
Analysis of risk factors for negative emotions in patients with severe pneumonia and their impact on prognosis
Highlight box
Key findings
• Negative emotions have a significant impact on the prognosis of patients with SP. According to individual differences, targeted nursing interventions should be supplemented to stabilize patients’ emotions, improve their prognosis, and enhance their quality of life.
What is known and what is new?
• Negative emotions (such as anxiety and depression) having many independent risk factors are known.
• Negative emotions having a significant impact on the prognosis of patients with SP is new. The finding that the presence of children and the presence of a spouse are independent risk factors for negative emotions in patients with severe pneumonia is new.
What is the implication, and what should change now?
• During treatment of SP, clinical efforts should pay attention to the patients’ emotions and demeanor, identification of relevant risk factors as early as possible, adoption of targeted measures to alleviate patients’ anxiety and depression, and prevention of complications.
Introduction
Pneumonia is an inflammatory lesion of the lungs caused by pathogenic infections such as viruses, bacteria or immune damage, physicochemical factors, drugs, or allergies. Pneumonia can occur at any age, but the incidence is higher in groups with low or impaired body immunity, smokers, and those within a poor living environment (1-3). Severe pneumonia (SP) is a common and critical medical condition with an acute onset, rapid progression in addition to many complications, and is one of the leading causes of death in the intensive care unit (ICU) (4). Patients with severe pneumonia in ICU often experience negative emotions such as irritability, anxiety, and depression due to the physical pain caused by the disease, the long duration of the disease, and the poor treatment outcome and prognosis of most of them, which cause great pressure and distress to the family economically and mentally plus the patient’s concern about the disease and fear of prognosis (5). Negative emotions such as anxiety and depression often lead to patients’ non-cooperation with treatment, which affects patients’ recovery and prolongs hospitalization, increases mortality and hospitalization costs, and seriously affects patients’ prognosis (6). In the elderly population, the morbidity and mortality rate of SP patients varies from 2% to 20%, and up to 50%, and varies according to health care facility, geographical area, patient’s physical condition, and age (7,8). Currently, more and more attentions are being paid to patient psychological interventions among the medical community. By improving the patient’s knowledge of the disease, the patient’s awareness of the necessity of the treatment measures implemented can be enhanced, thus improving the patient’s compliance during treatment. In addition, enhanced psychological interventions can improve patients’ negative thoughts and reduce their burden, effectively alleviating their negative emotions and improving clinical outcomes. So et al. (9) showed that humanistic care can improve the psychological status of pneumonia patients and enhance the treatment outcome. Thus, SP is associated with high morbidity and mortality (10), and its early detection and, timely and adequate antimicrobial therapy are essential for the recovery of patients (11).
Patients with SP generally have an acute onset, with respiratory symptoms such as fever, cough, dyspnea, and neurological symptoms such as depression, irritability, and drowsiness. Complications such as shock and hepatic and renal insufficiency often follow, and require long-term hospitalization (12-14). During hospitalization, patients are often required to receive invasive treatments such as mechanical ventilation, which may cause fear, worry, anxiety, and other adverse psychological effects, resulting in reduced compliance with treatment and seriously affecting the quality of life of patients. However, there are few studies on the effects of negative emotions on the prognosis of patients with SP. This study aims to analyze the risk factors of negative emotions in patients with SP and their effects on prognosis, to provide a reference for improving the prognosis and quality of life of patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-413/rc).
Methods
Research participants
The study included 270 patients with SP admitted to Hai’an Hospital from June 2017 to June 2021.
Inclusion criteria: (I) those who met the diagnostic criteria of CAP and were clearly diagnosed with SP; (II) complete information on laboratory findings; (III) age ≥18 years; (IV) those who voluntarily signed the informed consent form.
Exclusion criteria: (I) patients with malignant tumors such as lung cancer; (II) patients with other lung diseases such as tuberculosis and pulmonary embolism; (III) patients with other serious organ diseases such as myocarditis and renal failure, or serious cardiovascular and cerebrovascular diseases such as cerebral infarction and acute myocardial infarction; (IV) patients receiving anti-infection or hormone therapy in the last 2 weeks; (V) patients with untreated discharge, unclear diagnosis, and incomplete information; (VI) patients with consciousness and communication disorders, or those diagnosed with mental illness prior to admission to the hospital (Figure 1).
The purpose of this cross-sectional study was to investigate the relationship between severe pneumonia combined with negative emotions and prognosis. The prevalence of severe pneumonia combined with negative emotions was reported to be approximately 17% according to the group’s previous study and relevant literature. A tolerance error of 3% and a confidence level of 1-α=0.95 were specified, and the sample size N=235 cases to be investigated was calculated using PASS 15 software. Besides this, the general rule of logistic regression and multiple linear regression requires a ratio of item number to sample size of 1:5–1:10. Therefore, the sample size of the study population planned for this study was 270 cases. There were 27 cases of actual attrition and those with incomplete information, and 243 cases were finally counted. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Affiliated Hai’an Hospital of Nantong University (No. KYLC2017173) and informed consent was taken from all the patients.
General information questionnaire
The general information questionnaire included demographic data, including gender, age, education level, monthly household income, address, fertility or not, and spouse or not, and clinical data (respiration, temperature, heart rate, history of underlying disease such as hypertension, diabetes, etc.), Acute Physiology and Chronic Health Evaluation II (APACHE II) score on admission, partial pressure of oxygen (PaO2), oxygen saturation (SaO2), partial pressure of carbon dioxide (PaCO2), white blood cell count (WBC), procalcitonin (PCT), neutrophil to lymphocyte ratio (NLR), platelet count (PLT), albumin (Alb), C-reactive protein (CRP), fever reduction time, ICU treatment time, mechanical ventilation time, hospitalization time, and complications.
