceral pleura. In chemical pleurodesis, Picibanil and minocycline were used as sclerosing agents.
Recurrence was defined as that of radiologically confirmed pneumothorax regardless of the need for subsequent interventions. To evaluate recurrence, the last treatment was analyzed. Treatment that resulted in the cessation of the air leak was considered as the last treatment. If additional treatment intending to prevent recurrence was performed after cessation of air leak, the last treatment was the additional treatment. Recurrence on the ipsilateral side was only counted as recurrence. In patients treated with tube drainage, if re-collapsed lung was detected within two days after removal of the chest tube, we usually judged minor collapse as treatment failure and major collapse as recurrence.
Data collection
The following clinical data and radiological findings were collected from patients’ medical records and chest radiographs: age, sex, smoking history, previous episode of ipsilateral pneumothorax, side of pneumothorax, underlying lung disease, degree of lung collapse, radiological finding of bulla formation or pulmonary fibrosis confirmed by computed tomography (CT) in the ipsilateral lung, intervention for pneumothorax, and recurrence of pneumothorax. Information of the intervention from the initial management was collected along with the patient’s timeline as follows: (I) initial treatments; (II) further treatments to cease air leak in cases of failure, including second and third treatments; and (III) additional treatments to prevent recurrence after cessation of air leak. The recurrence-free interval was defined as the period between the day of the last treatment and the day of recurrence. When the patient was managed by only observation and cured, the recurrence-free interval was defined as the period of radiographically evaluated day between full expansion of the lung and the day of recurrence.
Statistical analysis
Analysis of treatment efficacy to manage air leaks was performed by initial treatment and that of recurrence was performed by last treatments. Continuous variables were reported as a mean with standard deviation, and categorical variables were expressed as number of patients. Statistical analysis was performed to investigate the risk factors for treatment failure on initial treatment and those for recurrence of ipsilateral pneumothorax after the last treatment. The Wilcoxon rank-sum test and Fisher’s exact test were used to compare continuous data and categorical data, respectively. Logistic regression analysis was used in univariate and multivariate analyses. Factors with a P value <0.2 in univariate analyses were used in multivariate analyses. With respect to age, patients were categorized at 50 years of age, as the age of onset of pneumothorax is bimodal. A Cox proportional hazard model was used to estimate the recurrence risk. The cumulative recurrence rate was estimated by the Kaplan-Meier method. A P value <0.05 was considered statistically significant. To assess prediction accuracy, receiver operating characteristic (ROC) curves and the area under the ROC curves (AUCs) were calculated. All statistical analyses were performed using JMP software version 13.2.1 (SAS Institute, Cary, NC, USA).
Results
Study subjects
This study included 668 episodes of 522 patients. Treatments performed for the 668 pneumothoraces and evaluation of the initial treatments are summarized in Figure 2. Of them, 198 events were initially treated by observation, 22 by needle aspiration, and 448 by tube drainage, and pneumothorax was cured in 170 (85.9%), 18 (81.8%), and 289 (64.5%) events, respectively. The duration of observation varied from 2 to 120 days (median, 13 days). Success of the needle aspiration group required a maximum of 2 times of aspiration (2 times in 4 cases). The duration of drainage varied from 1 to 80 days (median, 10 days), depending on the patient’s general condition and comorbidities. Various sizes of catheters were used for tube drainage according to the clinician’s preference. There were 47 events of 195 patients (24.1%) with a low degree of lung collapse treated by tube drainage due to acute symptoms, including 15 PSPs and 32 SSPs. When the air leak persisted after the initial treatment, further treatment was performed to manage the air leak. Except in 2 patients who died due to exacerbation of pneumonitis and progression of malignant tumor, air leaks were stopped by initial or further treatments. After the pneumothorax was cured, surgery and pleurodesis were performed for 47 and 11 patients, respectively, to prevent recurrence.
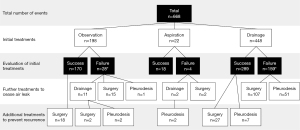
Initial treatments to manage air leak
The characteristics of the 3 groups classified according to the initial treatments are shown in Table 1. The clinical backgrounds of the 3 groups were significantly different in age (P<0.0001), sex (P<0.0001), smoking history (P<0.0001), type of spontaneous pneumothorax (P<0.0001), degree of lung collapse (P<0.0001), and bulla formation (P=0.0013). Aspiration or tube drainage was selected as initial treatment in most cases (88.3%) of high and middle degrees of pneumothorax, and observation was selected as the initial treatment in cases (73.3%) of low degree of pneumothorax.
