
Features of osteoporosis in male patients with bronchiectasis, a cross-sectional study
Highlight box
Key findings
• Factors associated with osteoporosis in males with bronchiectasis include older, low BMI, a smoking history, and a high BSI score.
What is known and what is new?
• Osteoporosis is reported as a comorbidity in patients with bronchiectasis. Studies related to osteoporosis is insufficient in bronchiectasis patients who were not affected by estrogen deficiency.
• The prevalence of osteoporosis was high in male bronchiectasis patients. In these patients not affected by estrogen deficiency, our study found that factors associated with osteoporosis included older, low BMI, a smoking history, and a high BSI score.
What is the implication, and what should change now?
• Considering the high prevalence and the potential disease burden of osteoporosis, early diagnosis of osteoporosis might be of great value in bronchiectasis patients.
Introduction
Bronchiectasis is a chronic airway inflammatory disease (1). Patients with bronchiectasis suffer from recurrent infection, decreased exercise ability, and deterioration of quality of life (2). Bronchiectasis is caused by many diseases, including bacterial infection, post tuberculosis, and due to airway obstruction, to name a few (3). The current treatment modality for stable bronchiectasis includes oral antibiotics and others to improve the healthy status and reduce the frequency of acute exacerbations (2,4). Some studies also reported the use of inhaled corticosteroids (ICS) in patients with bronchiectasis (5,6).
Osteoporosis is a skeleton metabolism disease. Patients with osteoporosis have decreased bone mineral density (BMD) and increased risk of fractures (7). They suffer from pain, a reduced ability to exercise, and a poor quality of life, burdening both themselves and the society. Osteoporosis increases the burden and disease related adverse events of comorbidities in chronic diseases like coronary heart disease, chronic obstructive pulmonary disease (COPD), and hypertension (8-11). Studies reveal osteoporosis as a comorbidity in patients with bronchiectasis (12-14). The reported prevalence of osteoporosis varies in regions with a range of 5.9–30.1% in patients with bronchietasis (12,15). The mechanism that links bronchiectasis and osteoporosis is still unclear. Some characteristics of bronchiectasis, such as chronic inflammation, use of ICS, malnutrition, and a reduced ability to exercise, may lead to an increased prevalence of osteoporosis (16-19). To improve the quality of life, it might be of great value to understand the prevalence and related factors of osteoporosis in patients with bronchiectasis.
Boonen et al. reported a rate of vertebral fracture of 4.9% for male osteoporosis patients (over 50 years old) in 24 months (20). Osteoporosis in men may be ignored despite reports of a high yearly fracture rate (21,22). Moreover, the understanding of osteoporosis in men is mostly inferred from that of osteoporosis in women. As osteoporosis is common in postmenopausal women due to estrogen deficiency, the aetiology of osteoporosis in men is different from that in women. In women, many clinical studies have revealed the disease characteristics of post-menopausal osteoporosis, leading to the development of new drugs to manage osteoporosis and complications. Given the lower prevalence, osteoporosis in men is underestimated, and its diagnosis and treatment are insufficient (21-23). There is evidence that men with osteoporosis prone to more complications and higher fracture-related mortality than women (21,24). Therefore, clarifying the characteristics of male osteoporosis is the key to promote personalized treatment, which is critical for disease management.
At present, there were lacking studies on osteoporosis in male patients with bronchiectasis. The purpose of our study was to explore the characteristics of osteoporosis in male patients with bronchiectasis. We also aimed to explore the risk factors of osteoporosis present in these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-887/rc).
