
A comprehensive clinical evaluation of first-line drugs for ALK-positive advanced non-small cell lung cancer
Highlight box
Key findings
• By comparing first-line drugs for ALK-positive advanced NSCLC using a comprehensive clinical evaluation of ALK-TKIs, we found that alectinib had the best comprehensive clinical value.
What is known and what is new?
• Studies evaluating the clinical value of ALK-TKIs based on one to two dimensions have already been conducted.
• This study focused on six dimensions of ALK-TKIs in treating ALK-positive advanced NSCLC patients and covered the safety, effectiveness, economy, suitability, accessibility, and innovation of ALK-TKIs.
What is the implication, and what should change now?
• This study’s comprehensive clinical evaluation of ALK-TKIs for patients with ALK-positive advanced NSCLC provides rational drug use in clinic and a basis for relevant policy formulation and decision-making.
• Real-world studies are needed to explore the clinical use of ALK-TKIs, and the system established remains to be further verified in clinical practice.
Introduction
Lung cancer ranks first in incidence and mortality of all malignant tumors in China (1). Non-small cell lung cancer (NSCLC) accounts for about 85% of lung cancers. The onset of NSCLC is insidious, and the disease is often at an advanced stage by the time of diagnosis, with a poor prognosis (2). The anaplastic lymphoma kinase (ALK) gene rearrangement is caused by a chromosomal inversion in NSCLC patients. Echinoderm microtubule-associated protein-like 4 (EML4) and ALK gene rearrangement formed fusion gene (EML4-ALK) are also known as ALK gene positive. EML4-ALK is an important tumor driver gene that promotes the occurrence and progression of NSCLC, with an incidence of 3–5% (3). Targeted therapy for advanced ALK-positive NSCLC can effectively prolong the survival time of patients with significant curative effects (4-8). Various ALK tyrosine kinase inhibitors (ALK-TKIs) have been used to treat EML4-ALK-positive NSCLC patients. Crizotinib was the first drug approved by the Food and Drug Administration (FDA) to treat patients with ALK-positive NSCLC (9). Subsequent second-generation inhibitors, like ceritinib, alectinib, ensartinib, and brigatinib, and a third-generation inhibitor, lorlatinib, have shown stronger inhibition and higher blood-brain barrier permeability (10-12). These drugs are already on the market in China.
Although ALK-TKIs have made breakthrough progress, the evidence level and basis for rapid drug approval are lower than for conventional drugs, thus potentially increasing the risks associated with ALK-TKI drug use. Additionally, ALK-TKIs are expensive, and the treatment duration is long, creating a heavy economic burden on the government and patients. The Chinese government has admitted some ALK-TKIs into the medical insurance scheme through price negotiation and other means, which provides more choices for ALK-positive NSCLC patients, but the disease burden remains high for most patients. The 2018 edition of the National Essential Medicine List has not covered ALK-positive therapeutic drugs. Therefore, it is essential to select accessible and affordable ALK-positive therapeutic drugs. A comprehensive clinical evaluation of drugs is the basis for the selection and dynamic adjustment of essential drugs and the promotion of a rational drug use in clinic. By carrying out a complete comprehensive clinical evaluation, a multi-dimensional decision-making basis can be provided for drug selection and clinical use. At present, a comprehensive clinical evaluation of antitumor drugs has been listed as one of the four major evaluation areas of national interest. There is no comprehensive clinical evaluation of ALK-TKIs in ALK-positive patients with advanced NSCLC. Therefore, this study aimed to combine the clinical application practice of antitumor drugs with the drug supply guarantee policy to integrate and analyze evidence-based medical data. The comprehensive clinical value of six ALK-TKIs was compared in terms of safety, effectiveness, economy, suitability, accessibility, and innovation. In this study, a comprehensive clinical evaluation system consistent with advanced ALK-positive NSCLC was established. This study provides a comprehensive clinical evaluation of antitumor drugs that is scientific, homogeneous, and standardized to establish a reference for clinical drug use and catalog access in hospitals and provides a basis for improving national policies and systems.
Methods
Selection of experts and establishment of an expert opinion interview consulting group
An expert opinion interview team was established, expert at senior associate levels and above with extensive experience in related work and research were selected to form an expert advisory group. They were recruited from level II or III medical institutions from five major regions (eastern, western, southern, northern, and central regions) of China, and each expert comes from a different medical institution. The team consisted of 24 experts, including five experts in oncology medicine, five in pharmacy, five in medical insurance, three in health economics, three in health administration, and three in drug bidding and purchasing.