Table 1
| Variables | Observation (n=198) | Aspiration (n=22) | Drainage (n=448) | P value |
|---|---|---|---|---|
| Age (years), mean | 39.7 | 44.3 | 51.3 | <0.0001 |
| ≥50 years, n (%) | 64 (68.1) | 8 (36.4) | 249 (55.6) | <0.0001 |
| Sex, n (%) | <0.0001 | |||
| Male | 141 (71.2) | 14 (63.6) | 383 (85.5) | |
| Female | 57 (28.8) | 8 (36.4) | 65 (14.5) | |
| Smoking history, n (%) (n=584) | <0.0001 | |||
| ≥10 packs-year | 35 (21.2) | 6 (40.0) | 183 (46.0) | |
| <10 packs-year | 130 (78.8) | 15 (60.0) | 215 (54.0) | |
| Previous episode of ipsilateral pneumothorax, n (%) | 59 (29.8) | 11 (50.0) | 147 (32.8) | 0.15 |
| Side, n (%) | 0.55 | |||
| Right | 99 (50.0) | 11 (50.0) | 244 (54.5) | |
| Left | 99 (50.0) | 11 (50.0) | 204 (45.5) | |
| Type of spontaneous pneumothorax, n (%) | <0.0001 | |||
| PSP | 109 (55.1) | 9 (40.9) | 156 (34.8) | |
| SSP | 89 (44.9) | 13 (59.1) | 292 (65.2) | |
| Degree of lung collapse defined by JSPCLD, n (%) (n=654) | <0.0001 | |||
| High | 3 (1.5) | 0 (0.0) | 171 (39.1) | |
| Middle | 51 (25.9) | 17 (77.3) | 219 (50.1) | |
| Low | 143 (72.6) | 5 (22.7) | 47 (10.8) | |
| Degree of lung collapse defined by ACCP, n (%) (n=654) | ||||
| Large | 54 (27.4) | 17 (77.3) | 389 (89.2) | <0.0001 |
| Small | 143 (72.6) | 5 (22.7) | 47 (10.8) | |
| Radiological finding, n (%) | ||||
| Bulla formation | 99 (50.0) | 14 (63.6) | 293 (65.4) | 0.0013 |
| Pulmonary fibrosis | 15 (7.6) | 1 (4.5) | 18 (4.0) | 0.16 |
PSP, primary spontaneous pneumothorax; SSP, secondary spontaneous pneumothorax; JSPCLD, Japan Society for Pneumothorax and Cystic Lung Disease; ACCP, American College of Chest Physician.
Results of univariate and multivariate analyses for predicting the cessation of air leak by initial treatment are shown in Table 2. Information on smoking history in 84 patients and degree of lung collapse in 14 patients could not be collected. In the univariate analysis, age (P<0.0001), sex (P=0.049), smoking history (P<0.0001), previous episode of ipsilateral pneumothorax (P<0.0001), type of spontaneous pneumothorax (P<0.0001), high and middle degrees of lung collapse (JSPCLD categorization; P<0.0001 and 0.0009, respectively), and treatment option of tube drainage as initial treatment (P<0.0001) were statistically significant for persistent air leak. However, in the multivariate analysis, previous episode of ipsilateral pneumothorax [odds ratio (OR) 1.9; 95% confidence interval (CI): 1.3–2.9; P=0.0022], high degree of lung collapse (OR 2.1; 95% CI: 1.1–4.2; P=0.032), and bulla formation (OR 2.6; 95% CI: 1.7–4.1; P<0.0001) were the significant risk factors for treatment failure. No treatment showed a significant difference for the outcome in multivariate analysis.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR* (95% CI) | P value | OR* (95% CI) | P value | ||
| Age (≥50 years) | 2.1 (1.5–2.9) | <0.0001 | 1.2 (0.6–2.5) | 0.54 | |
| Sex | 0.049 | 0.85 | |||
| Male | 1.6 (1.0–2.5) | 1.1 (0.6–1.9) | |||
| Female | 1 (reference) | 1 (reference) | |||
| Smoking history (≥10 packs-year) | 2.4 (1.6–3.4) | <0.0001 | 1.2 (0.7–2.2) | 0.48 | |
