
Improving airway management and tracheostomy care through interprofessional collaboration: aligning timing, technique, and teamwork
Tracheostomy is one of the oldest procedures in medicine, and vestiges of its long history can be traced back over 3,500 years to records in the ancient scrolls of Egypt and Vedic Sanskrit hymns of India. Legend has it that in the Hellenistic era, Alexander the Great used the tip of his sword to open the trachea of a choking soldier (1). Such depictions capture the role of tracheotomy as a heroic procedure reserved for life-threatening asphyxia or upper airway obstruction. Over the course of millenia, the indication and approach for tracheostomy have evolved to encompass a growing number of conditions, and the most common indications for tracheotomy now relate to respiratory failure and ventilatory support. The transition of tracheotomy to a largely elective procedure has coincided with a shift from a dramatized intervention by the individual to a carefully planned procedure engaging diverse team members.
Pandemic challenges in tracheostomy care
The coronavirus disease 2019 (COVID-19) pandemic heralded a new era for tracheotomy. During successive waves of pandemic, the procedure was performed in unprecedented numbers. Often, decisions regarding care were fraught with ethical questions around safety or allocation of scarce resources. The challenges that confronted clinicians across countries and continents posed new questions around infection control and timing. The high rates of mortality at overwhelmed hospitals underscored a need for coordinated, interprofessional care. An international quality improvement effort, the Global Tracheostomy Collaborative, has elevated awareness of preventable harm and used patient level-data to drive improvements in safety and quality of care (2). The collaborative also applied its guiding principles to improve quality of life, given the importance of recovery of speaking, swallowing, mobility, and other aspects of survivorship (3-6).
At the height of the COVID-19 pandemic, clinicians grappled with how to deliver patient-centered care without threatening the safety of healthcare professionals during aerosol-generating procedures. As concerns rose to a crescendo, fear and uncertainty constrained decision-making, but eventually scientific and clinical evidence accrued (7-9). The systemic stressors arising from hospital resource capacity strain, supply chain disruptions, and personnel shortages were an impetus for new strategies. COVID-inspired approaches ranged from deployment of healthcare professionals to using artificial intelligence to predict liberation from the ventilator. Virtual team rounds facilitated social distancing, and immersive technologies were developed to accelerate learning and onboarding of staff. This period coincided with the emergence of the largest global quality improvement effort to date, which has made measurable progress in enhancing safety and overall standards for tracheostomy care (1,10).
The pandemic reinvigorated longstanding controversies around timing, technique, and postoperative care for patients with a tracheostomy. Wide variations occurred in international practices and protocols for tracheostomy care (11-14). Timing of tracheostomy became the flash point because many speculated that performing tracheostomy before 10 or 14 days might increase the risk of viral transmission to healthcare professional. Whereas prolonged orotracheal intubation increased the risk of laryngotracheal stenosis, early tracheostomy afforded the prospect of accelerating liberation from the ventilator, thereby easing strain related to the scarcity of equipment, personnel, and intensive care unit (ICU) beds. Guidance documents and international protocols proliferated, but unexpected challenges could complicate decision-making even after a successful procedure. One key consideration is how to prevent or manage tracheostomy tubes that are poorly tolerated in critically ill patients.
Patterns of unplanned tube changes after tracheostomy in patients with COVID-19
In a recent issue of Journal of Thoracic Disease, McCauley and colleagues report on outcomes in ventilated patients with COVID-19. The study focused on unplanned tracheostomy tube exchange among 43 patients undergoing tracheostomy (15). The need for an unplanned tracheostomy tube exchange suggests an extreme safety risk of respiratory compromise, partial dislodgment, accidental decannulation, or device-related pressure injury. Incorrect caliber, angulation, or length can lead to unintended endobronchial placement, erosion of the luminal surface, and patient discomfort (16-19). Furthermore, an inadequate seal can result in loss of positive end-expiratory pressure (PEEP), derecruitment, and aerosolization of virus-laden particles, with risk of transmission to healthcare workers. The retrospective report of outcomes of tracheostomy for COVID-19 reveals the difficulty of standardizing tracheostomy care. Nearly one-third of patients (n=14) who underwent tracheostomy received an unplanned tracheostomy tube change, and the most common reason for tube change was persistent air leakage, as noted in 10 patients. Other reasons for tube change included patient-ventilator dyssynchrony, discomfort, inability to clear secretions, and accidental decannulation.
