
Long-term follow up and comparison between conservative and interventional therapy in postoperative bronchopleural fistula—a cohort study
Highlight box
Key findings
• Survival rates were better in the interventional therapy during the 28-day and the 90-day follow up (P=0.001, 43.40% vs. 76.92%; P=0.006, 35.85% vs. 66.67%).
What is known and what is new?
• Bronchopleural fistula (BPF) is a relatively rare postoperative complication with high mortality. The management is tough and controversial.
• We reviewed and followed postoperative BPF patients in a high-volume single center for ten years. Survival rates were better in the interventional therapy during the 28-day and the 90-day follow up.
What is the implication, and what should change now?
• Surgical and bronchoscopic interventions are recommendable in BPF as they guarantee better short and long-term outcomes compared with the conservative therapy.
Introduction
Bronchopleural fistula (BPF) is a relatively rare postoperative complication with high mortality. It is defined as a shunt between the pleural space and the bronchial tree or the lung parenchyma. The incidence ranges from 0.5% to 20% in different procedures and centers while the mortality rate could reach 70% (1). Surgical procedures, malignancy, poor nutritional status, neoadjuvant chemoradiation and diabetes are known as the risk factors of BPF (2). Since the management of BPF with continuous empyema, and the following infection remain challenging and controversial, we try to conclude our own strategy and experience of treatment in postoperative BPF. In this article, we reviewed and followed postoperative BPF patients in a high-volume single center (over 15,000 thoracic procedures per year and 21,353 thoracic procedures in 2021). We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1426/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by Shanghai Chest Hospital Clinical Research Ethics Committee (IS2191, 30th September, 2021). Every patient had signed the individual informed consents before surgery so that their history and examination data could be collected and analyzed.
Study design
From June 2011 to June 2020, 73,149 patients underwent general thoracic surgery in Shanghai Chest Hospital, Shanghai Jiao Tong University. Among them, 68,953 cases were non-esophageal thoracic surgery, and the others were esophageal surgery. All the operations were performed by well-experienced surgeons who had completed more than 500 thoracic operations.
The BPF was diagnosed according to clinical manifestation, radiographic imaging, and bronchoscopy. The inclusion criteria of our research were BPF patients with malignancies, aged from 18 to 80 years old. Transplantation or benign tumor patients were excluded. All patients were retrospectively analyzed with follow-up time ranged from 20 months to 10 years by telephone or outpatient department (Figure S1 and Table S1). All patients were divided into two groups: the conservative group and the interventional group. The interventional group received surgical or bronchoscopic reintervention besides medication and drainage, while the conservative group only received conservative medication (antibiotics, nutrition support), mechanical ventilation and drainage. We took a retrospective analysis on perioperative and follow-up data between the two groups. The overall flow chart is shown in Figure S2.
First surgical technique
The first surgical technique of BPF patients in our study were as follows: VATS right or left pulmonary lobectomy, pulmonary sleeve lobectomy, esophageal resection, and digestive tract reconstruction, tracheal resection and reconstruction, and pneumonectomy (Table 1).
Table 1
| First operation | BPF No./No. (%) |
|---|---|
| VATS right pulmonary lobectomy | 39/16,250 (0.24) |
| VATS left pulmonary lobectomy | 8/7,273 (0.11) |
| Pulmonary sleeve lobectomy | 14/7,050 (0.20) |
| Esophageal resection and digestive tract reconstruction | 18/4,196 (0.43) |
| Tracheal resection and reconstruction | 2/278 (0.72) |
| Pneumonectomy | 11/593 (1.85) |
The percentage means the ratio of BPF patients who underwent this procedure in our study and all patients of this procedure within 10 years in our center. BPF, bronchopleural fistula; VATS, video-assisted thoracoscopy.
