
Thinking outside the “Enhanced Recovery After Surgery” box: would a more progressive, patient-tailored approach in chest tube management be next?
As part of the special series “Prolonged air leak after lung surgery: prediction, prevention and management” published in the Journal of Thoracic Disease, Batchelor (2023) presents a narrative review on enhanced recovery after surgery (ERAS) and chest tube management (1). The author provides a clear overview of the relevance and benefits of the postoperative ERAS pathway after sublobar and lobar resection, focusing on three intertwined “key care elements”: chest tube management, pain relief, and early mobilization.
“Key care elements” approach to postoperative ERAS pathways
In 2019, the European Society of Thoracic Surgery (ESTS) introduced 45 evidence-based recommendations for the ERAS protocol. These recommendations were divided into 21 peri-operative interventions or care elements covering the pre-admission, admission, intraoperative and postoperative phases of the patient pathway (2). The effectiveness of an ERAS protocol has been described in a recent systematic review and meta-analysis reporting a mean decrease of two days in the length of hospital stay, as well as a decrease in complication- and readmission rates (3). However, strict protocol adherence is mandatory to achieve the suggested benefits of the ERAS guidelines after thoracic surgery (4,5).
Interestingly, wide variations are reported in literature reagarding the extent to which ERAS guidelines are implemented in clinical practice (6-8). As such, a Dutch national survey assessing 23 topics of the 45 recommendations demonstrated that 20 of the 43 responding surgical centers had an ERAS/ESTS score (defined as the amount of compliance) between 65% and 86% of the maximum score, indicating an intermediately high to high compliance in only half of centers (6). Not only does this survey show a large variation in perioperative care, it is important to notice that this percentage does not reflect a proper representation of the actual care delivered to the individual patient. Indeed, a study conducted in Switzerland showed that in only 48% of the patients who were treated by a dedicated ERAS team (certified by the ERAS Society) more than 12 out of 16 implemented ERAS process elements were met (>75% adherence) (7).
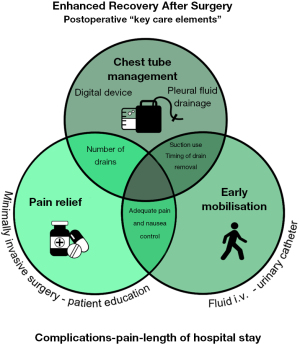
To increase protocol adherence, Batchelor suggested the “key care elements” approach: implementation of only a few ERAS key care elements which appear to be independently important factors associated with better postoperative outcomes (1,9). Figure 1 shows an overview of these postoperative “key care elements” mentioned in the narrative review by Batchelor, focusing on chest tube management intertwined with pain relief and early mobilization, including distinct ERAS recommendations per key care element and any associated other perioperative “key care elements” (e.g., minimally invasive surgery).
Early removal of chest tubes
According to Batchelor, chest tube management is one of the key care elements which significantly improves patient outcomes (1). Conservative chest tube management strategies should be replaced in an evidence-based way, focusing on the early removal of chest tubes. Implementation of a selected number of ERAS recommendations significantly reduces chest tube drainage duration: avoiding routine application of external suction, using digital drainage devices, and use of a higher pleural fluid drainage threshold (up to 450 mL/day) for chest tube removal. Of note, the optimal criteria of chest tube removal for digital drainage systems for air leak flow were not described (2). Published articles report wide variations in air flow criteria regarding the removal of digital drainage systems with volumes between 0 and 50 mL/min for time periods of at least 6 to 12 hours, with or without the presence of air spikes (10-17). These criteria could have a substantial impact on the duration of chest tube drainage, as well as the potential risk of postoperative complications (15). Aside from the chest tube removal criteria, postoperative chest tube drainage duration and the length of hospital stay are first and foremost determined by the frequency of clinical chest tube assessments, which can vary considerably between hospitals.
Thinking outside the ERAS box
An interesting, more progressive approach in chest tube management was addressed by Batchelor in his review, namely chest tube omission after lung resection in selected patients (1). Current literature reports contradictory results regarding its safety in terms of the risk of a postoperative pneumothorax requiring a reintervention (18-20). The differences in risk of reintervention after drainless lung resection could potentially be related to differences in patient selection criteria for chest tube omission.
Patient selection criteria for chest tube omission could be defined by an evidence-based risk model that predicts the risk of postoperative air leakage. Currently, there are validated risk models present in literature which predict the risk of prolonged air leakage after surgery, based on risk factors such as sex, body mass index (BMI), and pulmonary function (21-25). Preoperative estimation of the risk of prolonged air leakage can aid to tailor postoperative care and perioperative actions (e.g., pleurodesis, staple line reinforcement, or earlier discharge with an indwelling chest drain). If such a scoring system could be developed and validated for the risk of any postoperative air leakage, chest tubes may be omitted in selected patients. As scoring systems based on digital drainage data report promising results in the prediction of prolonged postoperative air leakage (25), it could be helpful in the development of a risk model to assess if the patient is eligible for chest tube omission or very early chest tube removal. An important next step to enhance the efficiency of chest tube management is the development of patient-tailored protocols that are based on validated evidence-based risk models for both chest tube omission and prolonged postoperative air leakage.