Determination of the focus of evaluation and construction of the comprehensive clinical evaluation index system
According to the Guideline for the Management of Comprehensive Clinical Evaluation of Pharmaceutical Products (in 2021) (13) and the Technical Guideline for the Comprehensive Clinical Evaluation of Antitumor Drugs (in 2022) (14), we constructed a comprehensive clinical evaluation system for six ALK-TKI drugs (crizotinib, ceritinib, alectinib, ensartinib, brigatinib, and lorlatinib) in the first-line treatment of advanced NSCLC. Using a literature review and expert survey and opinion, six dimensions and key evaluation indicators of each dimension were determined, including safety, effectiveness, economy, suitability, accessibility, and innovation (Table 1). Regarding safety, effectiveness, and economy, we used a systematic literature review and meta-analysis to conduct a quantitative analysis. We also conducted a qualitative analysis using supplementary reports, such as the health technology assessment reports (HTA reports) and the adverse drug reaction monitoring reports. Regarding suitability, accessibility, and innovation, domestic and foreign drug price data and other related data were quantitatively analyzed. A qualitative analysis was conducted based on drug instructions and multidisciplinary expert interviews.
Table 1
| Index | Indicators | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Primary index | Safety | Effectiveness | Economy | Suitability | Accessibility | Innovation |
| Secondary index | Grade 3 and above adverse event incidence, monitoring by drug supervisory and administrative departments | Clinical efficacy, quality of life, guideline recommendation | Cost-utility analysis, international HTA recommendation | Physician recommendation, patient compliance, health insurance directory, indication | Price, availability, affordability | Mechanism of drug, urgent clinical need, patent value, localization of technology |
| Tertiary index | – | OS, PFS, ORR | – | – | Market shares, urban affordability, rural affordability | – |
ALK, anaplastic lymphoma kinase; NSCLC, non-small-cell lung cancer; HTA, Health Technology Assessment; OS, overall survival; PFS, progression-free survival; ORR, objective response rate.
Comprehensive clinical evaluation of antitumor drugs in all dimensions
Safety evaluation
We evaluated the quality of antitumor drugs and the risks that may arise after marketing. The comprehensive clinical evaluation of all antitumor drugs required the evaluation of safety in clinical use (except in certain circumstances). Relative safety comparison is the comparison of the safety difference of the control drug with the same pharmacological classification or with the same indications (14). ALK-TKIs belong to the same pharmacological classification and indications. The selected indicators were the incidence of grade 3 and above adverse events (≥ grade 3 AEs). The post-marketing safety information includes the announcement issued by the State Medical Products Administration, the warning issued by the National Drug Administration of the United States and the European Union, and the withdrawal from the market (14). The selected indicators were the monitoring results of the drug supervision and administration departments. In this study, the incidence of ≥ grade 3 AEs and monitoring by drug regulatory authorities were selected as the two secondary indicators. A meta-analysis and systematic review was conducted to determine the incidence of ≥ grade 3 AEs. The incidence of ≥ grade 3 AEs was systematically compared between six ALK-TKI first-line drugs for ALK-positive advanced NSCLC. Clinical randomized controlled trials were included, excluding single-arm and unavailable full-text studies. The study population was ALK-positive patients with advanced NSCLC. The databases searched included PubMed, Web of Science, and the ClinicalTrial website. The retrieval period was from the establishment of the databases until June 30, 2022. Finally, nine studies were included (15-23) with a total of 2,508 patients.
Effectiveness evaluation
The effectiveness evaluation of antitumor drugs was based on the actual efficacy for patients after taking the drugs, including primary clinical outcome, secondary clinical outcome, and patient-reported outcome (PRO) (14). Indicators were measured based on the best available evidence and were optimized from clinical efficacy or actual effectiveness indicators. The primary clinical outcome reflects the long-term benefits for patients and the disease outcomes over the whole life course after medication, including overall survival (OS) and progression-free survival (PFS). Secondary clinical outcomes refer to other measurable clinical indicators, including disease control rate (DCR) and objective response rate (ORR). PRO was derived directly from patient reports on their health-related quality of life (HRQOL). In this study, three secondary indicators (clinical efficacy, quality of life, and guideline recommendation) were selected, and three tertiary indexes (OS, PFS, and ORR) were selected. The clinical efficacy of six ALK-TKIs in the first-line treatment of ALK-positive advanced NSCLC patients was compared and analyzed using a meta-analysis and systematic review. Clinical randomized controlled trials were included in the meta-analysis, excluding studies with incomplete data and one-arm studies. The study population was ALK-positive patients with advanced NSCLC. The databases searched included PubMed, Web of Science, and the ClinicalTrial website. The retrieval period was from the establishment of the databases to June 30, 2022. Finally, 10 studies (15-24) were included, with a total of 2,715 patients.
The Cochrane risk bias assessment tool was used to evaluate the quality of 10 studies included in the safety and effectiveness studies.