Interventional technique
Flexible covered stents by bronchoscopy, partial lung resection, BPF repair, debridement, amplaza occlusion, thoracoplasty, repair + flexible covered stent, sleeve resection + flexible covered stent, debridement + flexible covered stent were included in interventional treatment (Table 2). For surgical procedures, pericardium, intercostal muscle, serratus anterior muscle, or greater omentum were used for wrapping the fistula.
Table 2
| Reintervention surgery | Case |
|---|---|
| Tracheal or bronchial repair | 9 |
| Bronchoscopic stent implantation | 12 |
| Debridement and drainage | 2 |
| Partial lobectomy | 10 |
| Sleeve resection + stent implantation | 1 |
| Debridement + stent implantation | 2 |
| Fistula repair + stent implantation | 1 |
| Bronchoscopic Amplatzer implantation | 1 |
| Thoracoplasty | 1 |
BPF, bronchopleural fistula.
Statistical analysis
All statistical analyses were performed by SPSS 25.0 software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) and R language (R Version 4.2.1; CRAN; https://www.r-project.org/) for windows. The test of homogeneity variance was used for the determination of quantitative data distribution. When the distribution of variables was normal, independent samples Student’s t-test was used for comparison of quantitative sizes of two independent samples and continuous variables were stated as mean (standard deviation). Non-normally distributed variables were presented as median with interquartile range (IQR), and the Mann-Whitney U test was performed. The dependent of a categorical variable was evaluated by Chi-squared (χ2) criterion. When marginal observed frequencies were smaller than 5, Fisher’s exact test was used.
The baseline characters were analyzed by independent t-test analysis in 92 cases (Table 3). All analyses in our study were according to the guideline (3). Cox proportional hazards regression mode was adopted to identify significantly different factors between the two groups in the 90-day mortality for postoperative BPF. Survival was analyzed by the Kaplan-Meier method and log-rank test. A P value of less than 0.05 was considered statistically significant.
Table 3
| Characteristic | N (%) or (SD) or median [IQR] | P | |
|---|---|---|---|
| Conservative | Interventional | ||
| Age (years) | 62.98 (8.76) | 63.36 (7.27) | 0.827 |
| Gender (male) | 50 (94.34%) | 38 (97.44%) | 0.635 |
| Neoadjuvant chemotherapy | 3 (5.66%) | 2 (5.13%) | – |
| APACHE II score | 10.58 (4.61) | 9.92 (4.45) | 0.492 |
| TNM stage | 0.286 | ||
| 1 | 11 (20.75%) | 7 (17.95%) | |
| 2 | 13 (24.53%) | 14 (35.90%) | |
| 3 | 20 (37.74%) | 16 (41.03%) | |
| 4 | 9 (16.98%) | 2 (5.13%) | |
| BPF onset (days) | 9 [6, 13.5] | 11 [8, 21] | 0.895 |
| Intubation duration (days) | 2 [0.5, 7] | 1 [1, 9] | 0.341 |
| Start of intervention (days) | 11 [8, 19.5] | 16 [10, 25] | 0.845 |
Conservative: conservative treatment (n=53). Interventional: interventional treatment (n=39). BPF, bronchopleural fistula; APACHE II score, acute physiology and chronic health evaluation (acute physiology score, chronic health score and age); T, tumor; N, regional lymph node; M, metastasis; SD, standard deviation; IQR, interquartile range.
Results
From June 2011 to June 2020, 103 patients who had undergone general thoracic surgery were diagnosed as BPF in Shanghai Chest Hospital. The first surgery procedures are shown in Table 1. Incidence of BPF in pulmonary surgery was 0.299% while that of esophageal surgery was 0.43%. There were no significant differences in baseline characters after univariable analysis of 92 BPF cases. The patients ranged in age from 47 to 79 years old, with the mean age of 63.14 (8.12). Male accounts for 94.74%. The overall mortality of 28- and 90-day were 42.39% and 51.09% respectively. Reintervention types and case numbers are shown in Table 2. There are 39 cases underwent interventional treatment. Among 39 cases, 12 flexible covered stents, 10 partial lung resections, 9 BPF repairs, 2 debridements, 1 amplaza occlusion, 1 thoracoplasty, 1 repair + flexible covered stent, 1 sleeve resection + flexible covered stent, and 2 debridements + flexible covered stents were included.