Negative emotion assessment
The Self-Rating Anxiety Scale (SAS) was used to reflect the existence and degree of anxiety (15). SAS consisted of 20 items, and each item scored 1 to 4 points individually, resulting in a 20 to 80 raw score. The standard score was calculated by multiplying raw scores by 1.25, and as a result, the total standard score ranged 25 to 100. The evaluation criteria are SAS score >50, 50–59 for mild anxiety, 60–69 for moderate anxiety, and 70 and above for severe anxiety. The scale has good reliability and validity, and the Cronbach’s α coefficients are above 0.75.
The Self-Rating Depression Scale (SDS) was used to reflect the existence and degree of depression (16). This consists of 20 items and an SDS score >52 is used as the evaluation criterion. An SDS ≥53 is classified as depression, 53–62 as mild depression, 63–72 as moderate depression, and >72 as severe depression. The SDS has good reliability and validity, and the Cronbach’s α coefficients are above 0.75.
Prognostic evaluation
APACHE II Score
The APACHE II score is the acute physiology and chronic health status scoring system II, which includes the acute physiology score (temperature, mean arterial pressure, heart rate, respiratory rate, current oxygen concentration, serum Na+, serum K+, blood creatinine, erythrocyte volume, WBC, Glasgow coma score-eye opening, Glasgow coma score-verbal, and Glasgow coma score-movement), age score, and chronic health score (history of previous organ insufficiency or immunosuppression). Each item is scored according to the degree of deviation from normal values, with a total score of 71, higher scores indicating a more critical condition (17). The APACHE II score is a reliable indicator for the assessment of critical illness in internal medicine, and its score is significantly and positively correlated with the severity of SP patients. The score also correlates well with the actual morbidity and mortality rate in patients with SP and has become an important predictor of death.
Kaplan-Meier survival curve
The Kaplan-Meier survival curve is currently one of the most commonly used methods for survival analysis and was first proposed by Kaplan and Meier in 1958s. It is a continuous step-shaped curve plotted with survival time as the horizontal axis and survival rate as the vertical coordinate to illustrate the relationship between survival time and survival rate. A 1-year follow-up visit was conducted after surgery by telephone, SMS, and outpatient review. The follow-up visit included routine physical examination and general CT scan. If any patient experienced discomfort, they were seen at the hospital at any time. The patient’s death was considered as the follow-up outcome, and the last follow-up visit occurred in June 2022.
Statistical analysis
SPSS 26 statistical software (IBM SPSS, USA) was used for data process and statistical analysis. Data were described as mean with standard deviation (SD), or count (percentage). Statistical analysis between groups was performed using t-test, ANOVA, and chi-square test, and independent risk factors for the negative emotions and poor prognosis of patients were analyzed using binary logistics regression and multiple linear regression. Data for continuous variables were analyzed statistically between groups using t-test and ANOVA, and data for categorical variables were analyzed statistically between groups using chi-square test. Data with significant univariate tests were included in binary logistic regression to analyze independent risk factors for patients’ negative emotions. Factors that may affect patient prognosis were included in multiple linear regression to analyze independent risk factors affecting patient prognosis. A P value <0.05 was considered statistically significant.
Results
Baseline data
The baseline characteristics of patients are shown in Table 1. A total of 243 patients with SP were included in this study, including 156 (64.2%) with bacterial infection, 54 (22.2%) with viral infection, 19 (7.8%) with mycoplasma infection, and 14 (5.8%) with other pathogens, with SDS scores of 47.97±6.26, 46.11±5.63, 50.74±7.55, and 48.14±8.32, respectively, which indicates statistically significant differences (P=0.048). There were 127 cases (52.3%) in males and 116 cases (47.7%) in females. The SAS scores were 45.44±4.35 and SDS scores were 46.66±6.29 in males and 47.29±5.19 and 49.02±6.38 in females, showing the anxiety (P=0.003) and depression (P=0.004) scores were significantly different in both groups. A total of 70 (28.8%) of 243 patients had a temperature ≤37.3 ℃ and 173 (71.2%) had a temperature >37.3 ℃. In these two groups, the SAS scores were 44.86±4.55 and 46.92±4.85, respectively, with statistical differences (P=0.003). In addition, 122 patients (50.21%) had a history of underlying disease and 121 patients (49.79%) had no history of underlying disease, with SDS scores of 46.75±6.00 and 48.83±6.70, respectively, which was also statistically different (P=0.011). A total of 125 patients (51.44%) lived in urban areas and their SAS scores were 47.10±5.24, while 118 patients (48.56%) lived in rural areas and their SAS scores were 45.51±4.28, which was significantly lower than those in the urban group (P=0.010). In addition, there were 123 patients (50.62%) with monthly family income ≤5,000 yuan and 120 patients (49.38%) with >5,000 yuan, and the SDS scores of the two groups were 48.99±6.46 and 46.55±6.18, respectively, with a statistically significant difference (P=0.003). A total of 224 patients (92.18%) had children, with SAS scores of 46.08±4.69 and SDS scores of 47.46±6.42, while 19 patients (7.82%) had no children, with SAS scores of 49.16±5.92 and SDS scores of 51.68±5.31. There were 206 cases (84.77%) with a spouse, with an SAS score of 46.05±4.70 and SDS score of 47.41±6.32, and 37 cases (15.23%) without a spouse, with an SAS score of 47.84±5.44 and SDS score of 49.89±6.70. There were 131 cases (53.91%) with an APACHE II score ≤12 with an SAS score of 45.22±3.75 and SDS score of 46.98±5.50, while the APACHE II score >12 was 47.62±5.63 and the SDS score was 48.73±7.28 in 112 cases (46.09%), and the SAS score (P=0.000) and SDS score (P=0.038) were statistically different in these two groups.