Methods
The cross-sectional study was conducted between January 2017 and December 2019. Male patients, over 50 years old, with stable bronchiectasis were admitted to this study from Guangzhou First People’s Hospital. Bronchiectasis was diagnosed by high-resolution computed tomography (HRCT) as the presence of bronchial dilatation (4). Stable bronchiectasis refers to an absence of aggravation of symptoms or signs, or acute diseases at least 4 weeks before enrollment. Patients excluded from the study were those with an acute disease or uncontrolled chronic diseases including diabetes, hypertension, myocardial infarction, COPD, and malignancy. Male controls over 50 years old were admitted to our study. Controls subjects were matched for age group and selected from medical examination center in the same hospital. HRCT were performed in all controls, of whom with bronchiectasis were excluded. They suffered no acute diseases at least 4 weeks before enrollment. They did not have a history of uncontrolled chronic diseases including diabetes, hypertension, myocardial infarction, COPD, and malignancy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Guangzhou First People’s Hospital (No. K-2018-040-01) and informed consent was taken from all individual participants.
Dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, Massachusetts, USA) was used to test patients’ BMD of the Hip, the lumbar spine, and the femoral neck. The diagnosis of osteoporosis was based on BMD (25). In subjects age 50 and older, it is considered as normal BMD when T score ≥−1; osteopenia when −2.5 < T score < −1.0; and osteoporosis when T score ≤−2.5. The lowest T score were recorded and included in the final analysis.
All measurements were performed at the beginning of the study. Demographic and physical characteristics that were recorded included age, body-mass index (BMI), smoking history, alcohol history, exacerbations in previous year, and fracture history. Laboratory findings included sputum culture and assessment of serum levels of C-reactive protein (CRP) and calcium. The 6-minute walking distance (6-MWD) and lung function test were performed on the same day. Dyspnea was measured using the modified British medical research council questionnaire (mMRC). Bronchiectasis severity index (BSI) score was calculated to assess disease severity (26). The HRCT score was calculated to assess radiological severity using the modified Reiff score, with a possible range from 1 to 18 (27).
Statistical analysis
Normally distributed data were evaluated according to Shapiro-Wilk test. In the normal distribution, continuous variables were expressed as mean and standard deviation (SD). In the non-normal distribution, continuous variable were expressed as median and interquartile range (IQR). Independent t-test or Wilcoxon rank sum test was processed to test continuous variables. Counts and percentages were used to describe categorical variables. For categorical variables, Chi square test or Fisher exact test was processed. When a P value was 0.10 or less in univariable models, determinants were initially included in the multivariable model and then discarded via backward selection. P value was considered statistically significant if less than 0.05. SPSS 22.0 (IBM, Armonk, NY, USA) was used to process the data.
Results
Differences between patients with bronchiectasis and healthy controls
A total of 108 male patients with stable bronchiectasis and 56 healthy controls (Figure 1), average age 61.7 and 62.7 years, respectively (P=0.370), participated in this study (Table 1). Osteoporosis was found in 44 subjects, i.e., 34 bronchiectasis patients and 10 healthy controls (31.5% vs. 17.9%, P=0.001). The mean T score was lower in bronchiectasis patients than in healthy controls (−1.7 vs. −0.9, P<0.001). Compared to healthy controls, bronchiectasis patients were with lower BMI (21.6 vs. 23.0, P=0.007). They also had a less 6-MWD (472.8 vs. 562.3, P<0.001).