Economy evaluation
Economic evaluation refers to the identification, measurement, comparison, and analysis of the cost, benefit, effect, and utility of different drug treatment regimens using basic health economics or pharmacoeconomics methods. The clinical input-output ratio of drugs was evaluated comprehensively, and the economics of the clinical use of drugs was measured (14). It is recommended that cost-utility analysis (CUA) and cost-benefit analysis (CBA) be given priority. In this study, two secondary indicators of CUA and HTA recommendations were selected, and a systematic literature review was conducted.
Suitability assessment
Drug suitability refers to the precise application of a drug to the target population in an appropriate way under the guidance of an appropriate prescription (14). Since the course of antitumor treatment is generally long, drug suitability plays a vital role in improving patients’ long-term medication compliance. In this study, four secondary indicators, including physician recommendation, patient compliance, medical insurance directory, and indications were selected. We used expert interviews and access to the website of regulatory authorities to obtain the data. The CUA of the six ALK-TKIs were compared and analyzed using a systematic literature review to evaluate their economic benefits. The literature review included economic researches but excluded studies that lacked relevant data. The search databases included PubMed and Web of Science.
Accessibility evaluation
Accessibility assessment is a comprehensive and scientific assessment of drug supply capacity and patient burden using pharmacoeconomics and pharmacoepidemiology. Availability and affordability are recommended to evaluate the clinical accessibility of antitumor drugs (14). Availability refers to the potential opportunities for tumor patients to obtain targeted drugs, the types and quantities of marketed drugs, and the equipping capacity of medical institutions/drug retailers, etc. Affordability refers to the affordability of treatment costs for urban and rural patients’ families. In this study, three secondary indicators (price, availability, and affordability) and three tertiary indexes (market share, urban affordability, and rural affordability) were selected using the data retrieval method on the official website.
Innovation evaluation
The innovative evaluation of antitumor drugs is a professional, multi-tiered, multi-perspective gathering and analysis of information. Based on domestic and foreign literature and the actual situation in China, the guide recommends evaluation from three dimensions: clinical innovation, service innovation, and industrial innovation (14). Clinical innovation evaluates the degree to which antitumor drugs meet patients’ drug needs. Service innovation assesses the impact of drug use on health service systems. Industrial innovation assesses the production capacity of innovation and research and development. In this study, four secondary indicators, including drug action mechanism, urgent clinical need, patent value, and technology localization, were selected, and a systematic literature review was used.
Statistical analysis
Meta-analysis and systematic review were conducted. Indicators of relative risk ratio (RR), hazard ratio (HR) and 95% confidence interval (CI) were used.
Results
Evidence from the comprehensive clinical evaluation of ALK-TKI drugs in all dimensions
Safety
Meta-analysis showed that the incidence of ≥ grade 3 AEs was lower in alectinib than in crizotinib (OR =0.55, 95% CI: 0.40–0.74) (15-17), whereas the incidence of ≥ grade 3 AEs with ceritinib was higher than in Platinum-based chemotherapy regimens (OR =2.25, 95% CI: 1.51–3.35, P<0.05, Figure 1) (19,20). In addition to meta-analysis, clinical trials of brigatinib, ensartinib, lorlatinib, and crizotinib were reviewed. Grade 3 and above AEs were higher in brigatinib (78% vs. 64%), ensartinib (50.4% vs. 42.4%), and lorlatinib (72% vs. 56%) than in crizotinib (21-23).
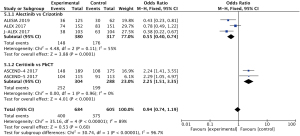
Regarding the monitoring reports from drug regulatory authorities, the United Kingdom and Japan suggested that crizotinib had safety risks of heart failure, and Europe suggested that crizotinib had safety risks of visual impairment in children.
Effectiveness
The results of the meta-analysis found that in terms of OS, alectinib was associated with a lower risk of death than crizotinib (HR 0.64, 95% CI: 0.44–0.92, P<0.05) (15-17). Crizotinib (HR 0.80, 95% CI: 0.61–1.05) (18,24) and ceritinib (HR 0.85, 95% CI: 0.64–1.11) (19,20) showed no significant difference in the risk of death compared with Platinum-based chemotherapy (P>0.05). In terms of PFS, alectinib was better than crizotinib (HR 0.40, 95% CI: 0.32–0.49) (15-17), and both crizotinib (HR 0.43, 95% CI: 0.35–0.53) (18,24) and ceritinib (HR 0.52, 95% CI: 0.43–0.64) (19,20) had longer PFS than Platinum-based chemotherapy regimens (P<0.05, respectively, Figure 2A,2B). In addition to the meta-analysis, a review of clinical trials (21-23) found that the PFS of brigatinib (30.8 months), ensartinib (25.8 months), and lorlatinib (PFS not reached) was significantly better than crizotinib (less than 13 months) (P<0.05, respectively). The OS was still changing at the data cutoff, but there was no statistical difference in OS between brigatinib and crizotinib, ensartinib and crizotinib, lorlatinib and crizotinib, respectively (P>0.05).