Table 1
| Items | N (%) | SAS | SDS | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | t/F | P | Mean ± SD | t/F | P | |||
| Types of pneumonia | 2.040 | 0.109 | 2.671 | 0.048 | ||||
| Bacterial pneumonia | 156 (64.2) | 46.26±4.71 | 47.97±6.26 | |||||
| Viral pneumonia | 54 (22.2) | 45.46±4.56 | 46.11±5.63 | |||||
| Mycoplasma pneumonia | 19 (7.8) | 48.26±5.54 | 50.74±7.55 | |||||
| Other types of pneumonia | 14 (5.8) | 47.79±5.93 | 48.14±8.32 | |||||
| Gender | 2.999 | 0.003 | 2.897 | 0.004 | ||||
| Male | 127 (52.3) | 45.44±4.35 | 46.66±6.29 | |||||
| Female | 116 (47.7) | 47.29±5.19 | 49.02±6.38 | |||||
| Age (year) | −0.313 | 0.754 | −1.007 | 0.315 | ||||
| ≤70 | 107 (44.0) | 46.21±4.78 | 47.32±6.59 | |||||
| >70 | 136 (56.0) | 46.41±4.92 | 48.15±6.30 | |||||
| Breathing rate (times per minute) | −0.696 | 0.487 | 1.461 | 0.145 | ||||
| ≤25 | 130 (53.5) | 46.12±4.75 | 48.35±6.57 | |||||
| >25 | 113 (46.5) | 46.56±4.98 | 47.14±6.23 | |||||
| Temperature (℃) | −3.053 | 0.003 | 0.044 | 0.965 | ||||
| ≤37.3 | 70 (28.8) | 44.86±4.55 | 47.81±5.55 | |||||
| >37.3 | 173 (71.2) | 46.92±4.85 | 47.77±6.76 | |||||
| HR (times per minute) | −1.295 | 0.197 | −0.295 | 0.769 | ||||
| ≤90 | 191 (78.60) | 46.12±4.73 | 47.72±6.36 | |||||
| >90 | 52 (21.40) | 47.10±5.26 | 48.02±6.74 | |||||
| Smoking history | 0.126 | 0.900 | −0.191 | 0.849 | ||||
| Yes | 133 (54.73) | 46.36±4.79 | 47.71±6.51 | |||||
| No | 110 (45.27) | 46.28±4.94 | 47.87±6.35 | |||||
| History of underlying disease | −1.076 | 0.283 | −2.561 | 0.011 | ||||
| Yes | 122 (50.21) | 45.99±4.61 | 46.75±6.00 | |||||
| No | 121 (49.79) | 46.66±5.08 | 48.83±6.70 | |||||
| Address | −2.595 | 0.010 | −1.568 | 0.118 | ||||
| City | 125 (51.44) | 47.10±5.24 | 48.41±7.08 | |||||
| Countryside | 118 (48.56) | 45.51±4.28 | 47.13±5.61 | |||||
| Monthly household income (yuan) | 0.793 | 0.428 | 3.010 | 0.003 | ||||
| ≤5,000 | 123 (50.62) | 46.57±4.82 | 48.99±6.46 | |||||
| >5,000 | 120 (49.38) | 46.08±4.89 | 46.55±6.18 | |||||
| Education level | 1.205 | 0.229 | 0.038 | 0.970 | ||||
| High school and above | 129 (53.09) | 46.60±4.72 | 47.72±6.39 | |||||
| High school or below | 113 (46.50) | 45.87±4.79 | 47.69±6.27 | |||||
| Fertility or not | 2.205 | 0.039 | 2.792 | 0.006 | ||||
| Have children | 224 (92.18) | 46.08±4.69 | 47.46±6.42 | |||||
| No children | 19 (7.82) | 49.16±5.92 | 51.68±5.31 | |||||
| Spouse or not | 2.075 | 0.039 | 2.181 | 0.030 | ||||
| Have spouse | 206 (84.77) | 46.05±4.70 | 47.41±6.32 | |||||
| No spouse | 37 (15.23) | 47.84±5.44 | 49.89±6.70 | |||||
| APACHE II score | −3.834 | 0.000 | −2.092 | 0.038 | ||||
| ≤12 | 131 (53.91) | 45.22±3.75 | 46.98±5.50 | |||||
| 13–25 | 112 (46.09) | 47.62±5.63 | 48.73±7.28 | |||||
SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; SD, standard deviation; HR, heart rate; APACHE II, Acute Physiology and Chronic Health Evaluation II.
In conclusion, gender, presence of children, presence of spouse, and APACHE II score significantly affected the SAS and SDS scores of patients. In addition, the patient’s body temperature and address significantly affected their SAS score, while the infective pathogen, a history of underlying disease, and a monthly household income significantly affected the SDS score.