Table 1
| Clinical characteristics | Bronchiectasis (n=108) | Controls (n=56) | P value* |
|---|---|---|---|
| Age, mean (SD), years | 61.7 (6.5) | 62.7 (7.2) | 0.370 |
| Body-mass index, mean (SD), kg/m2 | 21.6 (3.2) | 23.0 (2.7) | 0.007* |
| Smoking history, n (%) | 37 (34.3) | 17 (30.4) | 0.240 |
| Alcohol history, n (%) | 36 (33.3) | 12 (21.4) | 0.112 |
| 6-MWD, mean (SD), meters | 472.8 (92.4) | 562.3 (98.1) | <0.001* |
| Treatment with ICS, n (%) | 8 (7.4) | N/A | N/A |
| Daily ICS dose, median [IQR], μg# | 320 [320] | N/A | N/A |
| ICS duration, median [IQR], months | 34 [25] | N/A | N/A |
| Hip BMD T-score, mean (SD) | −0.8 (1.5) | −0.1 (1.5) | 0.003* |
| Lumbar spine BMD T-score, mean (SD) | −0.9 (1.5) | −0.2 (1.5) | 0.002* |
| Femoral neck BMD T-score, mean (SD) | −1.0 (1.6) | −0.5 (1.5) | 0.003* |
| Lowest T-score, mean (SD) | −1.7 (1.3) | −0.9 (1.5) | <0.001* |
| Normal, n (%) | 31 (28.7) | 33 (58.9) | 0.001* |
| Osteopenic, n (%) | 43 (39.8) | 13 (23.2) | |
| Osteoporotic, n (%) | 34 (31.5) | 10 (17.9) |
Smoking history: a history of smoking or ever smoking. #, different ICS were calculated into equivalent doses of budesonide; *, P<0.05. SD, standard deviation; 6-MWD, 6-minute walking distance; ICS, inhaled corticosteroids; N/A, not applicable; IQR, interquartile range; BMD, bone mineral density.
Differences between patients with osteoporosis and without
In comparison to those without osteoporosis, patients with osteoporosis were older (64.8 vs. 60.3 years, P=0.001; Table 2). They also had a lower BMI (20.4 vs. 22.2 kg/m2, P=0.004) and a less 6-MWD (441.4 vs. 487.3 meters, P=0.016). The disease severity evaluated by BSI score was more serious in patients with osteoporosis (9.0 vs. 4.0, P<0.001). They also suffered more acute exacerbations (1.0 vs. 0, P=0.023). The sputum culture (12 vs. 14, P=0.065) were not significantly different between the two groups. There was no difference in the use of ICS between the two groups (3 vs. 5, P>0.999).
Table 2
| Clinical characteristics | With osteoporosis (n=34) | Without osteoporosis (n=74) | P value* |
|---|---|---|---|
| Age, mean (SD), years | 64.8 (6.2) | 60.3 (6.2) | 0.001* |
| Body-mass index, mean (SD), kg/m2 | 20.4 (3.3) | 22.2 (2.9) | 0.004* |
| Smoking history, n (%) | 18 (55.9) | 19 (25.7) | 0.006* |
| Alcohol history, n (%) | 12 (35.3) | 24 (32.4) | 0.232 |
| 6-MWD, mean (SD), meters | 441.4 (81.2) | 487.3 (94.2) | 0.016* |
| mMRC score, median (IQR) | 2.5 (2.3) | 1.5 (2.0) | 0.019* |
| C-reactive protein >6.0 mg/dL, n (%) | 11 (32.4) | 16 (21.6) | 0.232 |
| Serum calcium, mean (SD), mmol/L | 2.42 (0.29) | 2.39 (0.26) | 0.700 |
| HRCT score, median (IQR) | 7.0 (4.3) | 7.0 (6.0) | 0.958 |
| AE in previous year, median (IQR) | 1.0 (3.0) | 0 (2.0) | 0.023* |
| Fracture history, n (%) | 1 (2.9) | 1 (1.4) | >0.999 |
| BSI, median (IQR) | 9.0 (7.5) | 4.0 (2.0) | <0.001* |
| Positive bacterial culture, n (%) | 12 (35.3) | 14 (18.9) | 0.065 |
| Pulmonary function, mean (SD) | |||
| FEV1 % pred | 63.5 (13.0) | 68.6 (12.6) | 0.055 |
| FEV1/FVC % pred | 73.7 (8.8) | 75.4 (7.9) | 0.307 |
| Treatment, n (%) | |||
| Mucoactive treatment | 14 (41.2) | 27 (36.5) | 0.641 |