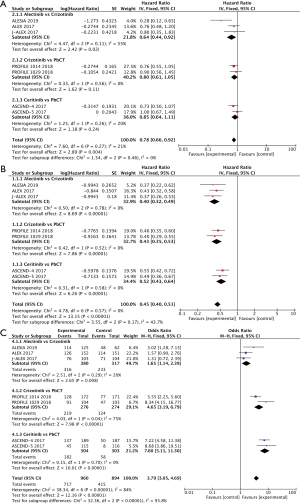
In terms of ORR, alectinib was superior to crizotinib (OR =1.65, 95% CI: 1.14–2.39) (15-17), and both crizotinib (OR =4.65, 95% CI: 3.19–6.79) (18,19) and ceritinib (OR =7.60, 95% CI: 5.11–11.30) were superior to Platinum-based chemotherapy (P<0.05, respectively, Figure 2C). In addition to the meta-analysis, our review of clinical trials (22,23) found no significant difference in ORR between ensartinib and crizotinib (P>0.05), while the ORR of lorlatinib was significantly better than that of crizotinib (76% vs. 58%, P<0.05).
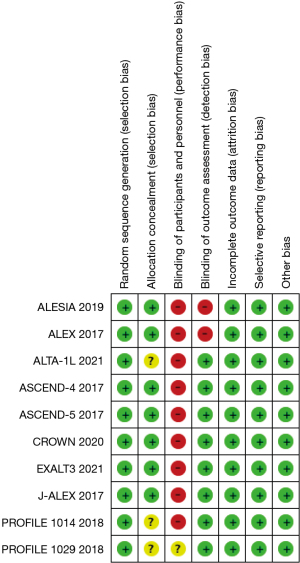
The Cochrane risk bias assessment results showed that most of the included studies had a low risk of bias, and the literature quality was good (Figure 3).
For quality of life, we used a literature review to summarize and analyze the effects of six first-line ALK-TKI drugs on quality of life in patients with ALK-positive advanced NSCLC. The retrieval databases were PubMed and Web of Science, and studies related to crizotinib, brigatinib, ceritinib, alectinib, and lorlatinib were retrieved (19,25-28). The results showed that lorlatinib significantly improved patients’ quality of life compared with crizotinib (P<0.05) (19). Compared with crizotinib and chemotherapy, brigatinib significantly improved patients’ quality of life (P<0.05, respectively) (25,26). Crizotinib and ceritinib improved patients’ quality of life better than chemotherapy (P<0.05, respectively) (10,27). There was no significant difference between alectinib and crizotinib in improving life quality (P>0.05) (28).
Regarding guideline recommendations, we retrieved the authoritative NSCLC treatment guidelines issued by authoritative institutions and industrial associations in China, the United States, Europe, and other countries (9,11,29-32). The NCCN Guidelines for Non-Small Cell Lung Cancer (2022 v3) recommend brigatinib, alectinib, and lorlatinib as first-line treatments for ALK-positive advanced NSCLC (9). The American Society of Clinical Oncology (ASCO) Dynamic Guidelines 2021 recommend alectinib and brigatinib for patients with ALK rearrangement, PS 0-2, and previously untreated NSCLC (29). The European Society of Oncology (ESMO) Guidelines (in 2020) recommend crizotinib for first-line drug treatment of ALK-positive advanced NSCLC. Alectinib and brigatinib are recommended as class IA for patients with central nervous system metastasis (30). The Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of Non-small Cell Lung Cancer (in 2022) recommend alectinib (priority recommendation), crizotinib, and ceritinib for first-line treatment of stage IV ALK-fused NSCLC. Brigatinib and lorlatinib are recommended for third-line treatment (11). The Chinese guidelines for the diagnosis and treatment of anaplastic lymphoma kinase positive and ROS1 positive non-small cell lung cancer (in 2018) recommend crizotinib as the first-line drug for patients newly diagnosed with ALK-positive advanced NSCLC (Class I recommendation) (3). The Guidelines for the Clinical Diagnosis and Treatment of Lung Cancer of the Chinese Medical Association Oncology Society (in 2021) recommend crizotinib, alectinib, and ceritinib as first-line treatments for ALK-rearranged NSCLC (31). The Chinese Guidelines for the Treatment of Brain Metastases in Lung Cancer (in 2021) recommend second-generation ALK-TKIs to treat ALK-rearranged advanced NSCLC with brain metastases (32).