Complications for patients with SP
Among patients with combined pulmonary edema, 11 (47.8%) had negative emotions and 12 (52.2%) did not, while among patients presenting with infectious shock, 10 (90.9%) had combined negative emotions and only one (9.1%) did not. Among patients presenting with hemoptysis, 10 (83.3%) had combined negative emotions and only two (16.7%) did not present negative emotions. Among all complications, there was a significant difference between the presence or absence of pulmonary edema (P=0.010), infectious shock (P=0.000), and hemoptysis (P=0.000), and the presence or absence of negative emotions (P<0.05), as shown in Table 2.
Table 2
| Item | Pulmonary edema | Bronchial dilation | Infectious shock | Respiratory tract infections | Hemoptysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |||||
| With negative emotions, n (%) | 11 (47.8) | 51 (23.2) | 7 (35.0) | 55 (24.7) | 10 (90.9) | 52 (22.4) | 7 (43.8) | 55 (24.2) | 10 (83.3) | 52 (22.5) | ||||
| No negative emotions, n (%) | 12 (52.2) | 169 (76.8) | 13 (65.0) | 168 (75.3) | 1 (9.1) | 180 (77.6) | 9 (56.3) | 172 (75.8) | 2 (16.7) | 179 (77.5) | ||||
| χ2 | 6.655 | 1.032 | 25.926 | 2.997 | 22.205 | |||||||||
| P | 0.010 | 0.310 | 0.000 | 0.083 | 0.000 | |||||||||
Risk factors of patients’ anxiety and depression analyzed by binary logistic regression
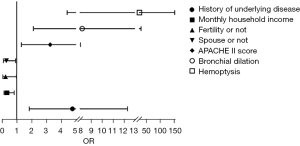
Binary logistics regression analysis showed gender (P=0.007), presence of children (P=0.048), presence of spouse (P=0.027), APACHE II score (P=0.000), infectious shock (P=0.016), and hemoptysis (P=0.008) were independent risk factors for anxiety (Table 3, Figure 2). A history of underlying disease (P=0.001), monthly household income (P=0.018), presence of children (P=0.047), presence of spouse (P=0.035), APACHE II score (P=0.010), bronchiectasis (P=0.002), and hemoptysis (P=0.000) were independent risk factors for depression (Table 4, Figure 3).
Table 3
| Related factor | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Gender | −1.326 | 0.489 | 7.346 | 0.007 | 0.265 | 0.102 | 0.693 |
| Temperature | 0.893 | 0.579 | 2.378 | 0.123 | 2.443 | 0.785 | 7.605 |
| Address | 0.734 | 0.469 | 2.451 | 0.117 | 2.083 | 0.831 | 5.222 |
| Fertility or not | −1.300 | 0.657 | 3.910 | 0.048 | 0.273 | 0.075 | 0.989 |
| Spouse or not | −1.278 | 0.577 | 4.899 | 0.027 | 0.279 | 0.090 | 0.864 |
| APACHE II score | 2.249 | 0.552 | 16.570 | 0.000 | 9.475 | 3.209 | 27.978 |
| Pulmonary edema | 0.477 | 0.714 | 0.447 | 0.504 | 1.612 | 0.398 | 6.531 |
| Bronchial dilation | 0.081 | 0.925 | 0.008 | 0.930 | 1.084 | 0.177 | 6.642 |
| Infectious shock | 2.345 | 0.973 | 5.806 | 0.016 | 10.433 | 1.549 | 70.276 |
| Respiratory tract infections | 1.019 | 0.753 | 1.831 | 0.176 | 2.771 | 0.633 | 12.125 |
| Hemoptysis | 2.142 | 0.809 | 7.015 | 0.008 | 8.513 | 1.745 | 41.529 |
SE, standard error; OR, odds ratio; CI, confidence interval; APACHE II, Acute Physiology and Chronic Health Evaluation II.
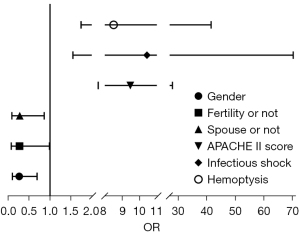
Table 4
| Related factor | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Types of pneumonia | 1.124 | 0.660 | 2.898 | 0.089 | 3.077 | 0.844 | 11.226 |
| Gender | −0.724 | 0.442 | 2.684 | 0.101 | 0.485 | 0.204 | 1.153 |
| History of underlying disease | 1.562 | 0.485 | 10.384 | 0.001 | 4.768 | 1.844 | 12.327 |
| Monthly household income | −1.075 | 0.454 | 5.605 | 0.018 | 0.341 | 0.140 | 0.831 |
| Fertility or not | −1.399 | 0.706 | 3.932 | 0.047 | 0.247 | 0.062 | 0.984 |
| Spouse or not | −1.195 | 0.568 | 4.422 | 0.035 | 0.303 | 0.099 | 0.922 |
| APACHE II score | 1.182 | 0.462 | 6.553 | 0.010 | 3.261 | 1.319 | 8.059 |
| Pulmonary edema | 0.838 | 0.605 | 1.920 | 0.166 | 2.311 | 0.707 | 7.556 |
| Bronchial dilation | 2.106 | 0.689 | 9.338 | 0.002 | 8.219 | 2.128 | 31.739 |
| Infectious shock | 1.131 | 1.125 | 1.009 | 0.315 | 3.098 | 0.341 | 28.120 |
| Respiratory tract infections | 1.081 | 0.779 | 1.925 | 0.165 | 2.949 | 0.640 | 13.585 |
| Hemoptysis | 3.259 | 0.906 | 12.947 | 0.000 | 26.012 | 4.409 | 153.472 |
SE, standard error; OR, odds ratio; CI, confidence interval.