The study also reflects the growing trend towards using percutaneous tracheostomy as a first-line treatment in critical settings (8,19-21). Most tube changes involved increasing tube length or caliber. Among the 14 patients requiring tube exchange, there was a change to a longer device [e.g., ShileyTM to Shiley Extended Length Tube (XLT)TM] in 8 patients; upsizing of the device in five patients, and both upsizing and transitioning to XLT device in the remaining patients. The timing for these unplanned tube changes ranged from <24 h to over a month, with a mean of 5.5 days. The authors highlight the counter-intuitive characteristics of International Organization for Standardization (ISO) 8.8 mm tracheostomy tubes, which correspond to inner diameters of 7.0 mm (Portex® Blue Line Ultra®), 7.5 mm (ShileyTM flexible adult TaperGuardTM), and 8.0 mm (Tracoe® Twist). The authors standardized their approach using the ShileyTM line of products, but the 33% incidence of unplanned tracheostomy changes underscores the need for a tailored approach to selecting tracheostomy devices based on patient anatomy, physiology, and ventilatory requirements.
The tracheostomy tube exchanges were performed with relatively few difficulties. From a patient safety perspective, there was no evidence of significant derecruitment, based on the absence of significant differences in FiO2, PEEP, or peak airway pressure assessed at days 1, 3, and 5 after placement of the tracheostomy tube. This favorable outcome may partly have reflected standardized protocols to exchange the tube efficiently and minimize the loss of PEEP, or perhaps the signs of derecruitment were measured too late since derecruitment typically occurs immediately. The parameters measured usually returned to baseline by days 1, 3, or 5. The authors also report routine use of cuff manometry to minimize the risk of ischemic injury or aspiration arising from over- or under-inflation of cuffs, respectively. Anecdotally, personnel concerned with viral transmission from an incompletely sealed circuit sometimes over-inflated cuffs, and reports of subglottic stenosis after prolonged intubations are increasing (22). The authors also used a defined protocol for enlarging the stoma site with a Blue Rhino® dilator when performing upsizing and did not report instances of false passages caused by devices, thus avoiding respiratory compromise, subcutaneous emphysema, and exposure of personnel to aerosols.
Predictors and management of unplanned tracheostomy tube exchange
Several insights regarding etiologic factors in unplanned tracheostomy tube exchange can be drawn from the present study. Consistent with prior literature, patients with obesity are more likely to have a longer distance from the skin or flange to the trachea and thus require a longer proximal length of the tracheostomy tube; therefore, initial placement of a proximal XLT device should be considered in patients with body mass index (BMI) >30 kg/m2, or if the skin-cricoid distance is >4 cm on computed tomography imaging (23). Even individuals with lower BMI may have sufficient soft tissue in the neck to result in suboptimal device position and reduction of ventilatory flow. This observation is borne out by findings in the present study that change to an XLT was the intervention most used to resolve problems with excessive air leakage, including several patients without elevated BMI. Ensuring adequate ventilation in patients with tracheostomy tubes is a perennial challenge due to anatomic variances.
The 2014, the United Kingdom National Confidential Enquiry of Outcomes and Death (UK NCEPOD) related to tracheostomy examined 2,199 new tracheostomy insertions (70% percutaneous) occurring in the National Health Service over an 11-week period in nearly 200 hospitals (24). Sixty-two percent of patients were overweight (24.8% obese; 4.8% morbidly obese), yet the rate of XLT tubes used at first insertion was 10.1% overall and 18.8% in obese patients. The authors comment that these factors almost certainly contributed to the 27% of tubes changed within the first seven days post-insertion amongst the critical care cohort, 50.4% of which were unplanned (25). Data from the present study corroborates these observations and emphasizes need for an individualized approach.
