
Perioperative outcomes of neoadjuvant immunotherapy plus chemotherapy and neoadjuvant chemoradiotherapy in the treatment of locally advanced esophageal squamous cell carcinoma: a retrospective comparative cohort study
Highlight box
Key findings
• Neoadjuvant immunotherapy plus chemotherapy (nICT) in the treatment of locally advanced esophageal malignancies has demonstrated good postoperative pathological response rates and controllable adverse reactions.
What is known and what is new?
• Neoadjuvant chemoradiotherapy is the standard modality of neoadjuvant therapy for locally advanced esophageal malignancies, but this modality has high complications, cumbersome procedures, and low clinical acceptance. Studies have shown the efficacy of immuno-checkpoint inhibitors in the treatment of advanced esophageal cancer.
• In this study, we explored the feasibility and usefulness of nICT in preoperative neoadjuvant therapy for locally advanced esophageal cancer.
What is the implication, and what should change now?
• nICT in the treatment of locally advanced esophageal malignancies is a potential new therapeutic model. However, studies with larger sample sizes are still needed to further assess the safety, feasibility, and efficacy of this treatment modality.
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the main pathological types of esophageal cancer. It frequently occurs in Central and Southeast Asia, Eastern and Southern Africa, South America, and other regions, and it has a high incidence and fatality rate that poses a serious threat to human life (1). For early-stage ESCC, surgical resection is the primary treatment option; for locally advanced cases, the current treatment modality is neoadjuvant therapy followed by surgery, which can improve survival outcomes in these patients (2). Platinum-based neoadjuvant chemotherapy (nCT) or neoadjuvant chemoradiotherapy (nCRT) is recommended for clinical neoadjuvant therapy. Compared with surgery alone, nCRT improves the R0 resection rate (92% vs. 69%) and 5-year overall survival (47% vs. 34%). As a result, it has become the main neoadjuvant therapy mode (3,4). In clinical practice, nCRT is characterized by a complicated clinical procedure with a low acceptance rate by clinicians and low compliance of patients, and a high incidence of adverse events. The survival prognosis of patients without pathological complete response (PCR) is also an issue (62.3 vs. 34.4 m2; P<0.001) (5). Based on these considerations, the discussion of the best mode of neoadjuvant therapy continues.
An existing study found that programmed cell death-ligand 1 (PD-L1) expression correlates with some esophageal cancer clinical characteristics, including tumor invasion depth, lymph node metastasis, pathologic differentiation, and TNM staging (6). The KEYNOTE-590 (7) and TENERGY studies (8) demonstrated the safety and benefits of advanced esophageal cancer treatment. The use of immune checkpoint inhibitors in preoperative adjuvant therapy to improve the prognoses of patients with locally advanced esophageal cancer is currently a focus of research, with an increasing number of clinical trials being conducted. Neoadjuvant immunotherapy plus chemotherapy (nICT) is commonly used in clinical treatment and has achieved good therapeutic effects (PCR: 39.2%) (9). In contrast to the complexity and relatively high complication rate of nCRT, nICT is seen to be relatively simple and has a low complication rate. However, due to the short duration of clinical applications, there have been few clinical reports on the merits and demerits of these two treatment modalities. Therefore, in this study, we conducted a retrospective analysis of Gaozhou People’s Hospital’s data to compare the perioperative outcomes of these 2 neoadjuvant therapy regimens. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-84/rc).
Methods
Patient selection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of Gaozhou People’s Hospital (No. GYLLPJ-2022104). Individual consent for this retrospective analysis was waived. This paper presents the results of a retrospective analysis. We collected the data of patients with ESCC who underwent neoadjuvant therapy (nICT or nCRT) at Gaozhou People’s Hospital between January 1, 2019, and September 1, 2022. Patients were included if they had ESCC that was confirmed by an electronic gastroscopy biopsy, at clinical stage cT1-3 N+M0 or cT4N0M0 identified by chest plus upper-abdominal enhanced computed tomography or positron emission tomography-computed tomography, with or without ultrasound gastroscopy, and that was assessed by surgeons as resectable.