Economical efficiency
The results showed that for first-line drug treatment of ALK-positive advanced NSCLC patients, second-generation ALK-TKIs (ceritinib, alectinib, and ensartinib) have a cost-utility advantage over first-generation crizotinib (33-35). Among second-generation ALK-TKI drugs, the cost-utility value of ceritinib was superior to that of alectinib (35). Compared with crizotinib, the current price of lorlatinib in China is not economical (36). Regarding HTA recommendations, the National Institute for Health and Clinical Excellence (NICE) recommended brigatinib, alectinib, ceritinib, crizotinib, and Platinum-containing two-drug chemotherapy as first-line drug treatments for ALK-positive advanced NSCLC (37). Based on relevant clinical studies and guidelines, Canada’s Drug and Health Technology Agency (CADTH) recommended alectinib and ceritinib as first-line drugs for treating ALK-positive advanced NSCLC due to better cost-effectiveness than crizotinib (38).
Suitability
In terms of physician recommendations and patient compliance, data were collected through interviews with five clinical experts from Shanghai Municipality, Jiangsu, Anhui, Sichuan, and Zhejiang provinces. The interview results showed that physicians highly recommended alectinib, and they reported high levels of patient compliance with this drug. Regarding the medical insurance list and indications, crizotinib, ceritinib, alectinib, and ensartinib are all on the current Medicare list. All six indications for ALK-TKIs covered locally advanced or metastatic NSCLC.
Accessibility
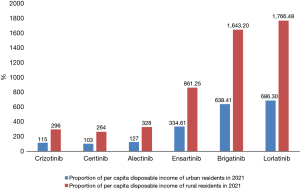
In terms of price, crizotinib, ceritinib, and alectinib have all been approved for first-line treatment of ALK-positive advanced NSCLC and entered the national medical insurance directory, and their prices are much lower than international prices. Ensartinib was approved for second-line treatment of ALK-positive advanced NSCLC in the medical insurance directory. Brigatinib and lorlatinib are not currently covered by the medical insurance directory and are relatively expensive. In terms of affordability, the annual cost of the six ALK-TKIs for advanced ALK-positive NSCLC is shown in Figure 4 (it was assumed that ensartinib 100 mg and 25 mg were used together, and brigatinib was administered at a 90 mg dose for the first 7 days and 180 mg dose after 7 days).
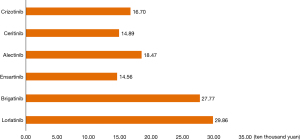
In terms of affordability for urban and rural residents, after being reimbursed by insurance, the annual cost of crizotinib, ceritinib, and alectinib in the treatment of ALK-positive advanced NSCLC was 50,100, 44,700 and 55,400 yuan, respectively. The proportion of the six ALK-TKIs in the per capita disposable income of urban and rural residents in 2021 is shown in Figure 5.
Innovation
In this study, the six ALK-TKIs evaluated are small molecule targeted drugs. Compared with chemotherapy, they can significantly improve treatment outcomes in ALK-positive advanced NSCLC patients. Crizotinib, the first generation of ALK-TKIs, significantly improves patients’ quality of life compared with standard chemotherapy, but with the extension of medication time, drug resistance and disease progression will inevitably occur (39). Compared with crizotinib, the second- and third-generation ALK-TKIs demonstrate stronger inhibitory capacity, better blood-brain barrier permeability, and more innovative drug mechanisms of action. In terms of clinical urgency, the data on clinical use and demand were collected by expert interviews (refer to method: “Suitability”). The results of the clinical expert interviews showed that ALK-TKIs are urgently required for tumor prevention and treatment, and the degree of urgent clinical need was high. Regarding patent value and technology localization, none of the six drugs have reached the patent expiry date, and the patent value is high. Among them, ensartinib is China’s first highly selective and efficient ALK-TKI with fully independent intellectual property rights. Among ALK-targeted drugs, there are still four new domestic drugs in phase III clinical trials (CT-707, TQ-B3139, XZP-3621, and SAF-189). It is vitally important to promote the localization of technology.
Discussion
Policies have been issued successively in China to guide and standardize the technical work and comprehensive clinical evaluation related to antitumor drugs for the prevention and control of major national diseases (13,14). As antitumor drugs, it is vitally important to comprehensively evaluate the clinical value of ALK-TKIs, which have high clinical demand and good targeted therapeutic effects in treating ALK-positive advanced NSCLC. Previous studies have mainly considered the clinical value of ALK-TKIs based on one or two dimensions, such as safety, efficacy (40), or economy (33,34). In addition, the study subjects included patients treated with ALK-TKIs in both first-line and later treatments. This study focused on patients receiving first-line treatment for ALK-positive advanced NSCLC and included patients in the Asian population. This study covered not only the safety, efficacy, and economics but also the suitability, accessibility, and innovation. Therefore, this study started from national policy and clinical practice needs and investigated the clinical value of six ALK-TKIs. We aimed to provide a reference for a rational use of antitumor drugs and medical insurance admission decision-making in hospitals.