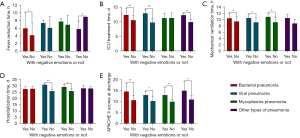
Comparison of prognostic indicators for patients with different types of pneumonia
There was a significant difference between the fever reduction time and the presence of negative emotions in patients with bacterial pneumonia (P=0.006) and other types of pneumonia (P=0.012) (Table 5, Figure 4A). There was a significant difference (P<0.05) between the duration of ICU treatment and the presence or absence of negative emotions in patients with bacterial (P=0.000), viral pneumonia (P=0.000), and other types of pneumonia (P=0.001), as shown in Table 5, Figure 4B. There was a significant difference between the mechanical ventilation time and the presence or absence of negative emotions in patients with bacterial(P=0.001) and viral pneumonia (P=0.015) and mycoplasma pneumonia (P=0.017), as shown in Table 5, Figure 4C. There was a significant difference between the length of hospitalization time and the presence or absence of negative emotions in patients with viral pneumonia (P=0.000) and mycoplasma pneumonia (P=0.002) (P<0.05), as shown in Table 5, Figure 4D. There was a significant difference (P<0.05) between APACHE II scores at discharge and the presence or absence of negative emotions in all four groups (P=0.000; P=0.001; P=0.019; P=0.024) of patients with pneumonia types, as shown in Table 5, Figure 4E.
Table 5
| Items | N (%) | Fever reduction time (h) | ICU treatment time (d) |
Mechanical ventilation time (h) | Hospitalization time (d) | APACHE II scores at discharge |
|---|---|---|---|---|---|---|
| Bacterial pneumonia | ||||||
| With negative emotions | 42 (26.92) | 5.98±4.09 | 12.33±2.18 | 10.43±1.63 | 27.40±1.86 | 14.64±3.80 |
| No negative emotions | 114 (73.08) | 4.19±3.33 | 10.54±1.64 | 9.43±1.55 | 27.63±1.70 | 10.69±2.32 |
| t | −2.783 | −5.542 | −3.351 | 0.722 | −7.839 | |
| P | 0.006 | 0.000 | 0.001 | 0.471 | 0.000 | |
| Viral pneumonia | ||||||
| With negative emotions | 8 (14.81) | 7.38±0.74 | 12.88±1.36 | 10.50±1.20 | 30.88±0.99 | 13.00±1.69 |
| No negative emotions | 46 (85.19) | 6.11±2.93 | 9.74±1.50 | 9.15±1.43 | 25.76±1.46 | 10.30±2.01 |
| t | −1.207 | −5.941 | −2.513 | −9.475 | −3.573 | |
| P | 0.233 | 0.000 | 0.015 | 0.000 | 0.001 | |
| Mycoplasma pneumonia | ||||||
| With negative emotions | 8 (42.11) | 7.75±0.89 | 11.25±1.58 | 10.88±1.13 | 29.13±1.73 | 13.00±3.63 |
| No negative emotions | 11 (57.89) | 6.91±2.47 | 11.27±1.85 | 9.18±1.54 | 25.82±1.99 | 10.00±1.18 |
| t | −0.916 | 0.028 | −2.635 | −3.772 | −2.586 | |
| P | 0.373 | 0.978 | 0.017 | 0.002 | 0.019 | |
| Other types of pneumonia | ||||||
| With negative emotions | 4 (28.57) | 5.80±2.10 | 12.25±0.50 | 10.50±1.19 | 28.00±2.16 | 15.00±4.08 |
| No negative emotions | 10 (71.43) | 9.00±0.000 | 9.90±0.99 | 8.80±1.14 | 27.90±1.45 | 11.00±1.89 |
| t | −2.978 | −4.430 | −2.094 | −0.102 | −2.586 | |
| P | 0.012 | 0.001 | 0.058 | 0.920 | 0.024 |
Data are expressed as mean ± SD. ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II; SD, standard deviation.
Comparison of laboratory indicators for patients with different types of pneumonia
Among the laboratory examination indexes, WBC (P=0.000), PCT (P=0.000), NLR (P=0.000), and CRP (P=0.000) were significantly different in the four groups of pneumonia types. In patients with bacterial pneumonia, WBC was (11.14±2.59)×109/L, PCT was 7.31±4.47 ng/mL, NLR was 3.79±1.30, and CRP was 12.59±4.06 mg/L. In patients with viral pneumonia, WBC was (6.89±1.75)×109/L, PCT was 1.33±0.75 ng/mL, NLR was 0.94±0.46 and CRP was 8.17±2.19 mg/L. The WBC of patients with mycoplasma pneumonia was (9.17±1.96)×109/L, PCT was 1.13±0.68 ng/mL, NLR was 2.93±1.26, and CRP was 10.73±3.07 mg/L. The WBC of patients with other types of pneumonia was (9.24±1.78)×109/L, PCT was 1.46±0.91 ng/mL, NLR was 2.12±0.63, and CRP was 11.96±3.48 mg/L (Table 6).