Neoadjuvant therapy
The radiotherapy regimen consisted of PTV-CTV (planning target volume-clinical target volume) with a total dose of 40–44 Gy in 20 sessions of 2.0 Gy each. The total dose of PTV-GTV (gross target volume) was 40–46 Gy in 20 sessions, 5 days per week. The chemotherapy regimen comprised two 3-week cycles of paclitaxel plus platinum administered on day 1 of the cycle. Each dose of medication included either docetaxel (75 mg/m2), paclitaxel (135 mg/m2), or paclitaxel albumin (260 mg/m2) plus either cisplatin (75 mg/m2), nedaplatin (80 mg/m2), or carboplatin (300–400 mg/m2). The immunotherapy regimen comprised two 3-week cycles of an intravenous programmed cell death protein 1 (PD-1) inhibitor (200 mg of camrelizumab, tislelizumab, or sintilimab) administered before chemotherapy on day 1.
Surgical treatment options
Operations in the nCRT group were performed 6–8 weeks after the last neoadjuvant treatment (10); those in the nICT group were performed at 4–6 weeks (11). All patients were operated upon by the same medical team. The surgical procedure was McKeown minimally invasive esophagectomy plus two-field lymph node dissection and gastric reconstruction.
Outcome measures
The primary endpoint of the study was the rate of PCR. Postoperative pathological tumor regression grading (TRG) was performed according to the Mandard criteria: TRG1, no residual cancer cells; TRG2, a small number of cancer cells scattered in the fibrosis; TRG3, fibroids are more numerous than residual cancer cells; TRG4, less fibrosis than residual cancer cells; and TRG5, no tumor regression change. The secondary study objectives included indicators related to the neoadjuvant therapy cycle, which were the number of patients who completed neoadjuvant therapy followed by surgery, their clinical evaluation after neoadjuvant therapy, and adverse events during the neoadjuvant therapy cycle. CTCAE version 5.0 (http://ctep.cancer.gov) was used for statistical analyses, including blood biochemistry test results such as hemoglobin (HGB), white blood cell count (WBC), absolute neutrophil count (ANC), platelet count (PLT), and total bilirubin (TBIL) and clinical symptoms or signs (nausea and vomiting, diarrhea, alopecia, and rash). Perioperative indices of the 2 groups were included for comparison, including the R0 resection rate, operation time, intraoperative blood loss, number of lymph nodes dissected, number of lymph node dissection stations, postoperative thoracic tube drainage time, thoracic fluid drainage volume, postoperative intensive care unit (ICU) time, postoperative proportion of returns to the ICU, postoperative hospitalization days, postoperative anastomotic/stomatogastric/tracheal fistulas, pulmonary infection, and hoarseness.
Statistical analysis
SPSS 19.0 (IBM Corp, Armonk, NY, USA) was used for the statistical analyses. Data on age, operation time, intraoperative blood loss, number of lymph nodes dissected, number of lymph nodes dissection stations, chest tube drainage time, chest tube drainage volume, ICU time, and postoperative hospital stay are all presented as the mean ± standard deviation. The 2 groups were compared using the Student’s t-test. The chi-square test was used for sex comparison. Fisher’s exact probability method was used to analyze the data on tumor locations, tumor clinical stages, tumor clinical T stages, tumor clinical N stages, chemotherapy regimens, the number of patients who completed neoadjuvant plus surgery according to the course of treatment, surgical plans, returns to the ICU, and postoperative complications. The rank sum test was used to analyze the data on adverse events during the neoadjuvant therapy, clinical evaluations after neoadjuvant therapy, and postoperative pathological response grades. A value of P value less than 0.05 was considered statistically significant.
Results
A total of 44 patients were enrolled in the study. According to their neoadjuvant treatment regimens, the patients were divided into the nCRT (n=23) and nICT groups (n=21). The proportion of patients at clinical stage III was 82.61% in the nCRT group and 61.90% in the nICT group. There were no significant differences between the 2 groups for the clinical stage, sub-T stage, or N stage (Table 1).