Owing to the possibility of experiencing adverse reactions, patients are more likely to adjust, skip or discontinue medication after antitumor treatment. Ensuring the safety of medication is the premise of long-term treatment. Safety evaluation results showed that the incidence of ≥ grade 3 AEs was lower in alectinib than in crizotinib, and the incidence of ≥ grade 3 AEs in both crizotinib and ceritinib was higher than in Platinum-based chemotherapy, and the incidence of ≥ grade 3 AEs with brigatinib, ensartinib, and lorlatinib was higher than in crizotinib. The second and third-generation ALK-TKIs showed better safety than the first generation ALK-TKIs. A meta-analysis also showed that alectinib and brigatinib had less discontinuation due to adverse reactions than ceritinib and crizotinib, and lorlatinib had the lowest discontinuation rate (40). The common adverse reactions to ALK-TKIs include gastrointestinal adverse reactions and hepatotoxicity (10,15,17-19,21-23). However, some ALK-TKIs may cause specific adverse reactions. For example, the related AEs of crizotinib include visual impairment, prolonged QT interval, and neutropenia. In addition, crizotinib also has a high incidence of gastrointestinal events (diarrhea, etc.), and elevated aminotransferase. These AEs limit its application to some extent. Besides, ceritinib related AEs include gastrointestinal side effects, hyperglycemia, elevated amylase, and lipase. Alectinib related AEs include elevated blood creatine phosphokinase, photosensitive dermatitis, and elevated total bilirubin. Ensartinib associated AEs include rash and elevated serum creatinine. Lorlatinib related AEs of hyperlipidemia and central nervous system function related toxicity. Brigatinib associated AEs include hypertension and elevated blood creatine phosphokinase. Specific adverse reactions of different ALK-TKIs may limit their application in some populations. It is necessary to conduct further studies on the safety of different ALK-TKIs since the proportion of patients who decreased their dose or stopped medication due to adverse reactions was not explained in the relevant studies.
Effectiveness evaluation showed that compared with first-generation ALK-TKIs, second- and third-generation ALK-TKIs significantly prolonged PFS. PFS, ORR, and OS were significantly better with alectinib than with crizotinib. The central nervous system permeability and inhibition ability of second- and third-generation ALK-TKIs are better, and thus the curative effect is better. A review of literature related with quality-of-life of ALK-TKIs found that brigatinib and lorlatinib significantly improved quality of life in comparison to crizotinib and chemotherapy. Patients often face a burden of symptoms and side effects during antitumor therapy, which greatly reduces their quality of life and affects their treatment compliance. Therefore, adverse reaction management is essential in improving medication compliance and optimizing HRQOL (41). It is necessary to conduct whole-process management of patients on the occurrence of adverse reactions and their quality of life. With changes to the function of pharmacists, pharmacists can play a positive role in the medication process. In addition, as far as the guidelines for ALK-TKI usage are concerned, alectinib (9,29,11,31,32) and crizotinib (3,11,30,31) have been recommended as first-line treatments by more clinical guidelines.
Generally, antitumor drugs are expensive, especially small-molecule targeted drugs and monoclonal antibodies, and are a major component of patient treatment costs. Reasonable price has a significant influence on whether patients will choose such drugs for treatment. For the economic evaluation, ceritinib, alectinib, and ensartinib have been recommended by more economic evaluation studies. At present, crizotinib, ceritinib, and alectinib have all been approved as first-line treatments of ALK-positive advanced NSCLC (11) and have been entered into the national medical insurance directory. Their cost is far lower than the international price, and their accessibility and affordability are good, which will largely affect the choice of medication for patients. Crizotinib, ceritinib, and alectinib, when used as first-line treatments for ALK-positive advanced NSCLC, accounted for a much lower proportion of the income of urban and rural residents in 2021 than ensartinib, brigatinib, and lorlatinib. This further reduced the economic burden on patients. The indication for ensartinib to enter the national medical insurance is already second-line treatment, but it is not economical for its first-line treatment. Although the clinical efficacy of brigatinib and lorlatinib is significantly better than crizotinib, their short time on the market and lack of inclusion in the national insurance directory mean their economic efficiency and accessibility are poor. Based on the current prices of brigatinib and lorlatinib in our country, they are not economical (in dollar terms, annual cost of NSCLC treatment with crizotinib, ceritinib, alectinib, ensartinib, brigatinib, and lorlatinib are $24,829, $22,138, $27,460, $21,647, $41,287, $44,394 respectively). A reasonable reduction in drug prices in the future will improve their economic viability. In terms of innovation, all six drugs are ALK-targeted therapeutic drugs and demonstrate better mechanistic innovation than chemotherapy drugs. Studies (10,31,32) have shown that the median PFS of the patients treated with crizotinib is less than 1 year, and more than 40% of patients are prone to brain metastasis. The control and prevention of brain metastasis are insufficient, resulting in worse prognosis. It is one of the most common reasons for treatment failure. The mechanism action of second- and third-generation ALK-TKIs are significantly more innovative. According to the six evaluation dimensions, alectinib performed better in the first-line treatment of ALK-positive advanced NSCLC.