Table 6
| Item | PaO2 (mmHg) | SaO2 (%) | PaCO2 (mmHg) | WBC (×109/L) | PCT (ng/mL) | PLT (×109/L) | NLR | Alb (g/L) | CRP (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Bacterial pneumonia | 66.38±10.90 | 83.69±7.81 | 43.16±7.019 | 11.14±2.59 | 7.31±4.47 | 291.50±65.13 | 3.79±1.30 | 28.45±2.85 | 12.59±4.06 |
| Viral pneumonia | 68.19±9.85 | 84.48±7.44 | 42.11±6.32 | 6.89±1.75 | 1.33±0.75 | 292.56±68.40 | 0.94±0.46 | 28.56±2.59 | 8.17±2.19 |
| Mycoplasma pneumonia | 69.74±10.68 | 85.79±86.5 | 43.47±8.30 | 9.17±1.96 | 1.13±0.68 | 300.89±65.83 | 2.93±1.26 | 29.38±3.11 | 10.73±3.07 |
| Other types of pneumonia | 67.86±10.19 | 83.21±7.82 | 42.50±6.15 | 9.24±1.78 | 1.46±0.91 | 285.36±49.51 | 2.12±0.63 | 27.61±2.11 | 11.96±3.48 |
| F | 0.837 | 0.530 | 0.365 | 45.299 | 51.108 | 0.171 | 86.922 | 1.150 | 17.576 |
| P | 0.475 | 0.662 | 0.778 | 0.000 | 0.000 | 0.916 | 0.000 | 0.330 | 0.000 |
Data are expressed as mean ± SD. PaO2, partial pressure of oxygen; SaO2, oxygen saturation of blood; PaCO2, pressure of carbon dioxide; WBC, white blood cell; PCT, procalcitonin; PLT, platelet; NLR, neutrophil to lymphocyte ratio; Alb, albumin; CRP, C-reactive protein.
Risk factors of prognosis of APACHE II scores by multiple linear regression
Multiple linear regression analysis showed Alb (P=0.026), CRP (P=0.014), mechanical ventilation time (P=0.000), and presence of negative emotions (P=0.000) were independent risk factors affecting patient prognosis (Table 7).
Table 7
| Related factor | B | SE | t | P |
|---|---|---|---|---|
| PaO2 | −0.010 | 0.029 | −0.333 | 0.740 |
| SaO2 | −0.009 | 0.024 | −0.390 | 0.697 |
| PaCO2 | −0.029 | 0.041 | −0.722 | 0.471 |
| WBC | 0.061 | 0.060 | 1.020 | 0.309 |
| PCT | 0.027 | 0.039 | 0.687 | 0.493 |
| PLT | 0.000 | 0.002 | −0.091 | 0.928 |
| NLR | −0.149 | 0.106 | −1.404 | 0.162 |
| Alb | 0.132 | 0.059 | 2.245 | 0.026 |
| CRP | 0.104 | 0.042 | 2.479 | 0.014 |
| Fever reduction time | −0.072 | 0.047 | −1.537 | 0.126 |
| ICU treatment time | 0.137 | 0.095 | 1.434 | 0.153 |
| Mechanical ventilation time | 0.527 | 0.107 | 4.907 | 0.000 |
| Hospitalization time | −0.125 | 0.081 | −1.536 | 0.126 |
| With negative emotions or not | 2.715 | 0.459 | 5.915 | 0.000 |
| Pulmonary edema | 0.679 | 0.524 | 1.295 | 0.197 |
| Bronchial dilation | 0.219 | 0.549 | 0.398 | 0.691 |
| Infectious shock | 1.373 | 0.800 | 1.716 | 0.088 |
| Respiratory tract infections | 0.248 | 0.623 | 0.398 | 0.691 |
| Hemoptysis | −1.122 | 0.787 | −1.426 | 0.155 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; SE, standard error; PaO2, partial pressure of oxygen; SaO2, oxygen saturation of blood; PaCO2, pressure of carbon dioxide; WBC, white blood cell; PCT, procalcitonin; PLT, platelet; NLR, neutrophil to lymphocyte ratio; Alb, albumin; CRP, C-reactive protein; ICU, intensive care unit.
Comparison of Kaplan-Meier survival curve between patients in different groups
This follow-up lasted one year, and the difference in cumulative survival function between the two groups of patients with and without negative emotions was statistically significant (P=0.013). In contrast, there was no significant difference (P=0.201) in the comparison of the cumulative survival functions of patients with the four pneumonia types (Figure 5).

Discussion
Pneumonia is a common acute respiratory tract infection involving the alveoli and distal airways. As a common respiratory disease in clinical practice, it is associated with high morbidity and short-term and long-term mortality in all age groups (10). SP is one of the common infectious critical illnesses in clinical practice, and if the inflammation is not effectively controlled, it may continue to worsen after a certain stage of development, affecting not only the function of the respiratory system, but also the circulatory and neurological systems, leading to multi-organ failure and even shock or death (18,19). Medical research statistics show SP is the first infectious disease in terms of morbidity and mortality (20). Patients often feel great uncertainty in the process of disease treatment because of severe symptoms such as high fever, generalized pain, shortness of breath, etc. They tend to feel fearful, worried, and anxious about their prognosis, thus easily inducing negative emotions.