Univariable analyses of all perioperative characteristics in two groups are shown in Table 3. Significant differences were identified in 28- and 90-day survivals between the two groups (P=0.001, 43.40% vs. 76.92%; P=0.006, 35.85% vs. 66.67%). Kaplan-Meier survival curve for 28-day, 90-day, and 5-year survivals is shown in Figures 1-3. Patients in the interventional group present better survival rate than that in the conservative group. No significant difference was found in the 5-year survival rate between the two groups (P=0.443). As shown in Table 4, Cox proportional hazards regression model analysis confirmed that simple conservative therapy was independently associated with postoperative 90-day mortality of BPF (P=0.002, HR =2.913, 95% CI: 1.480–5.731).
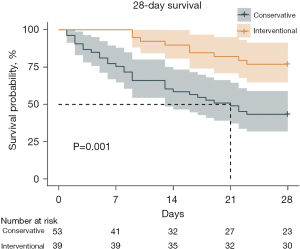
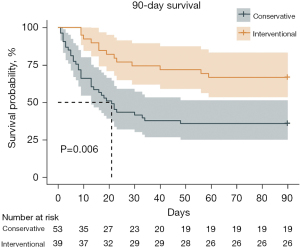
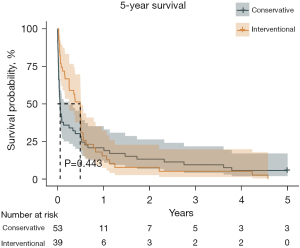
Table 4
| Variables | B | S.E. | Wals | df | P | HR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Step1a | Age | −0.001 | 0.018 | 0.001 | 1 | 0.971 | 0.999 | 0.965–1.035 |
| APACHE II | 0.027 | 0.033 | 0.664 | 1 | 0.415 | 1.028 | 0.963–1.097 | |
| TNM stage | – | – | 3.700 | 3 | 0.296 | – | – | |
| TNM I | −0.812 | 0.590 | 1.891 | 1 | 0.169 | 0.444 | 0.140–1.412 | |
| TNM II | 0.008 | 0.488 | 0.000 | 1 | 0.987 | 1.008 | 0.387–2.624 | |
| TNM III/IV | 0.178 | 0.434 | 0.168 | 1 | 0.682 | 1.194 | 0.511–2.794 | |
| BPF onset | 0.029 | 0.020 | 2.241 | 1 | 0.134 | 1.030 | 0.991–1.070 | |
| Start of intervention | −0.020 | 0.019 | 1.067 | 1 | 0.302 | 0.980 | 0.944–1.018 | |
| Intubation duration | −0.008 | 0.012 | 0.489 | 1 | 0.484 | 0.992 | 0.968–1.015 | |
| Conservative therapy | 1.069 | 0.345 | 9.582 | 1 | 0.002 | 2.913 | 1.480–5.731 |
a, variable(s) entered on step 1: age, APACHE II, TNM stage, BPF onset, start of intervention, intubation duration, non-intervention. BPF, bronchopleural fistula; APACHE II score, acute physiology and chronic health evaluation (acute physiology score, chronic health score and age); T, tumor; N, regional lymph node; M, metastasis; B, Regression coefficient; S.E., standard error; Wals, statistics of test; df, degree of freedom; HR, hazards ratio; 95% CI, 95% confidence interval.
