
Measuring cough: what really matters?
Introduction: why measure cough?
When you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot measure it, when you cannot express it in numbers, your knowledge is of a meagre and unsatisfactory kind. (1)—William Thomson, Lord Kelvin, 1824–1907
Cough as a subject of clinical and academic interest has grown substantially over the last 10–20 years. Whilst much remains to be explained about the physiology of the cough reflex, and the pathology of excessive coughing, a lot has been revealed. Recommended approaches to managing chronic cough are now supported by more evidence than ever before (2).
Chronic cough is a common, widespread, and often distressing clinical problem. In an internet-based survey of 1,122 subjects experiencing chronic cough across 29 European countries 78% replied either ‘frequently’ or ‘sometimes’ in response to the question ‘does your cough stop you doing the things you would like to do’, and 90% ‘frequently’ or ‘sometimes’ in response to ‘do you feel fed up or depressed because of your cough?’ (3).
Cough is important not only because of its significant impact on patient wellbeing, but also as an early feature, and marker of severity, of potentially serious disease (cancer, interstitial lung disease). Some such diseases, including tuberculosis and pandemic respiratory viral infections, also have strong implications for public health.
As expressed in the above quote by Kelvin in the context of the science of temperature (1), to measure something is the first step to understanding it. Many, if not all, of the recent advances in the field of cough would not have been possible without a means of measurement. A number of approaches have been taken to measure cough, and the method chosen depends on various factors, not least the particular reason for measurement, but also accuracy and precision, convenience, simplicity, cost, and acceptability to the patient. If cough is addressed wholly as a symptom, then subjective measurements are most important—the patient’s perception and the impact of cough on quality of life. Cough is of course though not simply perceived only by the patient, but is also a directly observable physiological and pathological phenomenon, usually obvious to others. Coughs can therefore be measured objectively, most obviously by frequency, but also in other ways.
A patient-centred approach
If we don’t ask, our care and what we do to people isn’t aligned with what matters most to them and then you get suffering. (4) —Atul Gawande, 1965
Cough-related quality of life
The subjective adverse effects of chronic cough have been well-documented for some time. The developers of the first specific tool for measuring the subjective impact of cough, the Cough Quality of Life Questionnaire (CQLQ), reported the following were frequent features in those suffering with chronic cough: worry about serious illness, concern something is wrong, frequent nausea, exhaustion, others thinking something is wrong, embarrassment or self-consciousness, difficulty speaking on the telephone, and urine incontinence (5). The resulting questionnaire has been validated in acute and chronic cough, and also used in chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) (6,7). Repeatability and internal consistency have been demonstrated, and the CQLQ has been used in clinical studies, including an early trial of the novel antitussive gefapixant (8).
Another cough-specific quality of life tool, the Leicester Cough Questionnaire (LCQ) was developed through a process of psychometric interviewing, and initial validation data were published in 2003 (9). It consists of 19 questions which assess physical, psychological and social domains of cough-related quality of life, and takes around 3 mins for patients to complete. The developers demonstrated concurrent validity, repeatability and responsiveness (9,10), and the Questionnaire has subsequently been translated into >50 languages (11,12). The LCQ has also been widely used as a primary and secondary endpoint in clinical trials of antitussives, helping to provide evidence of the effectiveness of therapies for chronic cough such as gabapentin (13) and gefapixant (14). The minimum clinically important difference in LCQ scores has been defined, as specifically has the minimal threshold for change in refractory chronic cough (RCC) (15).
Measurement of cough with the LCQ has led to better understanding of cough in a range of diseases including COPD (16), asthma (17), bronchiectasis (18) and IPF (19). A version of the questionnaire has also been developed for acute cough to enable study of interventions in this context (20).
Cough-severity symptom scales
Rather than making a measurement of the effect of chronic cough on global quality of life, symptom scales can be used to give a subjective rating of cough severity. The main advantage of such scales is ease and brevity, with the trade-off of not capturing the fuller impact of cough on the patient. A 100-mm (100-point) cough severity visual analogue scale (VAS) has been commonly employed in both routine clinical practice and research (Figure 1). The tool has been shown to be responsive, for example to the resolution of symptoms following acute upper respiratory tract infection (21).
A simple symptom scale is clearly distinct from a global measure of cough-specific quality of life. This is well-demonstrated, including recently in a group of patients by Martin Nguyen et al., where both cough severity diary and LCQ scores correlated only to a moderate degree with cough severity VAS scores (22). This less than strong correlation with quality of life scores is by no means a weakness of the cough severity VAS—the tool may be seen as measuring only one specific dimension of the subjective impact of cough. Limitations of the VAS though include poor intra-patient repeatability, with intra-class correlation (ICC) scores of around 0.5 (22). The lack of numbers or gradation markers on the simple plain line scale may be part of the reason for this, hence a cough severity numeric rating scale may be an alternative to consider.