| First or repeated episode of ipsilateral pneumothorax | <0.0001 | 0.0022 | |||
| First | 1 (reference) | 1 (reference) | |||
| Repeated | 2.3 (1.6–3.2) | 1.9 (1.3–2.9) | |||
| Side | 0.093 | 0.33 | |||
| Right | 1 (reference) | 1 (reference) | |||
| Left | 0.7 (0.5–1.1) | 0.8 (0.6–1.2) | |||
| Type of spontaneous pneumothorax | <0.0001 | 0.20 | |||
| PSP | 1 (reference) | 1 (reference) | |||
| SSP | 2.1 (1.5–3.0) | 1.6 (0.8–3.3) | |||
| Degree of lung collapse defined by JSPCLD | <0.0001 | 0.082 | |||
| High | 3.5 (2.1–5.7) | <0.0001 | 2.1 (1.1–4.2) | 0.032 | |
| Middle | 2.2 (1.4–3.5) | 0.0009 | 1.5 (0.8–2.7) | 0.21 | |
| Low | 1 (reference) | 1 (reference) | |||
| Radiological finding | |||||
| Bulla formation | 3.1 (2.1–4.6) | <0.0001 | 2.6 (1.7–4.1) | <0.0001 | |
| Pulmonary fibrosis | 0.9 (0.4–2.0) | 0.78 | |||
| Initial treatments | <0.0001 | 0.18 | |||
| Observation | 1 (reference) | 1 (reference) | |||
| Aspiration | 1.3 (0.4–4.3) | 0.61 | 0.7 (0.2–2.5) | 0.62 | |
| Drainage | 3.3 (2.1–5.2) | <0.0001 | 1.5 (0.8–2.8) | 0.19 | |
*, >1 indicates risk factor and <1 indicates preventive factor. OR, odds ratio; CI, confidence interval; PSP, primary spontaneous pneumothorax; SSP, secondary spontaneous pneumothorax; JSPCLD, Japan Society for Pneumothorax and Cystic Lung Disease.
Comparison of categorizations of lung collapse between JSPCLD and ACCP for predicting treatment failure showed that the AUCs of JSPCLD and ACCP were 0.62 and 0.59, respectively. The sensitivity and specificity of high-degree pneumothorax in JSPCLD were 0.78 and 0.38 (respectively) and 0.34 and 0.85 (respectively) for large pneumothorax in ACCP.
Recurrences after the last treatments
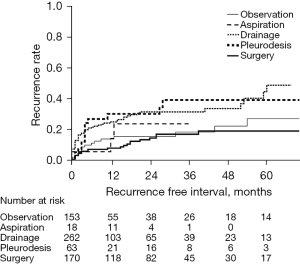
Eighteen events under observation and twenty-seven events with tube drainage underwent surgery after success in initial management to prevent recurrence because of previous episodes (shown in Figure 2). Recurrences of ipsilateral pneumothorax after the last treatments are shown in Table 3. Recurrence of ipsilateral pneumothorax was observed in 126 cases: 18 in the observation group, 3 in the aspiration group, 67 in the tube drainage group, 15 in the pleurodesis group, and 23 in the surgery group. The mean follow-up period was 18.4 months (range, 0–124 months). Figure 3 shows the Kaplan-Meier curve of recurrence according to the last treatments in all 666 pneumothoraces; the curve of the recurrence rate in the observation group overlaps with that in the surgery group. Statistical analysis by the log-rank test showed a significant relationship between observation and tube drainage (P=0.0029), tube drainage and surgery (P<0.0001), and pleurodesis and surgery (P=0.0024).
Table 3
| Treatment | Number of treatments, n (%) | Number of recurrences, n (recurrence rate, %) |
|---|---|---|
| Total | 666* (100.0) | 126 (18.9) |
| Observation | 153 (23.0) | 18 (11.8) |
| Aspiration | 18 (2.7) | 3 (16.7) |
| Drainage | 262 (38.3) | 67 (25.6) |
| Pleurodesis | 63 (9.5) | 15 (23.8) |
| Surgery | 170 (25.5) | 23 (13.5) |
*, two patients died before cessation of air leak were excluded.