Table 1
| Clinical feature | nCRT group (n=23) | nICT group (n=21) | T/χ2/Z | P value |
|---|---|---|---|---|
| Age, years, mean ± SD | 64.78±7.01 | 59.19±5.57 | 2.91 | 0.18 |
| Sex, n (%) | 0.10 | 0.74 | ||
| Male | 12 (52.17) | 12 (57.14) | ||
| Female | 11 (47.83) | 9 (42.86) | ||
| Tumor location, n (%) | 4.51 | 0.10 | ||
| Upper | 7 (30.43) | 2 (9.52) | ||
| Middle | 14 (60.87) | 13 (61.91) | ||
| Lower | 2 (8.70) | 6 (28.57) | ||
| Clinical staging of tumor, n (%) | −0.12 | 0.81 | ||
| II | 1 (4.45) | 3 (14.29) | ||
| III | 19 (82.61) | 13 (61.90) | ||
| IV | 3 (13.04) | 5 (23.81) | ||
| T stage, n (%) | −0.69 | 0.50 | ||
| T2 | 3 (13.04) | 4 (19.05) | ||
| T3 | 18 (78.26) | 16 (76.19) | ||
| T4 | 2 (8.70) | 1 (4.76) | ||
| N stage, n (%) | −0.20 | 0.86 | ||
| N1 | 14 (60.87) | 14 (66.67) | ||
| N2 | 7 (30.43) | 4 (19.05) | ||
| N3 | 2 (8.70) | 3 (14.28) | ||
| Chemotherapy regimen, n (%) | 5.49 | 0.09 | ||
| Docetaxel plus platinum | 8 (34.78) | 3 (14.29) | ||
| Paclitaxel plus platinum | 4 (17.39) | 1 (4.76) | ||
| Albumin-bound paclitaxel plus platinum | 11 (47.83) | 17 (80.95) |
nCRT, neoadjuvant chemoradiotherapy; nICT, neoadjuvant immunotherapy plus chemotherapy; SD, standard deviation.
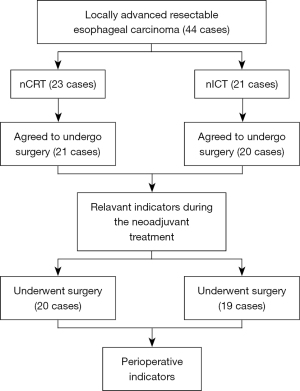
For subjective reasons, 4 patients did not agree to surgical treatment after neoadjuvant treatment (3 patients in the nCRT group; 1 patient in the nICT group). One patient in the nICT group was switched to radical radiotherapy after 2 courses of neoadjuvant therapy due to the progressive disease (PD) assessment by multidisciplinary treatment of esophageal cancer. Twenty patients in the nCRT group and 19 in the nICT group completed the surgery (Figure 1).
During neoadjuvant therapy, the nICT group had 2 cases of Grade 3 hemoglobin reduction. Hemoglobin reductions of Grades 1 and 2 were significantly higher in the nICT group compared to the nCRT group (P<0.05). However, the incidence of Grade 2 and 3 leukopenia adverse events in the nCRT group was significantly higher (P<0.05). The nICT group experienced varying degrees of a rash, including 1 case of Grade 3 that required additional hospitalization. The nCRT group had no cases of rash (P<0.05). The nCRT group had no incidence of diarrhea, while the nICT group had 2 occurrences, including a Grade 3 reaction (P>0.05). In addition, 1 patient developed an esophageal fistula after nCRT (Table 2).