This key limitation has led to advocates of the Cough Severity Diary, a 7-item measure of frequency, intensity and disruption. The scores seem more repeatable than those of the cough severity VAS (ICC 0.68), and a slightly stronger correlation than cough severity VAS scores with LCQ scores (23). Further evaluation is required, but the VAS, or a slightly modified version of it, will likely remain popular due to its simplicity.
Psychological impact
Another way of subjectively measuring cough is to focus on associated psychological morbidity. Diminished mental health is common in those with chronic cough. McGarvey et al. in a UK specialist cough clinic used the Hospital Anxiety and Depression Scale (HADS) and other validated tools to demonstrate rates of anxiety and depression of 33% and 16% respectively in those referred with chronic cough (24). Similarly, in a US-based study of patients with chronic cough using the Center for Epidemiologic Studies Depression Scale, 53% had depression (25). Recent data from our South London specialist cough clinic have also demonstrated high rates of significant depression (26).
Objective measures of cough
Cough is defined physiologically as an objective phenomenon, the rapid release of air following forced expiration against a closed glottis producing a characteristic sound (27). Coughs can clearly therefore be independently observed and objectively measured. Furthermore, asking a patient to give a subjective account of their cough will likely lead to a different version of events to that obtained by an independent observer. The basic pathophysiological action of coughing is unlikely the focus of concern of the patient; the subjective perception of cough will be strongly influenced by concerns about underlying illness, secondary physical effects (such as urinary stress incontinence and pain), social anxiety about the reactions of others, and other factors, as discussed in the previous section. Such perceptions and subjective awareness of cough will also vary depending on mood and distraction.
The advantage of objectively measuring cough is in providing a more reliable surrogate marker of the underlying pathological process. Objective measures, by definition, depend not on the observer, but on the tools and processes of measurement, and should therefore much be much-less variable within and between individuals than patient-reported metrics. For this reason, objective measures of cough should be expected to better reflect disease mechanisms. Objective measures therefore are preferred for research into the causes of cough, and for testing the effects of an intervention which aims to lessen coughing.
Cough frequency measurement
Although cough can be objectively measured in several ways, perhaps the most obvious characteristic of pathological coughing which lends itself to objective measurement is cough frequency. As has been reviewed elsewhere (28), attempts to objectively measure cough frequency have been made since the 1950s. This was initially driven by a desire to understand factors which exacerbate cough, such as smoking (29), to study infectiousness in tuberculosis (30), and to investigate cough suppressants (31).
Cough monitoring technology
Digitisation and miniaturisation of electronic technology over the last decades allows potential modern cough frequency monitors to be a lot smaller, more portable, and run for longer time periods than previously. Modern computerised signal processing techniques have also introduced the potential for automating the detection of coughs, based largely on their distinctive acoustic properties (28).
One such recent system is the Leicester Cough Monitor (LCM) (32). A lapel microphone is connected to a small MP3 digital audio recorder and worn by the subject for periods of 24 h or longer during usual day-to-day activity. The resultant recording, following brief calibration by a researcher, is then later analysed by bespoke software to give a report of numbers of individual coughs, and their timings. Compared to human listeners of recordings in patients with chronic cough, the developers initially reported the system had a sensitivity and specificity for cough counts of 91% and 99% respectively (32). An independent research group subsequently reported excellent correlation between human cough counts and those reported by the LCM software in a blinded analysis (correlation coefficient, r=0.97, P<0.001; intraclass correlation 0.98, P<0.001) (33).
The LCM has proven useful at providing cough counts in clinical trials of potential treatments for cough, both antitussive medications (13,34,35), and non-pharmacological interventions (36). The LCM system has also been used to investigate predictors of cough in sarcoidosis (37), and to assess the risk of transmitting infection in patients with pulmonary tuberculosis (38).
Another cough monitoring system is VitaloJAK, differing from the LCM in using a proprietary digital recorder, and recording sound through a chest wall contact microphone as well as one attached to the lapel (39). Cough counting is then not automated, but resulting recordings are compressed and shortened to remove silences and the majority of non-cough sounds, to be then processed by human cough counters aided by software providing a visual representation of the audio data. Validation of this compression process has been reported by the developers (39).
VitaloJAK has been widely used in antitussive clinical trials in the last decade, for example recently for novel classes of antitussives such as P2X3, NK1 and TRMP8 antagonists (14,40,41). The monitor has also been used in other contexts in cough research, such as studying cough in COPD, asthma and lung cancer (16,42,43).