Discussion
As is known to all, BPF resulted in continuous air leak. In our center, BPF was found by continuous air leak for more than three days after surgery or new onset air leak for more than 24 hours and then diagnosis was confirmed by bronchoscopy. All cases in our study were leakage between pleural and supralobar bronchus. There was no leakage from parenchyma to pleural which was also called alveoli pleural fistula in our data. The incidence of postoperative BPF is about 2–5% in lung cancer and tuberculosis (4-7). BPF is not a common complication (1,8). However, it remains challenging and leads to high mortality for a series of severe and tough consequences. The mortality rate of BPF ranges from 16% to 72%. In our center, the perioperative mortality is 42.39%. Besides, short-term (28-day) and mid-term (90-day) mortality were significantly related to postoperative BPF. Surgical procedures are the leading cause of BPF (9,10). As a high volume center, lobectomy and segmentectomy are the most popular operation for pulmonary tumor in our center. A meta-analysis showed the diabetes mellitus was an independent risk factor for BPF (4). Besides, malignancy, poor nutritional status, neoadjuvant chemoradiation and surgery played a potential impact on higher incidence of BPF (2,7,11-13). In the two groups, 41 fistula occurred within 7 days after surgery, 9 occurred after 30 days and the rest occurred between 7 and 30 days in our study. According to our data, anti-BPF prophylaxis such as muscle flap or pericardium wrapping were used in tracheal resection and carina reconstruction or pulmonary sleeve lobectomy during the first surgery. However, muscle flap, pericardium and omentum wrapping were regularly used in the second surgical procedure.
Our diagnosis of BPF is made by a combination of clinical, radiographic, and bronchoscopic findings. BPF occurs with a large quantity of sputum, sudden or progressive hypoxia, change of drainage, continuous air leakage and extensive pulmonary effusion in X-ray or CT. It characteristically manifests within two weeks postoperatively. Persistent air leakage and infection were associated with mechanical ventilation, prolonged ICU duration, prolonged hospital stays and higher morbidity or mortality as well. In our study, whenever related manifestation came out, surgeons and critical care medicine physicians confirmed the diagnosis by bronchoscopy. Although BPF is a life-threatening complication, patients may get a favorable turn by proper manipulation in this duration. In our study, there was no difference on age, APACHE II score, TNM stage, BPF onset, start of intervention and intubation duration between the two groups in the Cox’s hazards regression model. That is to say, BPF patients were almost in same critical condition before decisions were carried out.
The location and onset phase of BPF may greatly influence the types and timing of reintervention (14). Central BPFs which result from trauma or surgical procedures are usually bigger with high morbidity and mortality. Peripheral BPFs are smaller and typically caused by neoplasms, necrosis, bronchiectasis, or iatrogeny (4,5,7,15). BPF could also be classified by onset phase as early (1–7 days), intermediate (8–30 days), and late (>30 days) (6,8). Endoh et al. (16) showed their omental flap in treatment of BPF. Han et al. (17) reported 148 cases early stage BPF intervened by covered airway stent. Wang et al. (18) reviewed their 12 cases mid stage BPF treated by extra lobectomy. In our center, decisions were carried out according to the surgeons’ experiences. Central, early phase and localized BPF underwent tracheal or bronchial repair or residual lobectomy. Surgical reintervention may be performed within 48 hours after onset. When BPF patients performed as a poor clinical status, strategies might be conservative management rather than surgical intervention. Nowadays, even for patients with poor status, we choose trans-bronchoscopy stent to intervene BPF for better airway management (19,20). Reintervention strategy differed from person to person. Furthermore, mechanical ventilation and preoperative pleuropulmonary infection might be correlated with postoperative BPF (1,12,21,22). We decreased peak inspiratory pressure (PIP) by using lower tidal volumes, lower PEEP, decreasing the inspiratory time, and decreasing the respiratory rate to improve BPF patients’ ventilation.