Results of univariate and multivariate analyses for predicting the recurrence of ipsilateral pneumothorax according to the last treatment are shown in Table 4. In univariate and multivariate analyses, previous episode of ipsilateral pneumothorax was the significant risk factor for recurrence [hazard ratio (HR) 1.4; 95% CI: 1.0–2.0; P=0.047 and HR 1.8; 95% CI: 1.2–2.5; P=0.0032, respectively]. Tube drainage as the last treatment was a significant risk factor for recurrence in univariate analysis but not in multivariate analysis (HR 2.1; 95% CI: 1.2–3.6; P=0.0029 and HR 1.7; 95% CI: 0.9–3.2; P=0.10, respectively). Degree of lung collapse was not a significant risk factor for recurrence in univariate and multivariate analyses.
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR* (95% CI) | P value | HR* (95% CI) | P value | ||
| Age (≥50 years) | 0.9 (0.6–1.3) | 0.55 | |||
| Sex | 0.26 | ||||
| Male | 0.8 (0.5–1.2) | ||||
| Female | 1 (reference) | ||||
| Smoking history (≥10 packs-year) | 0.9 (0.6–1.3) | 0.59 | |||
| First or repeated episode of ipsilateral pneumothorax | 0.047 | 0.0032 | |||
| First | 1 (reference) | 1 (reference) | |||
| Repeated | 1.4 (1.0–2.0) | 1.8 (1.2–2.5) | |||
| Side | 1.0 | ||||
| Right | 1 (reference) | ||||
| Left | 1.0 (0.7–1.4) | ||||
| Type of spontaneous pneumothorax | 0.14 | 0.45 | |||
| PSP | 1 (reference) | ||||
| SSP | 1.3 (0.9–1.9) | 1.2 (0.8–1.7) | |||
| Degree of lung collapse defined by JSPCLD | 0.17 | 0.60 | |||
| High | 1.5 (0.9–2.4) | 0.13 | 1.3 (0.7–2.5) | 0.33 | |
| Middle | 1.5 (1.0–2.4) | 0.072 | 1.3 (0.7–2.2) | 0.36 | |
| Low | 1 (reference) | 1 (reference) | |||
| Radiological finding | |||||
| Bulla formation | 1.2 (0.9–1.8) | 0.27 | |||
| Pulmonary fibrosis | 0.3 (0.1–1.1) | 0.075 | 0.4 (0.1–1.4) | 0.20 | |
| Last treatment | 0.0003 | 0.0005 | |||
| Observation (n=153) | 1 (reference) | 1 (reference) | |||
| Aspiration (n=18) | 1.3 (0.4–4.6) | 0.65 | 0.9 (0.3–3.3) | 0.92 | |
| Drainage (n=262) | 2.1 (1.2–3.6) | 0.0029 | 1.7 (0.9–3.2) | 0.10 | |
| Pleurodesis (n=63) | 2.0 (1.0–3.9) | 0.060 | 1.4 (0.6–3.0) | 0.43 | |
| Surgery (n=170) | 0.8 (0.4–1.4) | 0.42 | 0.6 (0.3–1.2) | 0.11 | |
*, >1 indicates risk factor and <1 indicates preventive factor. HR, hazard ratio; CI, confidence interval; PSP, primary spontaneous pneumothorax; SSP, secondary spontaneous pneumothorax; JSPCLD, Japan Society for Pneumothorax and Cystic Lung Disease.
Discussion
In this study, we searched the independent risk factors leading treatment failure and recurrence of pneumothorax based on the initial treatment choice. The first analysis identified risk factors to predict treatment failure and the second identified risk factors to predict recurrence after the last treatments. In the multivariate analyses, no significant difference between observation and intervention was present either in the outcomes of treatment failure or recurrence. Moreover, the type of pneumothorax between primary and secondary did not differ in outcomes of either treatment failure or recurrence.