Other cough monitoring systems are in development (44-46), but there are several limitations to current technology. The above-mentioned devices, although small and lightweight, are intrusive to the patient to some degree. The wearer is physically aware of being attached to the monitor, and whilst in use activities such as swimming and showering are restricted. The wearer may also be conscious of being recorded, potentially altering the cough rate being measured—similar to the well-known phenomenon of ‘white coat hypertension’, whereby anxiety and other factors influence blood pressure when the subject is aware of being observed. As well as coughs, both the LCM and VitaloJAK pick up speech and all other background sound on the recordings which are sent for analysis. Whilst the subsequent automated components of cough frequency analysis circumvent the need for human observers to listen to recorded speech, the possibility of small portions of private conversations being ‘overheard’ exist, hence currently consent into intrusion of personal privacy is required prior to cough monitoring.
Validated cough frequency monitors up to now have very much been restricted to the research setting rather than routine clinical practice, mainly due to the necessary expense and expertise of analysing the recordings. The current technology has limited the maximum duration of ambulatory recordings to 2–4 days at most. Much longer periods of time would allow tracking of cough, for example as a potential early indicator of COPD exacerbations (47) or pandemic viral infections (48). Potential applications for cough frequency monitoring would be more widespread in clinical practice if technology was fully automated, and give results in real time. Cough monitoring mobile phone apps are one possibility (48), although several obstacles need to be overcome, including drain on battery power, interruption of detecting coughs during phone calls, and the need for a microphone or the phone to be in relatively close unobstructed range of the subject.
Measuring cough frequency: uncertainties
Aside from the technical and practical considerations surrounding cough frequency monitoring, there remain theoretical uncertainties. There has been debate about the definition and basic unit of cough for frequency monitoring (49). While individual coughs are now generally used for reporting cough counts (50), one recently used bespoke cough monitor, amongst others, has used ‘coughing episodes’, variously described (51). Coughing ‘bouts’ are currently defined by consensus amongst experts in the field as runs of closely spaced individual coughs separated by no more than 2 s (52). The concept of cough bouts is attractive as they may be more noticeable to the patient than individual coughs. Periods of closely-spaced prolonged coughing may also correlate better with pathology than single coughs (53), the latter perhaps more common in healthy individuals exposed to occasional inhaled irritants and other factors in daily life. Previous work from the Manchester group suggests that in chronic cough, number of cough bouts (or ‘epochs’), as defined above are closely correlated with the daily frequency of individual coughs, and that both are similarly related to patient cough-related quality of life (52). However, recent work from the same researchers has suggested that a looser definition of cough bouts which groups together individual component coughs separated by ≤3 s rather than by only ≤2 s into the same episode may be slightly more relevant in terms of patient perception of coughing severity (54).
The temporal distribution of coughs during a day may also be important. Cough is well-known to be less frequent during sleep than during the day (55), but particular diurnal patterns may indicate certain diagnoses and disease activity, for example excessive cough at night and immediately after waking in poorly-controlled asthma (42). In clinical trials in recent years both 24-h and daytime (‘awake’) cough frequency have been used as primary endpoints (56). It is not clear which should be preferred in general. Focusing on the waking hours results in higher hourly median cough counts, potentially amplifying positive effects of an intervention on cough, although perhaps with wider variation by excluding similar low numbers of coughs overnight. Not including nocturnal coughs may also miss the minority of individuals for whom coughing overnight is significant, particularly if having a large impact on sleep.
The timing of coughs throughout waking hours may potentially indicate precipitants and aetiology, such as environmental exposures, including occupational. This has been little explored (57). The temporal clustering of daily coughs likely has an influence on how subjects perceive cough independent to overall cough frequency (58); short bursts of intense coughing widely dispersed intermittently throughout a day may be more distressing than several coughs per hour spaced more regularly with the same overall average daily cough frequency.
At present, due to the limitations of current technology already mentioned, little information exists on inter-day variation in cough frequency, not only in stable disease states, but also in acute respiratory conditions, chronic conditions with varying activity over time, and in healthy individuals. This is a problem, not only in determining what is ‘normal’, for a particular individual, or for groups of particular types of individuals, but also for using 24-h cough frequency on one particular day to judge the effect of an intervention on cough. In the ‘stable’ state coughs occur sporadically during waking hours, with numbers per hour roughly approximating a Poisson distribution. Day-to-day variation in cough counts is determined not only by inherent biological influences, but also by environmental factors. A single day of recording will give little indication of ‘average’ daily cough frequency, especially if there is an ‘acclimatisation’ effect whilst the subject becomes comfortable to being monitored. Only through the accumulation of more data, from prolonged periods of cough frequency monitoring can this variation be better understood and statistically modelled (59,60). In 1965 Robert Loudon stated “the development of cough-counting devices measuring cough frequency in individual patients has made it important to estimate the frequency with which cough occurs in the general population” (61). This holds true now as much as it did nearly 60 years ago.