A variety of factors might explain the improvement observed after tracheostomy tube upsizing in this study. The increased tube diameter facilitates optimal bedside suctioning and pulmonary hygiene using bronchoscopy for patients with thick secretions that can occlude the device. It also decreases airway resistance allowing increased volumetric airflow and facilitating improved ventilation for patients with high ventilatory requirements. In addition, as the authors observe, malposition or partial dislodgement can contribute to inadequate airflow. Although tracheostomy tubes are designed to have the distal end flowing freely into the distal airway, approximately 10% of tracheostomy tubes have malposition that causes >50% blockage of the distal tube opening. This degree of malposition can cause irritation, granulation, or erosion of the trachea, in addition to increased resistance that prolongs the duration of ventilation and predisposes to potentially life-threatening dislodgement. The longer length of an upsized tube can bypass blockages, decreasing airway resistance and improving airflow.
Air-leakage may indicate suboptimal tube placement, but it can also be a consequence of high ventilatory airway pressures relative to the cuff seal pressure in the trachea. Bronchoscopy is recommended to assess any problems with the tube prior to changing the tube; doing so can provide important information about tube position and orientation (26). Making the correct initial tube choice requires experience, supplemented by physical and imaging assessments before placement. Meticulous post-insertion endoscopy can identify poorly positioned tubes at the time of insertion; however, it is important that the assessment of tube position occurs with the patient in the “resting” position (usually the head of the bed elevated at 30 degrees), which is the position that they will spend the following days or weeks in, rather than the hyperextended neck “insertion” position (27). The optimal time to identify and address a poorly positioned tube is when the insertion team is assembled at the bedside, and not when a problem occurs later.
Generalizability of findings
An important question is whether these data are generalizable across devices, institutions, and eras. The present study was conducted in a large tertiary center, and characteristics of patients or care delivery may differ from other centers. Hospitals and ICUs can differ in healthcare structure, available resources, and regulatory constraints, all of which can shape outcomes. In the context of a COVID-19 surge, the availability of ICU beds, personnel to promote pulmonary hygiene, and supplies may have been constrained. Approaches to acute respiratory distress syndrome (ARDS) in patients with COVID-19 need not deviate markedly from established best practices for ARDS, although patients with COVID-19 ARDS were likely to receive prone ventilation, which is often regarded as a contraindication to tracheostomy. The limited sample reflects challenges not only in analyzing outcomes during the COVID-19 pandemic but also difficulty in comprehensive data capture related to tracheostomy.
The retrospective analysis captured relevant discrete events, but it does not reflect the fluidity of tracheostomy care, including nuances of patient management and real-time corrections not necessarily documented in electronic health records. Care of critically ill patients with a tracheostomy is highly complex, and the outcome of exchange of a device affords relatively little insight into the severity of cuff leakage or other difficulties encountered. Furthermore, an experienced team can perform interventions to temporize tube dysfunction, potentially underestimating the frequency of difficulties. When an acute situation is resolved without harm, some adverse events, or re-insertions might not be documented. Prospective data capture is needed to iteratively improve decision-making and management. Such data can minimize the need for additional procedures, such as unplanned tube changes. Other contributing factors, such as tracheomalacia, airway bleeding, or malposition related to deterioration of stoma sites, were not necessarily detected.
High reliability tracheostomy care
A well-integrated interprofessional team is critical for achieving a high-reliability system of tracheostomy care. This approach draws on several fields of expertise in tracheostomy care (Figure 1). Despite notable progress in the past decade, tracheostomy-related adverse events remain a global problem. The conditions that necessitate an unplanned change of tracheostomy tube can, if not resolved, lead to permanent harm. Up to half of airway-related hypoxic brain injury and death in critical care units involves a tracheostomy (28,29). The Global Tracheostomy Collaborative (GTC), created in 2012, works to improve the safety and quality of care. Its guiding principles include standardized training for health professionals; multidisciplinary and interprofessional team collaboration; active engagement of patients and families; and collecting data to allow tracking of outcomes (1).
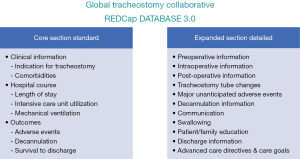
Having a specialized database on tracheostomy care has been instrumental in demonstrating improvements, such as reduction in mortality, reduced frequency and severity of adverse events, and reduced hospital and ICU length of stay. The patient-level data has also shown how teamwork leads to shorter times to functional goals such as speaking and swallowing. The database was built through collaboration between quality improvement specialists, health information technology experts, clinicians from nine disciplines, and legal counsel to assure compliance with global data privacy laws; the interface has allowed for prospective data capture and audit trails across over 10,000 patients. Patient-level data on demographics, comorbidities, and adverse events can facilitate benchmarking outcomes to peer institutions (1). In addition, an expanded data set allows for tracking of functional recovery outcomes, including speech, swallowing, decannulation, and other aspects of rehabilitation (Figure 2).