In the analyses of success in initial treatment, the success rates of the aspiration group and the drainage group were 81.5% (19 of 22 cases) and 64.5% (289 of 448 cases), respectively. The result that drainage failure rate is higher than aspiration is consistent with a previous report (9). The first multivariate analysis for predicting failure after initial treatments, which required additional treatment to cease air leak, showed statistical significances in previous episodes of ipsilateral pneumothorax, high degree of lung collapse, and radiological findings of bulla formation. Initial treatment was not an independent risk factor for the outcome of air leaks in this study. This result is not consistent with findings in previous reports (10,11). And very few reports evaluated success in cessation of air leak in comparison between observation and invasive treatment (11,12). Those studies did not adjust the outcomes with the degree of lung collapse. Size of pneumothorax may be a potential confounding factor, because that was used as an indication of treatment selection, in accordance with the clinical guidelines (3,4). Lung collapse on chest radiograph does not always represent ongoing air leak, although a high degree of lung collapse was a risk factor for treatment failure in this study. Recent research in PSP reported that observation was safe even for patients with moderate to large collapse in specific situations (7). Furthermore, recent report in SSP showed that more conservative management would be appropriate in largely collapsed lung, even though this goes against the recommendations in clinical guidelines (8). Less invasive management of patients with use of ambulatory device was reported to be effective (13). Conversely, tube drainage risks critical complications (7,14). Based on the findings of our analysis, less invasive treatments: conservative observation, would be attempted in patients with mild and moderate lung collapse without risk factors, i.e., previous episode and bulla formation. Although the presence of bulla can be confirmed by plain X-ray, CT is more reliable. Thus, as the presence of bulla is a predictor of air leak persistence, CT scan is recommended.
The second recurrence analysis showed that the previous episode of ipsilateral pneumothorax was the only risk factor for ipsilateral recurrence. This finding is consistent with recommendations in BTS guidelines 2010 (4). Kaplan-Meier analysis showed that observation and surgery were superior to tube drainage among the last treatments. When adjusted by clinical background factors in multivariate analysis, tube drainage showed a high hazard ratio for recurrence but was not statistically significant. This tendency is consistent with Brown’s report, which showed a two-fold higher recurrence rate in intervention compared to that in observation (7). Further, large pneumothorax was not an independent risk factor for recurrence. As we consider that initial management is intended to manage air leaks (9), recurrence prevention should be followed if necessary. Therefore, among patients with a previous episode of ipsilateral pneumothorax, those showed no air leak with tube drainage may be recommended to be treated with additional treatment to prevent recurrence.
The recurrence rate of the surgery group in our study was 13.5%, which was higher than expected. One reason for this high recurrence rate in the surgery group may involve the patients’ characteristics. A previous study showed that the postoperative recurrence rate in younger patients with PSP exceeds 20% in long-term follow-up (15). The recurrence rate of pleurodesis was as high as that of tube drainage in our analysis. Talc was not available in Japan during the study period; however, usage of talc would improve efficacy of pleurodesis.
Our study included PSP and SSP. In our cohort, PSP and SSP were not independent risk factors for either the treatment failure and recurrence, regardless of the initial management. These findings contradict those of a previous report; SSP has been reported to be associated with higher morbidity and mortality than PSP (16), and the BTS guideline states that a distinction between PSP and SSP should be made at the time of diagnosis to guide appropriate management (4). In clinical practice, we sometimes encounter difficulty in distinguishing PSP from SSP. These findings are consistent with recent opinions that pointed out that spontaneous pneumothorax should be initially treated the same way regardless of PSP or SSP distinction (6,17).
Our study has several limitations. Due to the retrospective design of this study, the choice of initial treatment would be affected by patient’s background. Although we performed multivariate analysis, further confirmation is required to clarify these findings. Body mass index and smoking habit during the follow-up period, which were reported as risk factors for recurrence of PSP in several studies (18,19), were unavailable in most patients of this study and, therefore, were not examined. Moreover, as this was a retrospective study from a single institution, unknown confounding factors may affect the prognosis. For example, several studies reported that the water seal setting was favorable in cessation of air leak compared to continuous suction (20-22), but the data regarding this was unavailable in this study. Thus, we could not separately evaluate the two types of management of tube drainage. Our recent study showed that measurement of the intrapleural pressure is useful for predicting persistent air leak determining the indication for initial intervention in pneumothorax (23). In this study, the presumption of air leak was determined clinically by the presence of symptoms and chest radiography findings. In a future study, considering the measurement of the intrapleural pressure may facilitate more appropriate management of air leaks.
Conclusions
The multivariate analyses with clinical background factors including size of the pneumothorax showed that risk factors for treatment failure were previous episode of ipsilateral pneumothorax, high degree of lung collapse, and radiological bulla formation. The risk factor for recurrence after the last treatment was a previous episode of ipsilateral pneumothorax. Observation was superior to tube drainage in success rate to cease air leak and recurrence rate, although this effect was not statistically significant. Owing to treatment invasiveness, observation may be recommended to be firstly attempted in patients without risk factors. Further confirmation is required to clarify these findings in a prospective study.