Binary Logistic regression analysis showed that gender, presence of children, presence of spouse, APACHE II score, infectious shock, and hemoptysis were independent risk factors for the development of anxiety in patients. Women have been shown to be a risk factor for negative mood in previous studies (21-23). The main reason for this is that women process emotions differently than men (24). Specifically, women are quick to recognize negative emotions, and people are more likely to fall into negative emotions due to the presence of emotional infection mechanisms. In addition, it has also been shown that women will show a greater response to mild negative events than men and have a longer startle response time to mild negative events; furthermore, even if the stimulus event is not emotionally charged, women show a large startle response as long as it appears suddenly with sudden characteristics (25,26). Because of this emotional processing characteristics of women, resulting in stronger negative emotional susceptibility to negative emotions, then the incidence of women suffering from mood disorders is greatly increased. Good family relationships can largely relieve patients’ mental and psychological pressure. Married patients have spouses and children to take care of their lives, and family members can give positive guidance to patients and observe their psychological conditions in time to help them set up expectations for a better future and stimulate their inner motivation; whereas patients without children and spouses are often more prone to negative emotions because they are alone with life pressure, economic pressure, and confusion and worries about their future lives. In addition, the results of this study found that history of underlying disease, monthly family income, presence of children, presence of spouse, APACHE II score, bronchiectasis, and hemoptysis were independent risk factors for depression, and patients with high APACHE II score or co-morbid underlying disease tended to be less tolerant and had a stronger physical stress response to pneumonia infection, thus making anxiety and fear more severe. Family income directly reflects the economic status of the family. Middle-aged patients have to bear the responsibility and obligation of supporting the elderly and raising children, with double economic pressure, and no source of income during surgery and recuperation, so the psychological pressure is also relatively high. Moreover, the presence of infectious shock, hemoptysis, and bronchiectasis are also independent risk factors for negative emotions in patients. The results suggest complications can affect the development of negative emotions, and their occurrence may increase the psychological burden of patients, resulting in anxiety, depression, and reduced treatment motivation, leading to poor treatment outcomes. A study has shown nursing by health care professionals can enhance the confidence and compliance of patients and reduce their fear, depression, and other negative emotions, which is important for their recovery (17). Therefore, in the clinical treatment process, in addition to paying attention to the primary disease a patient presents with, we should also attach great importance to effective communication and targeted treatment measures in a timely manner.
In recent years, the incidence of pneumonia has been consistently high with an increasing trend year by year. When pneumonia progresses to severe infection, the onset is often insidious and the lack of specific clinical manifestations makes it difficult to determine the optimal time for treatment, which may result in a poor prognosis (27-29). Therefore, it is particularly important to predict the development trend of SP as early as possible based on laboratory test indexes and clinical manifestations. The results of this study showed WBC, PCT, NLR, and CRP differed significantly among bacterial, viral, mycoplasma, and other types of pneumonia, which indicates these assessments are significant in determining the etiology of the disease and help determine appropriate treatment. In addition, common laboratory tests include PaO2, SaO2, PaCO2, and Alb should also be performed. Among the above laboratory indices, PCT and WBC levels are often used to assess the therapeutic effect of patients with SP, and PCT has high specificity and sensitivity in the diagnosis of bacterial infectious diseases (30). However, it has also been pointed out that in patients with SP, because of the additional effects of hypoxia and acidosis, the WBC may not accurately determine the prognosis due to the significantly lower level of the organism’s immunity (31), which is consistent with the results of the present study. The results of this study also found that Alb, CRP, duration of mechanical ventilation, and combined negative emotions were independent risk factors affecting the prognosis of patients. Among these, Alb often reflects the nutritional level of the patient, while CRP, as one of the common and important indicators of inflammation, can be also used to assess prognosis. It has also been shown that if serum infection indicators are chronically high in patients with severe infections, the likelihood of a poor prognosis or even death increase (32). In addition, mechanical ventilation is often used to improve blood oxygenation in patients with relatively critical conditions. However, invasive mechanical ventilation is an invasive procedure, which increases the risk of infection and may have an impact on the clinical outcome. This is also confirmed by the results of this study. Finally, and most importantly, in this study we found that combined negative emotions were an independent risk factor for poor prognosis in patients with SP. Therefore, in the treatment of patients with SP, besides paying attention to the clinical symptoms and examination indexes, we should also focus on the psychological condition of patients, and attach importance to communication and exchange with patients to make them feel care and respect, actively encourage them, answer their doubts, and give them correct guidance to reduce their negative emotions such as nervousness and fear. In conclusion, the combination of negative emotions can significantly affect all aspects of SP patients, and if they develop anxiety and depression, their prognosis and regression are easily affected.
The shortcomings of this study were the inclusion of few cases and a shorter follow-up period of only one year due to limited manpower and time, and it is recommended that a longer follow-up period be used in future studies.