Comprehensive clinical evaluations of drugs have been increasingly applied in rational drug use in clinic and for improving essential drug list systems and other research fields. This study followed the standard procedures and evaluation methods of the clinical evaluation guidelines (13,14) and combined with various disciplinary tools, such as HTA, to comprehensively evaluated the clinical value of six ALK-TKIs, and the results were reliable with a high level of evidence. This study also had some limitations: it mainly integrated and analyzed literature data from published clinical trials. Real-world studies of ALK-TKIs have been conducted in Caucasians (42). There may be some differences in the application practices of ALK-TKIs in different countries. Besides, the marketing time of ALK-TKIs in domestic and foreign countries is different, and the use of ALK-TKIs in various countries is also different. For example, brigatinib was launched abroad in 2017, and lorlatinib was launched abroad in 2018. However, they were not launched in China until 2022. Therefore, the actual application of these drugs in the real world in China is worth exploring. Besides, whether the comprehensive clinical evaluation index system established in this study truly reflects clinical practice usage remains to be further verified.
Conclusions
In conclusion, this study initially explored the clinical value of ALK-TKIs, and alectinib proved to have higher comprehensive clinical value. The results provide better drug choice for patients with ALK-positive advanced NSCLC. Besides, the results provide evaluation procedures and methods for the comprehensive evaluation of other types of antitumor drugs (cytotoxic drugs, novel antitumor drugs, etc.) in the future, and ensure rational drug use in clinic and offer a basis for decision-making and improvement of the national antitumor drug supply security system and relevant policy formulation.
Acknowledgments
Funding: The study was supported by Suzhou science and technology development plan (Medical and health science and technology innovation - Applied Basic Research) project (SKJYD2021160) and Hospital Pharmaceutical Research Fund of Chia Tai Tianqing, Jiangsu Pharmaceutical Association in 2022 (Q202230).
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-380/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-380/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zheng R, Sun K, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Chin J Oncol 2019;41:19-28. [PubMed]
- Zhang X, Lu X, Zhang L, et al. Chinese guidelines for the diagnosis and treatment of anaplastic lymphoma kinase positive and ROS1 positive non-small cell lung cancer. Chin J Pathol 2018;47:241-7.
- Ma Z, Wang Y, Sun Y, et al. Efficacy of crizotinib Combined with Chemotherapy in Treating Advanced Non-Small-Cell Lung Cancer and Effect on Patients’ Quality of Life and Adverse Reaction Rate. J Healthc Eng 2022;2022:7898737. [Crossref] [PubMed]
- Vavalà T, Novello S. Alectinib in the treatment of ALK-positive non-small cell lung cancer: an update on its properties, efficacy, safety and place in therapy. Ther Adv Med Oncol 2018;10:1758835918789364. [Crossref] [PubMed]
- Yang Y, Zhou J, Zhou J, et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med 2020;8:45-53. [Crossref] [PubMed]
- Passaro A, Prelaj A, Pochesci A, et al. Brigatinib for the treatment of ALK-positive advanced non-small cell lung cancer patients. Drugs Today (Barc) 2017;53:435-46. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Working Committee of the Guidelines of the Chinese Society of Clinical Oncology. Guidelines of Chinese society of clinical oncology (CSCO) Non-Small Cell Lung Cancer. Beijing: People’s Medical Publishing House, 2020.
- Jin He, Yang Li, Li Chungang, et al. Progress in targeted therapy of non-small cell lung cancer. Med & Pharm J Chin PLA 2020;32:105-11.