As a result of poor clinical status, strategies might be conservative management rather than surgical intervention. However, chest drainage, antibiotics, obliteration of residual pleural cavity might help less. In other words, treatment might be delayed. Size of fistula and detect of BPF onset are two critical points for prognosis and management (6,7,14,21,23,24). Surgical management might not be feasible in patients with severe hypoxemia or significant comorbidities. Encouragingly, less invasive new procedures by bronchoscopic closure is full of potential (9,14,21,23-26). There is no evidence in the literature regarding the timing of intervention which could be the key to the favorable outcome (9). In our study, simple conservative therapy was independently associated with the 90-day mortality which means surgical or bronchoscopic intervention played an essential role in the 90-day survival of postoperative BPF. Moreover, in Kaplan-Meier curve, interventional treatment presented better outcome than that of conservative treatment in 28- and 90-day survivals. However, we found that long-term outcome had less relationship with postoperative BPF. Long-term outcome may be much more influenced by tumor or intrinsic disease of patients.
This research has several limitations. First, the patient population enrolled for analysis was small since BPF is a rare and high mortality complication. Second, this is a single-center investigation, although our center has been one of the high-volume centers of thoracic surgery. Third, owing to their critical status, the patients in this study did not undergo full examinations. Finally, as this is a retrospective study, potential bias from misclassification could not be completely excluded.
Conclusions
In conclusion, BPF is one of the most critical complications after general thoracic surgery. Muscle flap or pericardium wrapping were used in tracheal resection and carina reconstruction or pulmonary sleeve lobectomy in first surgery to prevent BPF in our center. In our study, the interventional group presented better outcome in 28- and 90-day follow up. That is to say, surgical or bronchoscopic intervention of BPF played important roles in 28- and 90-day outcomes. Surgical and bronchoscopic interventions are recommendable in postoperative BPF as they guarantee better short-term and long-term outcomes compared with the conservative therapy.
Acknowledgments
We would like to thank all the surgeons in the Department of Thoracic Surgery in Shanghai Chest Hospital, who perform more than 15,000 surgeries every year. We would also give great gratitude to Ms. Liu Yuan of the statistical center in Chest Hospital and our friend Ms. Wang Jie as a professional editor in polishing our manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1426/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1426/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1426/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shanghai Chest Hospital Clinical Research Ethics Committee (IS2191, 30th September, 2021) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grotberg JC, Hyzy RC, De Cardenas J, et al. Bronchopleural Fistula in the Mechanically Ventilated Patient: A Concise Review. Crit Care Med 2021;49:292-301. [Crossref] [PubMed]
- Peng Z, Mei J, Liu C, et al. Risk factors and outcomes of bronchopleural fistula after bronchoplasty in patients with non-small cell lung cancer: a retrospective multivariate analysis. Transl Lung Cancer Res 2022;11:744-56. [Crossref] [PubMed]
- Hickey GL, Dunning J, Seifert B, et al. Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur J Cardiothorac Surg 2015;48:180-93. [Crossref] [PubMed]
- Li SJ, Fan J, Zhou J, et al. Diabetes Mellitus and Risk of Bronchopleural Fistula After Pulmonary Resections: A Meta-Analysis. Ann Thorac Surg 2016;102:328-39. [Crossref] [PubMed]