Table 2
| Clinical data | nCRT (n=23), n (%) | nICT (n=21), n (%) | T/χ2/Z | P value |
|---|---|---|---|---|
| Completed neoadjuvant plus surgery | 20 (86.96) | 19 (90.48) | – | >0.05 |
| Clinical evaluation | −1.14 | 0.24 | ||
| CR | 4 (17.39) | 1 (4.76) | ||
| PR | 15 (65.22) | 15 (71.43) | ||
| SD | 4 (17.39) | 4 (19.05) | ||
| PD | 0 (0.00) | 1 (4.76) | ||
| Hemoglobin decreased | −2.15 | 0.03 | ||
| Grade 1 | 6 (26.09) | 9 (42.86) | ||
| Grade 2 | 4 (17.39) | 5 (23.81) | ||
| Grade 3 | 0 (0.00) | 2 (9.52) | ||
| Leukopenia | −2.10 | 0.03 | ||
| Grade 1 | 6 (26.09) | 9 (42.86) | ||
| Grade 2 | 10 (43.48) | 5 (23.81) | ||
| Grade 3 | 5 (21.74) | 2 (9.52) | ||
| Neutropenia | −0.78 | 0.44 | ||
| Grade 1 | 9 (39.13) | 6 (28.57) | ||
| Grade 2 | 2 (8.70) | 2 (9.52) | ||
| Grade 3 | 0 (0.00) | 3 (14.29) | ||
| Thrombocytopenia | −0.59 | 0.57 | ||
| Grade 1 | 3 (13.04) | 3 (14.29) | ||
| Grade 2 | 0 (0.00) | 1 (4.76) | ||
| Total bilirubin increased | −0.69 | 0.47 | ||
| Grade 1 | 1 (4.35) | 1 (4.76) | ||
| Grade 2 | 0 (0.00) | 1 (4.76) | ||
| Elevated serum creatinine | 0.57 | 0.56 | ||
| Grade 1 | 2 (8.70) | 3 (14.29) | ||
| Emesis | −1.13 | 0.33 | ||
| Grade 1 | 22 (95.65) | 18 (85.71) | ||
| Grade 2 | 1 (4.35) | 3 (14.29) | ||
| Diarrhea | −1.49 | 0.22 | ||
| Grade 1 | 0 (0.00) | 1 (4.76) | ||
| Grade 3 | 0 (0.00) | 1 (4.76) | ||
| Alopecia | −1.01 | 0.36 | ||
| Grade 1 | 13 (56.52) | 15 (71.43) | ||
| Grade 2 | 10 (43.48) | 6 (28.57) | ||
| Erythema | −2.71 | 0.01 | ||
| Grade 1 | 0 (0.00) | 3 (14.29) | ||
| Grade 2 | 0 (0.00) | 2 (9.52) | ||
| Grade 3 | 0 (0.00) | 1 (4.76) | ||
| Esophageal perforation | 1 (4.35) | 0 (0.00) | – | >0.05 |
nCRT, neoadjuvant chemoradiotherapy; nICT, neoadjuvant immunotherapy plus chemotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Both groups were compared in terms of perioperative-related indicators and postoperative complications. PCR was achieved in 50% of the patients in the nCRT group and 36.84% of the patients in the nICT group. In the nCRT group, 1 patient underwent Sweet surgery because of an infection in an esophageal fistula, and 2 patients died during perioperative care (Table 3). The cause of death was esophagogastric fistula and lung infection.
Table 3
| Clinical data | nCRT group (n=20) | nICT group (n=19) | T/χ2/Z | P value |
|---|---|---|---|---|
| Intraoperative indicators | ||||
| Surgery program (%) | – | >0.999 | ||
| Sweet | 1 (5.00) | 0 (0.00) | ||
| MIE-McKeown | 19 (95.00) | 19 (100.00) | ||
| Operation time, min | 292.60±56.33 | 251.16±41.57 | 2.60 | 0.36 |
| Intraoperative blood loss | 59.50±30.34 | 52.47±29.60 | 0.73 | 0.41 |
| Number of dissected lymph nodes | 24.20±10.34 | 30.16±12.35 | −1.63 | 0.68 |
| Number of dissected lymph nodes stations | 8.05±3.15 | 9.58±5.57 | −1.06 | 0.68 |
| Mandard criteria (%) | −1.29 | 0.19 | ||
| TRG1 | 10 (50.00) | 7 (36.84) | ||
| TRG2 | 6 (30.00) | 5 (26.31) | ||
| TRG3 | 3 (15.00) | 2 (10.53) | ||
| TRG4 | 1 (5.00) | 3 (15.79) | ||
| TRG5 | 0 (0.00) | 2 (10.53) | ||
| Postoperative related indicators | ||||
| Chest tube drainage time (d) | 5.75±6.05 | 5.56±5.57 | 0.10 | 0.55 |
| Chest tube drainage volume (mL) | 595.75±303.12 | 812.12±790.22 | −1.14 | 0.16 |
| ICU stay (h) | 19.93±3.64 | 22.63±11.77 | −0.97 | 0.21 |
| Return to the ICU (%) | 2 (10.00) | 0 (0.00) | – | 0.48 |
| Postoperative hospital stay (d) | 16.60±10.24 | 13.50±6.16 | 1.11 | 0.51 |