Conclusions
Batchelor can be commended for his well-written review of the ERAS guidelines. He presents an interesting view on a “key care element” approach as an implementation strategy regarding the postoperative ERAS pathway, highlighting the importance of postoperative chest tube management. Although significant impressive improvements in patient outcomes can be achieved using chest tube strategies recommended by the ERAS, it is important to keep in mind that new and more progressive chest tube protocols are concurrently being implemented in clinical practice. The omission of chest tubes, for example, may lead to even better postoperative patient outcomes, and future studies should gain insight into patient selection criteria. More and better evidence is needed to support a patient-tailored chest tube protocol after lung resections.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-340/coif). KH reports consulting fees from Johnson&Johnson for training in uniportal VATS lobectomy. KH is a Board member of the Dutch Federation of Medical Specialists. YV reports consulting fees for training in uniportal VATS lobectomy, honorarium for teaching lectures in thoracic oncology, and payments for testimony on reducing complications in lung surgery from Johnson&Johnson. YV is a Board member of the Dutch Society for Lung Surgery. EdL reports consulting fees from Johnson&Johnson for training in uniportal VATS lobectomy. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Batchelor TJP. Enhanced recovery after surgery and chest tube management. J Thorac Dis 2023;15:901-8. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Khoury AL, McGinigle KL, Freeman NL, et al. Enhanced recovery after thoracic surgery: Systematic review and meta-analysis. JTCVS Open 2021;7:370-91. [Crossref] [PubMed]
- von Meyenfeldt EM, van Nassau F. Implementing an enhanced recovery after thoracic surgery programme: just having a protocol is not enough. J Thorac Dis 2023;15:1-4. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- von Meyenfeldt EM, de Betue CTI, van den Berg R, et al. Wide Variation in Perioperative Care in Anatomical Lung Resections in the Netherlands: A National Survey. Semin Thorac Cardiovasc Surg 2020;32:1101-10. [Crossref] [PubMed]
- Forster C, Doucet V, Perentes JY, et al. Impact of Compliance With Components of an ERAS Pathway on the Outcomes of Anatomic VATS Pulmonary Resections. J Cardiothorac Vasc Anesth 2020;34:1858-66. [Crossref] [PubMed]
- Budacan AM, Mehdi R, Kerr AP, et al. National survey of enhanced recovery after thoracic surgery practice in the United Kingdom and Ireland. J Cardiothorac Surg 2020;15:95. [Crossref] [PubMed]
- Petersen RH, Huang L, Kehlet H. Guidelines for enhanced recovery after lung surgery: need for re-analysis. Eur J Cardiothorac Surg 2021;59:291-2. [Crossref] [PubMed]
- Brunelli A, Salati M, Refai M, et al. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: a prospective randomised trial. Eur J Cardiothorac Surg 2010;37:56-60. [Crossref] [PubMed]
- De Waele M, Agzarian J, Hanna WC, et al. Does the usage of digital chest drainage systems reduce pleural inflammation and volume of pleural effusion following oncologic pulmonary resection?—A prospective randomized trial. J Thorac Dis 2017;9:1598-606. [Crossref] [PubMed]
- Gilbert S, McGuire AL, Maghera S, et al. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. J Thorac Cardiovasc Surg 2015;150:1243-9. [Crossref] [PubMed]
- Lijkendijk M, Licht PB, Neckelmann K. Electronic versus traditional chest tube drainage following lobectomy: a randomized trial. Eur J Cardiothorac Surg 2015;48:893-8; discussion 898. [Crossref] [PubMed]
- Mendogni P, Tosi D, Marulli G, et al. Multicenter randomized controlled trial comparing digital and traditional chest drain in a VATS pulmonary lobectomy cohort: interim analysis. J Cardiothorac Surg 2021;16:188. [Crossref] [PubMed]
- Plourde M, Jad A, Dorn P, et al. Digital Air Leak Monitoring for Lung Resection Patients: A Randomized Controlled Clinical Trial. Ann Thorac Surg 2018;106:1628-32. [Crossref] [PubMed]
- Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [Crossref] [PubMed]
- Takamochi K, Nojiri S, Oh S, et al. Comparison of digital and traditional thoracic drainage systems for postoperative chest tube management after pulmonary resection: A prospective randomized trial. J Thorac Cardiovasc Surg 2018;155:1834-40. [Crossref] [PubMed]
- Laven IEWG, Daemen JHT, Janssen N, et al. Risk of Pneumothorax Requiring Pleural Drainage after Drainless VATS Pulmonary Wedge Resection: A Systematic Review and Meta-Analysis. Innovations (Phila) 2022;17:14-24. [Crossref] [PubMed]
- Li R, Qiu J, Qu C, et al. Comparison of perioperative outcomes with or without routine chest tube drainage after video-assisted thoracoscopic pulmonary resection: A systematic review and meta-analysis. Front Oncol 2022;12:915020. [Crossref] [PubMed]
- Huang L, Kehlet H, Holbek BL, et al. Efficacy and safety of omitting chest drains after video-assisted thoracoscopic surgery: a systematic review and meta-analysis. J Thorac Dis 2021;13:1130-42. [Crossref] [PubMed]
- Brunelli A, Varela G, Refai M, et al. A scoring system to predict the risk of prolonged air leak after lobectomy. Ann Thorac Surg 2010;90:204-9. [Crossref] [PubMed]
- Pompili C, Falcoz PE, Salati M, et al. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: An analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957-65. [Crossref] [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8; discussion 1068. [Crossref] [PubMed]
- Gonzalez M, Karenovics W, Bédat B, et al. Performance of prolonged air leak scoring systems in patients undergoing video-assisted thoracoscopic surgery segmentectomy. Eur J Cardiothorac Surg 2022;62:ezac100. [Crossref] [PubMed]
- Klement W, Gilbert S, Maziak DE, et al. Chest Tube Management After Lung Resection Surgery using a Classifier. 2019 IEEE International Conference on Data Science and Advanced Analytics (DSAA) 2019:432-41.