| Theophylline | 9 (26.5) | 21 (28.4) | 0.837 |
| Long-term oral antibiotics | 7 (20.6) | 10 (13.5) | 0.348 |
| Inhaled corticosteroids | 3 (8.8) | 5 (6.8) | >0.999 |
| Long β2-receptor agonists | 3 (8.8) | 4 (5.4) | >0.999 |
Smoking history: a history of smoking or ever smoking. *, P<0.05. SD, standard deviation; 6-MWD, 6-minute walking distance; mMRC, modified British medical research council questionnaire; IQR, interquartile range; HRCT, high resolution computed tomography; AE, acute exacerbation; BSI, bronchiectasis severity index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Factors associated with osteoporosis
All possible variables associated with osteoporosis were assessed by univariable analysis. These variables comprised age, BMI, smoking history, 6-MWD, mMRC score, sputum culture results, exacerbations in the previous year, and BSI score (Table 3). Variables included in the multivariable analysis were age ≥65 years, BMI <18.5 kg/m2, smoking history, 6-MWD ≤425 meters, mMRC ≥4, BSI score ≥9, and positive bacterial culture. In the multiple regression model (Table 4), BSI score ≥9 was a major factor associated with osteoporosis [odd ratio (OR) =4.52; 95% confidence interval (CI): 1.57–12.96; P=0.005]. Other factors associated with osteoporosis included BMI <18.5 kg/m2 (OR =3.44; 95% CI: 1.13–10.46; P=0.030), age ≥65 years (OR =2.87; 95% CI: 1.01–7.55; P=0.033), and a smoking history (OR =2.78; 95% CI: 1.04–7.47; P=0.042).
Table 3
| Determinants | With osteoporosis (n=34) | Without osteoporosis (n=74) | Univariable models, OR (95% CI) | P value |
|---|---|---|---|---|
| Age ≥65 years | 20 | 22 | 3.38 (1.45–7.87) | 0.005* |
| BMI <18.5 kg/m2 | 14 | 10 | 4.48 (1.73–11.63) | 0.002* |
| Smoking history | 18 | 19 | 3.26 (1.39–7.63) | 0.007* |
| 6-MWD, meters | ||||
| ≤425 | 16 | 20 | 2.40 (1.03–5.60) | 0.043* |
| 426–550 | 15 | 36 | 0.83 (0.37–1.89) | 0.661 |
| ≥550 | 3 | 18 | 0.30 (0.08–1.10) | 0.070 |
| mMRC ≥4 | 12 | 13 | 2.56 (1.02–6.45) | 0.046* |
| BSI score | ||||
| 0–4 | 4 | 13 | 0.63 (0.19–2.08) | 0.445 |
| 5–8 | 12 | 51 | 0.24 (0.10–0.58) | 0.001 |
| >9 | 18 | 10 | 7.20 (2.79–18.57) | <0.001* |
| AE ≥2/year | 14 | 20 | 1.89 (0.80–4.44) | 0.144 |
| Positive bacterial culture | 12 | 14 | 2.34 (0.94–5.82) | 0.068* |
| FEV1 % pred | ||||
| <50 | 5 | 8 | 1.42 (0.43–4.72) | 0.565 |
| 50–80 | 23 | 49 | 1.07 (0.45–2.53) | 0.884 |
| ≥80 | 6 | 17 | 0.72 (0.26–2.02) | 0.531 |
Smoking history: a history of smoking or ever smoking. *, variables included in the multivariable analysis. OR, odd ratio; CI, confidence interval; BMI, body-mass index; 6-MWD, 6-minute walking distance; mMRC, modified British medical research council questionnaire; BSI, bronchiectasis severity index; AE, acute exacerbation; FEV1, forced expiratory volume in one second.
Table 4
| Determinants | OR | 95% CI | P value |
|---|---|---|---|
| Age ≥65 years | 2.87 | 1.01–7.55 | 0.033 |
| BMI <18.5 kg/m2 | 3.44 | 1.13–10.46 | 0.030 |
| Smoking history | 2.78 | 1.04–7.47 | 0.042 |
| BSI score ≥9 | 4.52 | 1.57–12.96 | 0.005 |
Smoking history: a history of smoking or ever smoking. OR, odd ratio; CI, confidence interval; BMI, body-mass index; BSI, bronchiectasis severity index.