Acknowledgments
We thank Editage (www.editage.jp) for English language editing. No funding was obtained for this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1486/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1486/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1486/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Kansai Medical University (approval date: May 28, 2018; approval No. 2017320). The requirement for informed consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leigh-Smith S, Harris T. Tension pneumothorax--time for a re-think? Emerg Med J 2005;22:8-16. [Crossref] [PubMed]
- Simpson G. Spontaneous pneumothorax: time for some fresh air. Intern Med J 2010;40:231-4. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Carson-Chahhoud KV, Wakai A, van Agteren JE, et al. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev 2017;9:CD004479. [Crossref] [PubMed]
- Thelle A, Gjerdevik M. Randomised comparison of needle aspiration and chest tube drainage in spontaneous pneumothorax. Eur Respir J 2017;49:1601296. [Crossref] [PubMed]
- Brown SGA, Ball EL, Perrin K, et al. Conservative versus Interventional Treatment for Spontaneous Pneumothorax. N Engl J Med 2020;382:405-15. [Crossref] [PubMed]
- Gerhardy BC, Simpson G. Conservative versus invasive management of secondary spontaneous pneumothorax: a retrospective cohort study. Acute Med Surg 2021;8:e663. [Crossref] [PubMed]
- Kaneda H, Nakano T, Taniguchi Y, et al. Three-step management of pneumothorax: time for a re-think on initial management. Interact Cardiovasc Thorac Surg 2013;16:186-92. [Crossref] [PubMed]
- Andrivet P, Djedaini K, Teboul JL, et al. Spontaneous pneumothorax. Comparison of thoracic drainage vs immediate or delayed needle aspiration. Chest 1995;108:335-9. [Crossref] [PubMed]
- Kelly AM, Kerr D, Clooney M. Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest 2008;134:1033-6. [Crossref] [PubMed]
- Chen JS, Hsu HH, Kuo SW, et al. Management of recurrent primary spontaneous pneumothorax after thoracoscopic surgery: should observation, drainage, redo thoracoscopy, or thoracotomy be used? Surg Endosc 2009;23:2438-44. [Crossref] [PubMed]
- Hallifax RJ, McKeown E, Sivakumar P, et al. Ambulatory management of primary spontaneous pneumothorax: an open-label, randomised controlled trial. Lancet 2020;396:39-49. [Crossref] [PubMed]
- Harris A, O'Driscoll BR, Turkington PM. Survey of major complications of intercostal chest drain insertion in the UK. Postgrad Med J 2010;86:68-72. [Crossref] [PubMed]
- Hung WT, Chen HM, Wu CH, et al. Recurrence rate and risk factors for recurrence after thoracoscopic surgery for primary spontaneous pneumothorax: A nationwide population-based study. J Formos Med Assoc 2021;120:1890-6. [Crossref] [PubMed]
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [Crossref] [PubMed]
- Bintcliffe OJ, Hallifax RJ, Edey A, et al. Spontaneous pneumothorax: time to rethink management? Lancet Respir Med 2015;3:578-88. [Crossref] [PubMed]
- Lippert HL, Lund O, Blegvad S, et al. Independent risk factors for cumulative recurrence rate after first spontaneous pneumothorax. Eur Respir J 1991;4:324-31. [Crossref] [PubMed]
- Cook CH, Melvin WS, Groner JI, et al. A cost-effective thoracoscopic treatment strategy for pediatric spontaneous pneumothorax. Surg Endosc 1999;13:1208-10. [Crossref] [PubMed]
- Ayed AK. Suction versus water seal after thoracoscopy for primary spontaneous pneumothorax: prospective randomized study. Ann Thorac Surg 2003;75:1593-6. [Crossref] [PubMed]
- Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg 2001;71:1613-7. [Crossref] [PubMed]
- Marshall MB, Deeb ME, Bleier JI, et al. Suction vs water seal after pulmonary resection: a randomized prospective study. Chest 2002;121:831-5. [Crossref] [PubMed]
- Kaneda H, Nakano T, Murakawa T. Measurement of intrapleural pressure in patients with spontaneous pneumothorax: a pilot study. BMC Pulm Med 2019;19:267. [Crossref] [PubMed]