Many aspects of care modify the relationship between tracheostomy tubes and patient outcomes. At the intersection of interventional pulmonology and critical care is therapeutic bronchoscopy, which can improve pulmonary hygiene, facilitating liberation from the ventilator and improving quality of life (30,31). Effective care requires partnership across all professionals participating in tracheostomy care, and the GTC scaffolds quality improvement efforts on a global scale across institutions, allowing for benchmarking of outcomes. Data are also emerging on the role for tracheostomy in patients receiving extracorporeal membrane oxygenation (32). In the future, artificial intelligence and machine learning will likely improve safety and allow predictive modeling to assist clinicians with decision-making.
Harnessing the power of data allows a deeper understanding of the factors that contribute to favorable recovery and untoward outcomes. In the present study, unplanned tracheostomy tubes are a potential marker for variations in anatomy or physiology that predispose to hypoxia, bleeding, or erosion (33). Currently, the GTC database can provide institutional comparisons to norms, identify predictors of adverse events, and quantify events per 1,000 bed days across tracheostomy-specific outcomes such as accidental decannulation, tracheostomy-related hemorrhage, tube obstruction, skin breakdown, cuff-related airway injury, infection, tracheomalacia, or tracheoesophageal fistula. Data can also be extracted on causes and outcomes of these complications. For example, data can be used to identify predisposing factors for hemorrhage across the age continuum, with or without anticoagulation. Precision patient care is made possible by collecting data on specific contexts, for example in patients with COVID-19 or in individuals with altered anatomy, for example, patients with cancer or head and neck reconstruction.
Humanizing care in the ICU and improving survivorship
Interprofessional teamwork also has an essential role in humanizing care. Doing so involves engaging patients, families, and caregivers to promote successful recovery (4). A cohesive team provides not only technically sound care but also makes effective use of interprofessional communication systems to avoid inconsistent messages or fragmented care. Engaging patients as partners in care can be fostered by using eye contact, augmentative and alternative communication systems, and therapeutic touch (34,35). Patients with tracheostomy are susceptible to feelings of isolation and stigma; therefore, welcoming family members to engage in tracheostomy hygiene, tube feeding, swallowing, or other aspects of rehabilitation can promote recovery. Care such as suctioning, stoma care, and tracheostomy tube changes should be done at times that minimize disruption of sleep, eating, or family visits. Modifications to the ICU environment (reducing noise, harsh lighting, or extremes of temperature) can improve comfort and reduce stress. Furthermore, providing décor reminiscent of a home environment, including personal items, and minimizing disruption of circadian rhythm can improve well-being.
A holistic approach to tracheostomy care requires transcending traditional siloes. Often, data collection is restricted to a particular segment of the patient journey, such as the procedure or ICU stay, but this approach is limiting. A whole-systems approach spans from prior to the performance of tracheostomy procedure, through the intensive care unit stay, and to the transition to home. Many patients with a tracheostomy will experience post-intensive care syndrome (PICS), which includes physical, cognitive, and mental health impairments after ICU stay; these barriers to resuming a meaningful life degrade survivorship experience. In ICU settings, optimal timing and management of tracheostomy can reduce the morbidity of orotracheal intubation, reducing risk of pressure injuries to mucosal and cartilaginous structures. During rehabilitation, identification and intervention for scarring of the larynx or trachea can restore ability to breathe, speak, and swallow normally. Appropriate placement and management of tracheostomy tubes and cuffs can mitigate airway risks. Last, in this era of digital health, using telehealth and wearable technology, such as pulse oximetry or cameras, can facilitate remote assessment.