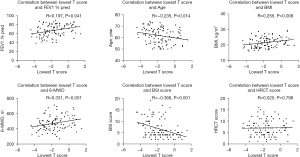
Correlations between T score and others
T score associated with the disease severity evaluated by BSI score (R=−0.336, P<0.001; Figure 2). It was also consistent with physical exercise ability, as it positively correlated with FEV1 % pred (R=0.197, P=0.041) and 6-MWD (R=0.331, P<0.001). Other factors correlated with T score included age (R=−0.235, P=0.014) and BMI (R=0.225, P=0.008). There was no correlation between T score and HRCT score.
Discussion
Our study illustrated the prevalence of osteoporosis in male bronchiectasis patients. The prevalence was 31.5%, significantly higher than that in healthy controls. The present study revealed that BMD was low in patients with bronchiectasis. Previous studies have indicated that potential factors might lead to low BMD, including poor nutritional status, reduced physical activity, chronic inflammation, and glucocorticoid use (18,19,28,29), which overlap with the characteristic features associated with bronchiectasis. In our study, patients with bronchiectasis exhibited low BMI and a reduced ability to exercise compared to healthy controls. This indicates that bronchiectasis might increase the risk of osteoporosis.
Several studies reported osteoporosis as a comorbidity in patients with bronchiectasis (12-14). Choi et al. found that the prevalence of osteoporosis in bronchiectasis was higher than that in COPD (13). The prevalence of osteoporosis varies in regions or age. Lee et al. reported the prevalence was highest in Australians (23.1%), followed by Koreans (11.7%), Europeans (7.4%) and Indians (5.9%) (14). In the study performed by Diehl et al., 30.1% bronchiectasis patients were with osteoporosis in America (15). Our study found that osteoporosis was present in 31.5% of male patients in South China. The prevalence of osteoporosis increases with age. In the study by Contreras-Bolívar et al., osteoporosis was present in 38.9% patients over 65 years old, 18.7% patents aged 45–65, and 2.7% under 45 years old (30).
To further investigate the factors related to osteoporosis, we conducted a multivariable analysis. We found that factors associated with osteoporosis included age, low BMI, smoking history, and high BSI score, consistent with results of previous studies (30,31). This suggests that the comorbidity of osteoporosis and bronchiectasis may be linked to a variety of mechanisms. It might also provide us with some potential interventions, such as enhancing nutrition, increasing physical activity, and smoking cessation. In fact, some of these measures have proven effective in treating osteoporosis (7,25).
In our study, the treatment for stable bronchiectasis included ICS and others. ICS are widely used in respiratory inflammatory diseases including asthma and COPD. They were also reported in the treatment of bronchiectasis in previous studies (5,6). Osteoporosis induced by glucocorticoids remains common and complex (32). Whether ICS can lead to osteoporosis remains controversial (32,33). Previous studies showed that osteoporosis induced by glucocorticoids is related to the daily dosages of glucocorticoids (32). In our study, the median daily doses of ICS were about 320 mg/day, and the median duration were 37 months. The daily dose of ICS were defined as low dose according to the standard of American College of Rheumatology (32). The low daily doses might be the reason why there was no relationship between ICS and low BMD.
Comorbidities of chronic diseases are common and increase with age. In osteoporosis, the comorbidities include cardiovascular disease, hypertension, diabetes mellitus, COPD, and others (34,35). Osteoporosis increases the burden of comorbidities in chronic diseases (8). It also increases disease related adverse events and mortality. Similar with previous studies (31,32), we reveal osteoporosis as a comorbidity of bronchiectasis. Osteoporosis and bronchiectasis show overlapping characteristics, including low BMI and a reduced ability to exercise. Bronchiectasis patients with osteoporosis have more severe disease and decreased exercise ability. Therefore, actions should be taken to prevent or treat osteoporosis in bronchiectasis patients. Despite its higher prevalence, osteoporosis in male patients is easily ignored (22). Some patients with osteoporosis may be asymptomatic or lack typical symptoms (36,37) that lead to the misdiagnosis of osteoporosis. Therefore, studies on the relationships between osteoporosis and bronchiectasis are needed. To benefit the patients with associated factors, early screening and diagnosis might be reasonable and appropriate.