| Postoperative complications (%) | ||||
| Death | 2 (10.00) | 0 (0.00) | – | 0.48 |
| Anastomotic leakage | 3 (15.00) | 2 (10.53) | – | >0.05 |
| Thoracogastric fistula | 2 (10.00) | 2 (10.53) | – | >0.999 |
| Bronchial fistula | 1 (5.00) | 0 (0.00) | – | >0.999 |
| Pulmonary infection | 2 (10.00) | 2 (10.53) | – | >0.999 |
| Stroke | 1 (5.00) | 0 (0.00) | – | >0.999 |
| Hoarseness | 4 (20.00) | 3 (15.79) | – | >0.999 |
Data are presented as n (%) or mean ± SD. nCRT, neoadjuvant chemoradiotherapy; nICT, neoadjuvant immunotherapy plus chemotherapy; MIE, McKeown minimally invasive esophagectomy; TRG, tumor regression grading; ICU, intensive care unit; SD, standard deviation.
Discussion
For patients with locally advanced resectable ESCC, the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) study showed a pathological PCR rate of 49% after surgery with a 10-year overall survival benefit of 13% (38% vs. 25%). Based on carboplatin plus paclitaxel nCRT cases, nCRT reduced the risk of esophageal cancer mortality (hazard risk: 0.60, 95% CI: 0.46–0.80) (12). The NEOCRTEC5010 study of ESCC showed a PCR rate of 43.2% with nCRT and a better 5-year survival rate (59.9%, 95% CI: 52.9–66.1%) compared with surgery alone (49.1%, 95% CI: 42.3–55.6%) (10). In our study, nCRT had a 50% PCR rate. This good PCR rate shows that nCRT improves the treatment of locally advanced resectable ESCC. Based on the favorable response to immunotherapy in advanced esophageal cancer and the increased expression of invasive immune cells and PD-L1 in resected gross specimens of ESCC, these findings may indicate that treatment of esophageal cancer benefits from immunotherapy.
There are a growing number of clinical studies of neoadjuvant immunotherapy in treating locally advanced resectable ESCC (8,9,11,13). Considering the short clinical time-to-effect of neoadjuvant immunotherapy, the efficacy of this treatment mode is currently being assessed, and PCR rates are widely used in clinical practice. Several phase II clinical studies have shown that nICT has a good pathological response rate. Yang et al. (14) reported that the neoadjuvant PCR of camrelizumab with chemotherapy was 25%, and 50% of the patients had a major pathological response. Yang et al. (10) reported a 50% PCR rate and a 75% major pathologic response (MPR) rate for tislelizumab used in combination with chemotherapy as neoadjuvant therapy. In our study, 36.84% of the patients in the nICT group achieved a PCR, and 89.47% of patients showed varying degrees of tumor regression in gross pathology specimens, indicating a good rate of tumor regression. Compared with nCRT, there was no statistically significant difference. However, according to the pathological results, 2 patients had almost no pathological regression, suggesting a need to further explore which patients will benefit from neoadjuvant immunotherapy (15).
In addition to the favorable pathological response rate, there are clinical concerns regarding the practicability of neoadjuvant treatment regimens from both the point of view of medical teams and patient compliance. To achieve neoadjuvant therapy for esophageal cancer, a well-established multidisciplinary team for esophageal cancer is required, which requires close collaboration between surgeons, oncology chemotherapy or radiotherapy departments, and medical imaging departments within the team and a complete process for managing patients (16).