Conclusions
McCauley and colleagues reveal how unplanned exchange of tracheostomy tubes provides a window into tracheotomy outcomes after COVID-19. The study emphasizes the role for integrated interprofessional care before, during, and after the index procedure. Seeming isolated complications, such as malfunctioning devices, erosion of luminal structures, or laryngotracheal stenosis, are often symptomatic of siloed or fragmented care. By integrating patients and family into the work of multidisciplinary, interprofessional teams, it is possible to minimize the risk of adverse events and accelerate recovery. Increasingly, standardized protocols, prospective data tracking, and preemptive airway surveillance are allowing high reliability tracheostomy teams to improve acute tracheostomy care and survivorship.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-205/coif). VP receives research funding from the NIH [National Institute of Nursing Research, NINR (R01NR017433-01A, 2018-2023)]. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brenner MJ, Pandian V, Milliren CEGlobal Tracheostomy Collaborative, et al. data-driven improvements in patient safety through multidisciplinary teamwork, standardisation, education, and patient partnership. Br J Anaesth 2020;125:e104-18. [Crossref] [PubMed]
- Bedwell JR, Pandian V, Roberson DW, et al. Multidisciplinary Tracheostomy Care: How Collaboratives Drive Quality Improvement. Otolaryngol Clin North Am 2019;52:135-47. [Crossref] [PubMed]
- Martin KA, Cole TDK, Percha CM, et al. Standard versus Accelerated Speaking Valve Placement after Percutaneous Tracheostomy: A Randomized Controlled Feasibility Study. Ann Am Thorac Soc 2021;18:1693-701. [Crossref] [PubMed]
- McCormick ME, Ward E, Roberson DW, et al. Life after Tracheostomy: Patient and Family Perspectives on Teaching, Transitions, and Multidisciplinary Teams. Otolaryngol Head Neck Surg 2015;153:914-20. [Crossref] [PubMed]
- Moser CH, Freeman-Sanderson A, Keeven E, et al. Tracheostomy care and communication during COVID-19: Global interprofessional perspectives. Am J Otolaryngol 2022;43:103354. [Crossref] [PubMed]
- Pandian V, Hopkins BS, Yang CJ, et al. Amplifying patient voices amid pandemic: Perspectives on tracheostomy care, communication, and connection. Am J Otolaryngol 2022;43:103525. [Crossref] [PubMed]
- Berges AJ, Lina IA, Ospino R, et al. Quantifying Viral Particle Aerosolization Risk During Tracheostomy Surgery and Tracheostomy Care. JAMA Otolaryngol Head Neck Surg 2021;147:797-803. [Crossref] [PubMed]
- Farlow JL, Park PK, Sjoding MW, et al. Tracheostomy for COVID-19 respiratory failure: timing, ventilatory characteristics, and outcomes. J Thorac Dis 2021;13:4137-45. [Crossref] [PubMed]
- Mahmood K, Cheng GZ, Van Nostrand K, et al. Tracheostomy for COVID-19 Respiratory Failure: Multidisciplinary, Multicenter Data on Timing, Technique, and Outcomes. Ann Surg 2021;274:234-9. [Crossref] [PubMed]
- McGrath BA, Wallace S, Lynch J, et al. Improving tracheostomy care in the United Kingdom: results of a guided quality improvement programme in 20 diverse hospitals. Br J Anaesth 2020;125:e119-29. [Crossref] [PubMed]
- Bier-Laning C, Cramer JD, Roy S, et al. Tracheostomy During the COVID-19 Pandemic: Comparison of International Perioperative Care Protocols and Practices in 26 Countries. Otolaryngol Head Neck Surg 2021;164:1136-47. [Crossref] [PubMed]
- McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med 2020;8:717-25. [Crossref] [PubMed]
- Meister KD, Pandian V, Hillel AT, et al. Multidisciplinary Safety Recommendations After Tracheostomy During COVID-19 Pandemic: State of the Art Review. Otolaryngol Head Neck Surg 2021;164:984-1000. [Crossref] [PubMed]
- Zaga CJ, Pandian V, Brodsky MB, et al. Speech-Language Pathology Guidance for Tracheostomy During the COVID-19 Pandemic: An International Multidisciplinary Perspective. Am J Speech Lang Pathol 2020;29:1320-34. [Crossref] [PubMed]