Chalmers et al. provided a clinical prediction tool named BSI to assess the severity of the disease (26). It performed well in predicting mortality and hospitalization. A high BSI score indicates a more severe disease and a worse prognosis. The BSI score of patients with osteoporosis is high, which means a higher risk of mortality and hospitalization in these patients. Therefore, more attention should be paid to them.
Our study has the following limitations. First, the single-center nature limits the scope of the conclusion. Second, the sample size in this study is small. Third, the nature of the cross-sectional study makes it difficult to elucidate the causal relationship between bronchiectasis and osteoporosis. Hence, more intervention studies, with large sample size, should be performed to confirm the causal relationships and clarify the underlying mechanisms of association between bronchiectasis and osteoporosis.
Conclusions
The prevalence of osteoporosis in male patients with bronchiectasis was high. Factors associated with osteoporosis in males with bronchiectasis include older, low BMI, a smoking history, and a high BSI score. These data provides valuable insights into osteoporosis associated with bronchiectasis in males.
Acknowledgments
Funding: This work was supported by the Guangzhou Municipal Health Commission (Nos. 20191A010014, 201704020105).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-887/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-887/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-887/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-887/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Guangzhou First People’s Hospital (No. K-2018-040-01) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sibila O, Laserna E, Shoemark A, et al. Airway Bacterial Load and Inhaled Antibiotic Response in Bronchiectasis. Am J Respir Crit Care Med 2019;200:33-41. [Crossref] [PubMed]
- Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J 2015;45:1446-62. [Crossref] [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Aetiology of bronchiectasis in Guangzhou, southern China. Respirology 2015;20:739-48. [Crossref] [PubMed]
- Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019;74:1-69. [Crossref] [PubMed]
- Martínez-García MÁ, Soler-Cataluña JJ, Catalán-Serra P, et al. Clinical efficacy and safety of budesonide-formoterol in non-cystic fibrosis bronchiectasis. Chest 2012;141:461-8. [Crossref] [PubMed]
- Tsang KW, Tan KC, Ho PL, et al. Inhaled fluticasone in bronchiectasis: a 12 month study. Thorax 2005;60:239-43. [Crossref] [PubMed]
- Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785-95. [Crossref] [PubMed]
- Thayer SW, Stolshek BS, Gomez Rey G, et al. Impact of osteoporosis on high-cost chronic diseases. Value Health 2014;17:43-50. [Crossref] [PubMed]
- Laroche M, Pécourneau V, Blain H, et al. Osteoporosis and ischemic cardiovascular disease. Joint Bone Spine 2017;84:427-32. [Crossref] [PubMed]
- Adas-Okuma MG, Maeda SS, Gazzotti MR, et al. COPD as an independent risk factor for osteoporosis and fractures. Osteoporos Int 2020;31:687-97. [Crossref] [PubMed]
- Ma L, Chhetri JK, Liu P, et al. Epidemiological characteristics and related factors of frailty in older Chinese adults with hypertension: a population-based study. J Hypertens 2020;38:2192-7. [Crossref] [PubMed]
- Yang B, Choi H, Lim JH, et al. The disease burden of bronchiectasis in comparison with chronic obstructive pulmonary disease: a national database study in Korea. Ann Transl Med 2019;7:770. [Crossref] [PubMed]
- Choi H, Yang B, Nam H, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J 2019;54:1900194. [Crossref] [PubMed]