Patients who did not undergo surgery after neoadjuvant therapy showed significant adverse events from neoadjuvant treatment. Four patients among the 2 groups were unable to undergo surgery due to subjective or objective factors (e.g., PD). The surgical treatment rate was 86.96% in the nCRT group and 90.48% in the nICT group. Esophageal perforation is a serious adverse event of nCRT. van Hagen et al. (17) reported 1 case (1/171) of esophageal perforation during neoadjuvant therapy. Although the incidence of esophageal perforation is low, it significantly affects the patient’s subsequent treatment and is a leading cause of death during neoadjuvant therapy. In our study, 1 patient had esophageal perforation during nCRT and underwent left thoracic surgery. This was the only case that resulted in perioperative death. In addition, Grade 3 or 4 leukopenia and neutropenia were major complications of nCRT, with rates of 48.9% and 45.7%, respectively, as reported in NEOCRTEC5010 (10). However, the incidence of bone marrow suppression in nICT is 20% (18). A meta-analysis showed a 16.3% incidence of immune-related adverse events of Grade 3 or higher, including serious adverse events, such as immune-related myocarditis and pneumonitis (19). For these reasons, using immune checkpoint inhibitors to treat esophageal malignancies requires additional monitoring for immune-related complications, which range from mild to life-threatening and may limit cancer therapy (20). In our study, there was 1 case of Grade 3 immune-related dermatitis in the nICT group, requiring additional hospitalization and resulting in delayed surgery. Another patient had Grade 3 diarrhea, which was one reason the patient did not consent to surgery.
Implementation of surgery and the occurrence of perioperative complications are also safety and efficacy indicators for evaluating treatment modalities following nCRT for esophageal cancer. After neoadjuvant immunotherapy, Sihag et al. (21) analyzed the perioperative conversion rate, pulmonary infections, anastomotic leakage, and 30-day mortality compared to standard nCRT. The preliminary conclusions indicated that neoadjuvant immunotherapy did not increase perioperative complications and was safe and feasible. After nCRT for esophageal cancer, fibrosis develops over time in the esophageal and peri-esophageal tissues. Surgical procedures, especially minimally invasive ones, have a learning curve and require preoperative planning (22,23). In the study, the duration of surgery was shorter in the nICT group than in the nCRT group, but the difference was not statistically significant. According to the current study, adequate lymph node dissection and the number of lymph node dissections were factors that affected patient survival and prognosis. Our study identified that the number of lymph nodes dissected was greater in the nICT group than in the nCRT group. Anastomotic or stomatogastric fistulas are serious complications of esophageal cancer surgery and can cause other serious complications. Two patients treated with nCRT developed anastomotic and gastric fistulas; one had a right bronchial fistula, and both had pulmonary infections. In the nICT group, 2 patients developed fistulas, but no further serious complications were induced. Further results are pending on whether nICT is more beneficial in reducing the incidence of severe complications following surgery.
Mungo et al. (20) studied the American College of Surgeons’ National Surgery Quality Improvement Program database [2005–2011], comparing 708 patients with esophageal cancer who received neoadjuvant therapy with 1,231 patients with esophageal cancer who did not receive neoadjuvant therapy. There was no statistically significant difference in the primary endpoint of death at 30 days after surgery. nCT or nCRT did not increase the risk of death at 30 days after surgery for esophageal cancer.
Finally, it should be noted that this study was conducted retrospectively in order to compare perioperative outcomes between nICT and nCRT. The sample size in this study was small. If the differences between nICT and nCRT are to be further evaluated, clinical validation with a larger sample size is needed. In the meantime, there is a lack of long-term data on the survival of patients in this study. Future studies should aim to provide follow-up data on patient outcomes.
Conclusions
Compared to standard nCRT, the administration of nICT to treat locally advanced resectable esophageal cancer is associated with a controlled incidence of adverse events during the neoadjuvant therapy and perioperative period. However, monitoring for the occurrence of immune-related complications should be performed. In addition, nICT has a good PCR, suggesting that it has the potential to be developed as a neoadjuvant treatment of esophageal cancer.
Acknowledgments
We thank the esophageal cancer multidisciplinary team (MDT) team at the Gaozhou People’s Hospital for their support, as well as Director Wen Zunbei, Cai Maode, and Director Zhang Kunqiang for their guidance on the management of patients with esophageal cancer during radiotherapy and chemotherapy in this study.