- Wu G, Zeng Y, Yin M, et al. A novel treatment for bronchopleural fistula after pulmonary resection by interventional technique. J Thorac Dis 2019;11:E135-7. [Crossref] [PubMed]
- Oki M, Seki Y. A customized, covered metallic stent to repair a postoperative bronchopleural fistula: a promising endobronchial approach. J Thorac Dis 2019;11:1088-90. [Crossref] [PubMed]
- van de Pas JM, van Roozendaal LM, Wanders SL, et al. Bronchopleural Fistula After Concurrent Chemoradiotherapy. Adv Radiat Oncol 2020;5:511-5. [Crossref] [PubMed]
- Ho E, Srivastava R, Hegde P. Bronchopleural Fistula Closure With Amplatzer Device: Our Case and Reviewing a Decade of Experience. J Bronchology Interv Pulmonol 2020;27:e41-e45. [Crossref] [PubMed]
- Cusumano G, Alifano M, Lococo F. Endoscopic and surgical treatment for bronchopleural fistula after major lung resection: an enduring challenge. J Thorac Dis 2019;11:S1351-6. [Crossref] [PubMed]
- Yang YH, Park SY, Kim HE, et al. Postoperative bronchopleural fistula repair: Surgical outcomes and adverse factors for its success. Thorac Cancer 2022;13:1401-5. [Crossref] [PubMed]
- Bribriesco A, Patterson GA. Management of postpneumonectomy bronchopleural fistula. Thorac Surg Clin 2018;28:323-35. [Crossref] [PubMed]
- Andreetti C, Menna C, D'Andrilli A, et al. Multimodal Treatment for Post-Pneumonectomy Bronchopleural Fistula Associated With Empyema. Ann Thorac Surg 2018;106:e337-9. [Crossref] [PubMed]
- Mazzella A, Bertolaccini L, Sedda G, et al. Pneumonectomy and broncho-pleural fistula: predicting factors and stratification of the risk. Updates Surg 2022;74:1471-8. [Crossref] [PubMed]
- Zhang C, Pan Y, Zhang RM, et al. Late-onset bronchopleural fistula after lobectomy and adjuvant chemotherapy for lung cancer: A case report and review of the literature. Medicine (Baltimore) 2019;98:e16228. [Crossref] [PubMed]
- Marques P, Andrade G, Granadas J, et al. Iatrogenic Bronchopleural Fistula. Cureus 2020;12:e12187. [PubMed]
- Endoh H, Yamamoto R, Nishizawa N, et al. Thoracoscopic surgery using omental flap for bronchopleural fistula. Surg Case Rep 2019;5:5. [Crossref] [PubMed]
- Han X, Yin M, Li L, et al. Customized airway stenting for bronchopleural fistula after pulmonary resection by interventional technique: single-center study of 148 consecutive patients. Surg Endosc 2018;32:4116-24. [Crossref] [PubMed]
- Wang Y, Zhuang W. Treat bronchopleural fistula after right lower lobectomy by extra right middle lobectomy—a neglected approach. INTERACT CARDIOV TH 2020;31:63-70. [Crossref] [PubMed]
- Skrzypczak P, Roszak M, Kasprzyk M, et al. The technique of stump closure has no impact on post-pneumonectomy bronchopleural fistula in the non-small cell lung cancer—a cross-sectional study. J THORAC DIS 2022;14:3343-51. [Crossref] [PubMed]
- Li X, Wang S, Yin M, et al. Treatment of peripheral bronchopleural fistula with interventional negative pressure drainage. Ther Adv Respir Dis 2022;16:17534666221111877. [Crossref] [PubMed]
- Motus IY, Bazhenov AV, Basyrov RT, et al. Endoscopic closure of a bronchopleural fistula after pneumonectomy with the Amplatzer occluder: a step forward? Interact Cardiovasc Thorac Surg 2020;30:249-54. [PubMed]
- Bi Y, Zhu X, Yu Z, et al. Clinical outcomes of metallic Y-shaped covered stents for bronchopleural fistula around upper carina after lobectomy. BMC Pulm Med 2019;19:199. [Crossref] [PubMed]
- Harris K. Customized airway stenting for bronchopleural fistula after pulmonary resection by interventional technique: single-center study of 148 consecutive patients. J Thorac Dis 2019;11:S1274-6. [Crossref] [PubMed]
- Fruchter O. Innovating customized stents for the treatment of bronchopleural fistula. J Thorac Dis 2019;11:1097-9. [Crossref] [PubMed]
- S S. I T, H K. Endoscopic treatment of bronchopleural fistula using ethyl-2-cyanoacrylate: A report of two cases. Respir Med Case Rep 2020;30:101123. [Crossref] [PubMed]
- Dugan KC, Laxmanan B, Murgu S, et al. Management of Persistent Air Leaks. Chest 2017;152:417-23. [Crossref] [PubMed]