- Lee H, Choi H, Chalmers JD, et al. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology 2021;26:619-21. [Crossref] [PubMed]
- Diehl N, Johnson MM. Prevalence of Osteopenia and Osteoporosis in Patients with Noncystic Fibrosis Bronchiectasis. South Med J 2016;109:779-83. [Crossref] [PubMed]
- Buehring B, Viswanathan R, Binkley N, et al. Glucocorticoid-induced osteoporosis: an update on effects and management. J Allergy Clin Immunol 2013;132:1019-30. [Crossref] [PubMed]
- Hardy R, Cooper MS. Bone loss in inflammatory disorders. J Endocrinol 2009;201:309-20. [Crossref] [PubMed]
- Amarasekara DS, Yu J, Rho J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J Immunol Res 2015;2015:832127. [Crossref] [PubMed]
- O'Brien CA, Jia D, Plotkin LI, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 2004;145:1835-41. [Crossref] [PubMed]
- Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 2012;367:1714-23. [Crossref] [PubMed]
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726-33. [Crossref] [PubMed]
- Gruntmanis U. Male osteoporosis: deadly, but ignored. Am J Med Sci 2007;333:85-92. [Crossref] [PubMed]
- Porcelli T, Maffezzoni F, Pezzaioli LC, et al. Management of endocrine disease: Male osteoporosis: diagnosis and management - should the treatment and the target be the same as for female osteoporosis? Eur J Endocrinol 2020;183:R75-93. [Crossref] [PubMed]
- Bliuc D, Alarkawi D, Nguyen TV, et al. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 2015;30:637-46. [Crossref] [PubMed]
- Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017;12:43. [Crossref] [PubMed]
- Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85. [Crossref] [PubMed]
- Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000;162:1277-84. [Crossref] [PubMed]
- Riaudel T, Guillot P, De Decker L, et al. Nutrition and osteoporosis in elderly. Geriatr Psychol Neuropsychiatr Vieil 2011;9:399-408. [PubMed]
- Min CY, Yoo DM, Choi HG. Associations between Physical Activity, Sunshine Duration and Osteoporosis According to Obesity and Other Lifestyle Factors: A Nested Case-Control Study. Int J Environ Res Public Health 2021;18:4437. [Crossref] [PubMed]
- Contreras-Bolívar V, Olveira G, Porras N, et al. Osteopenia and Osteoporosis in Patients with Bronchiectasis: Association with Respiratory Parameters, Body Composition, Muscle Strength and Bone Remodeling Biomarkers. Sci Rep 2019;9:14496. [Crossref] [PubMed]
- Huang HY, Sheng TF, Lin CW, et al. Oxygen desaturation during the 6-min walk test as a risk for osteoporosis in non-cystic fibrosis bronchiectasis. BMC Pulm Med 2019;19:28. [Crossref] [PubMed]
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62:1515-26. [Crossref] [PubMed]
- Ozcakir S, Sigirli D, Ursavas A, et al. COPD and Osteoporosis: Associated Factors in Patients Treated with Inhaled Corticosteroids. Int J Chron Obstruct Pulmon Dis 2020;15:2441-8. [Crossref] [PubMed]
- Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011;305:2184-92. [Crossref] [PubMed]
- Mishra BH, Mishra PP, Mononen N, et al. Uncovering the shared lipidomic markers of subclinical osteoporosis-atherosclerosis comorbidity: The Young Finns Study. Bone 2021;151:116030. [Crossref] [PubMed]
- Bhat KA, Kakaji M, Awasthi A, et al. High Prevalence of Osteoporosis and Morphometric Vertebral Fractures in Indian Males Aged 60 Years and Above: Should Age for Screening Be Lowered? J Clin Densitom 2018;21:517-23. [Crossref] [PubMed]
- Brennan SL, Pasco JA, Cicuttini FM, et al. Bone mineral density is cross sectionally associated with cartilage volume in healthy, asymptomatic adult females: Geelong Osteoporosis Study. Bone 2011;49:839-44. [Crossref] [PubMed]