- National Health Commission of the People’s Republic of China. Guidelines for the management of comprehensive clinical evaluation of drugs (2021 version). Available online: https://www.nhc.gov.cn/yaozs/s2908/202107/532e20800a47415d84adf3797b0f4869.shtml
- China National Health Development Research Center. Technical Guidelines for Clinical Comprehensive Evaluation of Antitumor Drugs (2022 version). Available online: https//www.nhei.cn/nhei/znfb/202206/c01d87a290664b01bf42a9dad769d69f.shtml
- Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med 2019;7:437-46. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol 2019;14:1233-43. Erratum in: J Thorac Oncol 2019;14:2023. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Soria J C, Tand S W, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. Erratum in: Lancet 2017;389:908. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J Thorac Oncol 2021;16:2091-108. [Crossref] [PubMed]
- Horn L, Wang Z, Wu G, et al. Ensartinib vs crizotinib for Patients With Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol 2021;7:1617-25. [Crossref] [PubMed]
- Shaw AT, Bauer TM, de Marinis F, et al. First-Line lorlatinib or crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. [Crossref] [PubMed]
- Wu YL, Lu S, Lu Y, et al. Results of PROFILE 1029, a Phase III Comparison of First-Line crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1539-48. [Crossref] [PubMed]
- Garcia Campelo MR, Lin HM, Zhu Y, et al. Health-related quality of life in the randomized phase III trial of brigatinib vs crizotinib in advanced ALK inhibitor-naive ALK + non-small cell lung cancer (ALTA-1L). Lung Cancer 2021;155:68-77. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Tan DSW, Soria J, de Castro Jr G, et al. PROs with ceritinib versus chemotherapy in patients with previously untreated ALK-rearranged nonsquamous NSCLC (ASCEND-4). In: Vienna, Austria: World Conference on Lung Cancer, 2016.
- Pérol M, Pavlakis N, Levchenko E, et al. Patient-reported outcomes from the randomized phase III ALEX study of alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer. Lung Cancer 2019;138:79-87. [Crossref] [PubMed]
- Singh N, Temin S, Baker S Jr, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO Living Guideline. J Clin Oncol 2022;40:3323-43. Update in J Clin Oncol 2023;41:e1-9. [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-iv237. Erratum in: Ann Oncol 2019;30:863-70. [Crossref] [PubMed]
- Oncology Society of Chinese Medical Association, Chinese Medical Association Publishing House. Oncology Society of Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2021 version). Natl Med J China 2021;101:1725-57.
- Chinese Association for Clinical Oncologists, Medical Oncology Branch of Chinese International Exchange and Promotion Association for Medical and Healthcare. Clinical practice guideline for brain metastases of lung cancer in China (2021 version). Chin J Oncol 2021;43:269-81. [PubMed]
- Ckloong HH, Wong CKH, Leung LKS, et al. Cost effectiveness analysis of ceritinib vs. crizotinib in previously untreated anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) in Hong Kong. Cost Eff Resour Alloc 2020;18:50. [Crossref] [PubMed]
- Carlson JJ, Suh K, Orfanos P, et al. Cost Effectiveness of alectinib vs. crizotinib in First-Line Anaplastic Lymphoma Kinase-Positive Advanced Non-Small-Cell Lung Cancer. Pharmacoeconomics 2018;36:495-504. [Crossref] [PubMed]
- Zhang L, Huang Q, Qiu L, et al. Cost-effectiveness Analysis of ceritinib Versus alectinib for First-line Treatment of ALK-positive Advanced Non-small Cell Lung Cancer. Strait Pharmaceutical Journal 2019;31:86-9.
- Sun Lei, Chen Pingyu, Ma Aixia, et al. Pharmacoeconomic evaluation of loratinib in the first-line treatment of anaplastic lymphoma kinase positive advanced non-small cell lung cancer. China Pharmacy 2022;33:1102-8.
- National Institute for Health and Care Excellence. Anaplastic lymphoma kinase inhibitors for genetically rearranged non-small cell lung cancer: a review of the clinical effectiveness. Available online: https://www.nice.org.uk
- Canada’s Drug and Health Technology Agency. ALK Inhibitors for Non-Small Cell Lung Cancer. Available online: https://www.cadth.ca/alk-inhibitors-non-small-cell-lung-cancer
- Haratake N, Toyokawa G, Seto T, et al. The mechanisms of resistance to second- and third-generation ALK inhibitors and strategies to overcome such resistance. Expert Rev Anticancer Ther 2021;21:975-88. [Crossref] [PubMed]
- Fan J, Fong T, Xia Z, et al. The efficacy and safety of ALK inhibitors in the treatment of ALK-positive non-small cell lung cancer: A network meta-analysis. Cancer Med 2018;7:4993-5005. [Crossref] [PubMed]
- Westeel V, Bourdon M, Cortot AB, et al. Management of lung cancer patients' quality of life in clinical practice: a Delphi study. ESMO Open 2021;6:100239. [Crossref] [PubMed]
- Poh ME, How SH, Ho GF, et al. Real-World Treatment and Outcomes of ALK-Positive Metastatic Non-Small Cell Lung Cancer in a Southeast Asian Country. Cancer Manag Res 2023;15:31-41. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