Funding: This study received funding from the Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2021Y9057).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-84/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-84/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-84/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-84/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of Gaozhou People’s Hospital (No. GYLLPJ-2022104). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DiSiena M, Perelman A, Birk J, et al. Esophageal Cancer: An Updated Review. South Med J 2021;114:161-8. [Crossref] [PubMed]
- Yu Y, Xu L, Chen X, et al. Neoadjuvant therapy combined with surgery is superior to chemoradiotherapy in esophageal squamous cell cancer patients with resectable supraclavicular lymph node metastasis: a propensity score-matched analysis. Ann Transl Med 2022;10:349. [Crossref] [PubMed]
- Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Li J, Chen B, Wang X, et al. Prognosis of patients with esophageal squamous cell carcinoma undergoing surgery versus no surgery after neoadjuvant chemoradiotherapy: a retrospective cohort study. J Gastrointest Oncol 2022;13:903-11. [Crossref] [PubMed]
- Macedo FI, Mesquita-Neto JW, Kelly KN, et al. Utility of Radiation After Neoadjuvant Chemotherapy for Surgically Resectable Esophageal Cancer. Ann Surg Oncol 2020;27:662-70. [Crossref] [PubMed]
- Jiang Y, Lo AWI, Wong A, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget 2017;8:30175-89. [Crossref] [PubMed]
- Kato K, Shah MA, Enzinger P, et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol 2019;15:1057-66. [Crossref] [PubMed]
- Bando H, Kotani D, Tsushima T, et al. TENERGY: multicenter phase II study of Atezolizumab monotherapy following definitive Chemoradiotherapy with 5-FU plus Cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Cancer 2020;20:336. [Crossref] [PubMed]
- Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e004291. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg 2021;156:721-9. [Crossref] [PubMed]
- Yan X, Duan H, Ni Y, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg 2022;103:106680. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Li C, Li B, Yang Y, et al. Stratified treatment of localized cervical esophageal squamous cell carcinoma induced by neoadjuvant immunotherapy plus chemotherapy (SCENIC). J Thorac Dis 2022;14:3277-84. [Crossref] [PubMed]
- Yang W, Xing X, Yeung SJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e003497. [Crossref] [PubMed]
- Yu Y, Wang W, Qin Z, et al. A clinical nomogram for predicting tumor regression grade in esophageal squamous-cell carcinoma treated with immune neoadjuvant immunotherapy. Ann Transl Med 2022;10:102. [Crossref] [PubMed]
- Shirakawa Y, Noma K, Maeda N, et al. Early intervention of the perioperative multidisciplinary team approach decreases the adverse events during neoadjuvant chemotherapy for esophageal cancer patients. Esophagus 2021;18:797-805. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Jiang B, Yang X, Zhang J, et al. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable esophageal squamous carcinoma: an open-label, single-arm study. Cancer Res 2022;82:5230. [Crossref]
- Wang J, Zhang K, Liu T, et al. Efficacy and safety of neoadjuvant immunotherapy combined with chemotherapy in locally advanced esophageal cancer: A meta-analysis. Front Oncol 2022;12:974684. [Crossref] [PubMed]
- Mungo B, Molena D, Stem M, et al. Does neoadjuvant therapy for esophageal cancer increase postoperative morbidity or mortality? Dis Esophagus 2015;28:644-51. [Crossref] [PubMed]
- Sihag S, Ku GY, Tan KS, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg 2021;161:836-843.e1. [Crossref] [PubMed]
- Borggreve AS, Kingma BF, Domrachev SA, et al. Surgical treatment of esophageal cancer in the era of multimodality management. Ann N Y Acad Sci 2018;1434:192-209. [Crossref] [PubMed]
- Ikebe M, Morita M, Yamamoto M, et al. Neoadjuvant therapy for advanced esophageal cancer: the impact on surgical management. Gen Thorac Cardiovasc Surg 2016;64:386-94. [Crossref] [PubMed]
(English Language Editor: C. Mullens)