- McCauley P, Mohammed A, Casey M, et al. Tracheostomy insertion in COVID-19: insertion practice and factors leading to unplanned tube exchange. J Thorac Dis 2023;15:410-22. [Crossref] [PubMed]
- Cramer JD, Graboyes EM, Brenner MJ. Mortality associated with tracheostomy complications in the United States: 2007-2016. Laryngoscope 2019;129:619-26. [Crossref] [PubMed]
- Jung DTU, Grubb L, Moser CH, et al. Implementation of an evidence-based accidental tracheostomy dislodgement bundle in a community hospital critical care unit. J Clin Nurs 2022; Epub ahead of print. [Crossref] [PubMed]
- Moser CH, Peeler A, Long R, et al. Prevention of Tracheostomy-Related Pressure Injury: A Systematic Review and Meta-analysis. Am J Crit Care 2022;31:499-507. [Crossref] [PubMed]
- Zouk AN, Batra H. Managing complications of percutaneous tracheostomy and gastrostomy. J Thorac Dis 2021;13:5314-30. [Crossref] [PubMed]
- Ghattas C, Alsunaid S, Pickering EM, et al. State of the art: percutaneous tracheostomy in the intensive care unit. J Thorac Dis 2021;13:5261-76. [Crossref] [PubMed]
- Barash M, Kurman JS. Patient selection and preoperative evaluation of percutaneous dilation tracheostomy in the intensive care unit. J Thorac Dis 2021;13:5251-60. [Crossref] [PubMed]
- Palacios JM, Bellido DA, Valdivia FB, et al. Tracheal stenosis as a complication of prolonged intubation in coronavirus disease 2019 (COVID-19) patients: a Peruvian cohort. J Thorac Dis 2022;14:995-1008. [Crossref] [PubMed]
- Pandian V, Hutchinson CT, Schiavi AJ, et al. Predicting the need for nonstandard tracheostomy tubes in critically ill patients. J Crit Care 2017;37:173-8. [Crossref] [PubMed]
- McGrath B, Wilkinson K, Shah RK. Notes from a Small Island: Lessons from the UK NCEPOD Tracheotomy Report. Otolaryngol Head Neck Surg 2015;153:167-9. [Crossref] [PubMed]
- McGrath BA, Wilkinson K. The NCEPOD study: on the right trach? lessons for the anaesthetist. Br J Anaesth 2015;115:155-8. [Crossref] [PubMed]
- McGrath BA, Lynch K, Templeton R, et al. Assessment of scoring systems to describe the position of tracheostomy tubes within the airway - the lunar study. Br J Anaesth 2017;118:132-8. [Crossref] [PubMed]
- Chandrasena AN, Goswamy J, Calder N, et al. Our experience: Quantifying changes in tracheostomy tube position and orientation with repositioning of 14 patients (the Lunar positioning study). Clin Otolaryngol 2020;45:143-7. [Crossref] [PubMed]
- Thomas AN, McGrath BA. Patient safety incidents associated with airway devices in critical care: a review of reports to the UK National Patient Safety Agency. Anaesthesia 2009;64:358-65. [Crossref] [PubMed]
- Cook TM, Woodall N, Harper J, et al. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth 2011;106:632-42. [Crossref] [PubMed]
- Benn BS. Therapeutic bronchoscopy facilitates liberation from mechanical ventilation and improves quality of life for critically ill patients with central airway obstruction. J Thorac Dis 2021;13:5135-8. [Crossref] [PubMed]
- Kurman JS, Sachdeva A, Nanchal R. The intersection of interventional pulmonology and critical care. J Thorac Dis 2021;13:5123-4. [Crossref] [PubMed]
- Durak K, Zayat R, Grottke O, et al. Extracorporeal membrane oxygenation in patients with COVID-19: 1-year experience. J Thorac Dis 2021;13:5911-24. [Crossref] [PubMed]
- Alsunaid S, Holden VK, Kohli A, et al. Wound care management: tracheostomy and gastrostomy. J Thorac Dis 2021;13:5297-313. [Crossref] [PubMed]
- Haring CT, Farlow JL, Leginza M, et al. Effect of Augmentative Technology on Communication and Quality of Life After Tracheostomy or Total Laryngectomy. Otolaryngol Head Neck Surg 2022;167:985-90. [Crossref] [PubMed]
- Freeman-Sanderson AL, Togher L, Elkins M, et al. Quality of life improves for tracheostomy patients with return of voice: A mixed methods evaluation of the patient experience across the care continuum. Intensive Crit Care Nurs 2018;46:10-6. [Crossref] [PubMed]