Conclusions
Patients with severe pneumonia have serious conditions and are prone to complications and psychological disorders such as anxiety and depression, which seriously affect the treatment outcome. Therefore, the negative emotions of patients and independent risk factors should be identified in a timely manner in clinical work, and targeted and effective measures should be actively taken to improve prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-413/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-413/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-413/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-413/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Affiliated Hai’an Hospital of Nantong University (No. KYLC2017173) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ghembaza A, Vautier M, Cacoub P, et al. Risk Factors and Prevention of Pneumocystis jirovecii Pneumonia in Patients With Autoimmune and Inflammatory Diseases. Chest 2020;158:2323-32. [Crossref] [PubMed]
- Furman CD, Leinenbach A, Usher R, et al. Pneumonia in older adults. Curr Opin Infect Dis 2021;34:135-41. [Crossref] [PubMed]
- Long ME, Mallampalli RK, Horowitz JC. Pathogenesis of pneumonia and acute lung injury. Clin Sci (Lond) 2022;136:747-69. [Crossref] [PubMed]
- Andrés-Martín A, Escribano Montaner A, Figuerola Mulet J, et al. Consensus Document on Community-Acquired Pneumonia in Children. SENP-SEPAR-SEIP. Arch Bronconeumol 2020;56:725-41. (Engl Ed). [Crossref] [PubMed]
- Kalvas LB, Harrison TM. State of the science in pediatric ICU delirium: An integrative review. Res Nurs Health 2020;43:341-55. [Crossref] [PubMed]
- Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 2007;33:66-73. [Crossref] [PubMed]
- Ramirez JA, Wiemken TL, Peyrani P, et al. Adults Hospitalized With Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin Infect Dis 2017;65:1806-12. [Crossref] [PubMed]
- Heo JY, Song JY. Disease Burden and Etiologic Distribution of Community-Acquired Pneumonia in Adults: Evolving Epidemiology in the Era of Pneumococcal Conjugate Vaccines. Infect Chemother 2018;50:287-300. [Crossref] [PubMed]
- So M, Oda K, Ota K, et al. Retrospective Analysis of Factors Decreasing the Efficacy of Tazobactam/Piperacillin for Pneumonia in Elderly Patients. Yakugaku Zasshi 2018;138:581-8. [Crossref] [PubMed]
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers 2021;7:25. [Crossref] [PubMed]
- Cilloniz C, Pericàs JM, Rojas J. Ceftaroline in severe community-acquired pneumonia. Rev Esp Quimioter 2022;35:28-30. [Crossref] [PubMed]
- Omori M, Chojin Y, Hashiki S, et al. A Simple Assessment of the Eating and Swallowing Functions in Elderly Patients with Pneumonia. J UOEH 2019;41:283-94. [Crossref] [PubMed]
- Jiang J, Hu C, Li Y, et al. Transmission Electron Microscopy Improves the Diagnostic Sensitivity in Nonbacterial Etiology of Severe Pneumonia: A Retrospective Study. Am J Med Sci 2019;357:289-95. [Crossref] [PubMed]
- Li HY, Guo Q, Song WD, et al. Priority for Treatment and Intensive Care of Patients With Non-Severe Community-Acquired Pneumonia. Am J Med Sci 2018;356:329-34. [Crossref] [PubMed]
- Gainotti G, Cianchetti C, Taramelli M, et al. The guided self-rating anxiety-depression scale for use in clinical psychopharmacology. Act Nerv Super (Praha) 1972;14:49-51. [PubMed]
- Zung WW, Gianturco JA. Personality dimension and the Self-Rating Depression Scale. J Clin Psychol 1971;27:247-8. [Crossref] [PubMed]
- Wang D, Sun S, Hu S. The therapeutic efficacy of high-dose ambroxol and the nursing effects in the treatment of severe pneumonia. Pak J Pharm Sci 2019;32:1409-13. [PubMed]
- Kim WY, Jo EJ, Eom JS, et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: Propensity score-based analysis of a before-after cohort study. J Crit Care 2018;47:211-8. [Crossref] [PubMed]
- Yang L, Liu T, Liu BC, et al. Severe Pneumonia Advanced to Lung Abscess and Empyema Due to Rothia Mucilaginosa in an Immunocompetent Patient. Am J Med Sci 2020;359:54-6. [Crossref] [PubMed]
- Artero A, Atienza A, Correa S, et al. Ertapenem therapy for pneumonia requiring hospital admission in elderly people. Rev Esp Quimioter 2016;29:8-14. [PubMed]
- Kindler CH, Harms C, Amsler F, et al. The visual analog scale allows effective measurement of preoperative anxiety and detection of patients’ anesthetic concerns. Anesth Analg 2000;90:706-12. [Crossref] [PubMed]
- Laufenberg-Feldmann R, Kappis B. Assessing preoperative anxiety using a questionnaire and clinical rating: a prospective observational study. Eur J Anaesthesiol 2013;30:758-63. [Crossref] [PubMed]
- Gonçalves KK, Silva JI, Gomes ET, et al. Anxiety in the preoperative period of heart surgery. Rev Bras Enferm 2016;69:397-403. [PubMed]
- Mak AK, Hu ZG, Zhang JX, et al. Sex-related differences in neural activity during emotion regulation. Neuropsychologia 2009;47:2900-8. [Crossref] [PubMed]
- Yuan J, Luo Y, Yan JH, et al. Neural correlates of the females’ susceptibility to negative emotions: an insight into gender-related prevalence of affective disturbances. Hum Brain Mapp 2009;30:3676-86. [Crossref] [PubMed]
- Gard MG, Kring AM. Sex differences in the time course of emotion. Emotion 2007;7:429-37. [Crossref] [PubMed]
- Bodnar VA, Koval TI, Pryimenko NO, et al. Some clinical and epidemiological features of influenza-associated pneumonia depending on the etiological agent. Wiad Lek 2020;73:1410-4. [Crossref] [PubMed]
- Aldás I, Menéndez R, Méndez R, et al. Early and Late Cardiovascular Events in Patients Hospitalized for Community-Acquired Pneumonia. Arch Bronconeumol 2020;56:551-8. (Engl Ed). [Crossref] [PubMed]
- Cho H, Tsuchida K, Iwasaki K, et al. Risk factors of post-operative pneumonia in elderly patients with gastric cancer: a retrospective cohort study. Jpn J Clin Oncol 2021;51:1044-50. [Crossref] [PubMed]
- Ito A, Ishida T, Tachibana H, et al. Utility of procalcitonin for differentiating cryptogenic organising pneumonia from community-acquired pneumonia. Clin Chem Lab Med 2019;57:1632-7. [Crossref] [PubMed]
- Gautam S, Cohen AJ, Stahl Y, et al. Severe respiratory viral infection induces procalcitonin in the absence of bacterial pneumonia. Thorax 2020;75:974-81. [Crossref] [PubMed]
- Liu D, Su LX, Guan W, et al. Prognostic value of procalcitonin in pneumonia: A systematic review and meta-analysis. Respirology 2016;21:280-8. [Crossref] [PubMed]