Cough frequency: relation to patient-reported outcomes
Objective cough counts correlate to only a limited degree with subjects’ own perceptions of their cough. This has been known for over 50 years. Cough frequencies in a mixture of patients with respiratory disease measured using sound recording equipment related poorly to responses to the question “Do you have a cough at the present time?” (62). In keeping with other recent data, we have similarly shown at most only a moderate correlation of 24-h cough frequency across a range of diseases with both subjectively-reported cough severity (VAS scores) and cough-related quality of life (LCQ-scores) (Figure 2) (53).
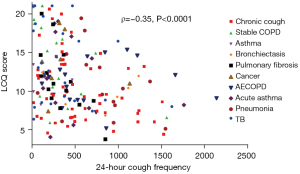
Data from recent clinical trials of novel antitussives in patients with RCC also give interesting insights in this area. VOLCANO-2 was a Phase 2b randomised controlled trial of the neurokinin-1 (NK1) antagonist orvepitant in 315 patients with refractory cough. Although those in the active treatment arms demonstrated significant improvements in the patient-reported secondary endpoints of cough-severity VAS and LCQ scores compared to placebo, the primary endpoint of improvement in awake cough frequency was not met (63). Overall, cough-related symptoms improved with the drug whilst cough frequency appeared to change no more than with placebo, a positive effect on cough as reported by the patient that was not explained by improvements in the objective measure of cough.
There are several possible explanations for this discrepancy, which are not mutually exclusive. One is that the drug altered patients’ perception of their cough in a positive way, making it appear less bothersome, whilst having no effect on the underlying objective nature of the cough itself. The second possibility relates to a potential statistical anomaly, including the stochastic nature of cough frequency alluded to above. The third possibility is that the drug did have an objective positive impact on cough, only not on the objective measure (cough frequency) chosen in this study.
In support of this first idea, of the drug affecting only the subjective appreciation of cough, is that NK1 inhibitors probably have action on the central nervous system. Partly for this reason, the related drug aprepitant is effective as an antiemetic. Orvepitant could therefore be having effects on cortical centres involved in the perception of cough, without necessarily altering the frequency of coughing. Regarding the second, possibly statistical explanation for the mismatch between cough symptoms and cough frequency following treatment with orvepitant, is a signal from the study data for an effect on a pre-defined subgroup of patients with cough frequency higher than the study median (63).
Large inter-individual variability has been observed in daily cough frequency in many contexts amongst patients who report troublesome cough (62). In VOLCANO-2 there was a 230% difference in the median cough frequency between the groups with lower vs higher cough frequency (Table 1). At the same time, there was only a 13% difference in mean cough severity VAS scores between these two groups (Steve Pawsey, Nerre Pharmaceuticals, personal communication). The much larger variation here in baseline cough frequency than in subjective cough severity may have been a reason for the difficulty in detecting meaningful change in the objective measure, even with >300 study subjects.
Table 1
| Variable | Lower frequency cough group (n=150) | Full analysis set (n=315) | Higher frequency cough group (n=156) |
|---|---|---|---|
| Awake cough frequency (coughs/hour) | 20.2 | 43.0 | 66.7 |
| Cough severity VAS, day (mm) | 63.6 | 67.6 | 71.8 |
Sub-groups prospectively defined according to study population median frequency (32 coughs/hour). Summary data are medians. VAS, visual analogue scale. Previously unpublished data reproduced with permission of Nerre Pharmaceuticals.
Cough frequency therefore is not the same thing as cough ‘severity’ but is likely only a component of it. Returning to the third suggested reason for the apparent discordant effects of orvepitant on cough frequency and cough symptoms, there are presumably factors besides average numbers of coughs over unit time that influence subjective perceptions and impact of coughing. The previous section suggested one of these factors—the way coughs are distributed in time throughout the day; clusters in time may be relevant as well as overall average cough frequency per unit time. However, variables of cough other than its frequency can also be objectively measured, and may be of relevance.
Other objective measures of cough
Cough intensity
Patients describe their coughs in varied ways, using adjectives including harsh, strong, intense, loud, painful (64). The strength or intensity of cough may therefore be as important to patients as its frequency. Hence attempts have been made at measuring this characteristic. Approaches have included oesophageal manometry, electromyography of the respiratory muscles, cough peak flow, and cough sound amplitude (65). There has been interest in measuring cough peak flow as a surrogate of cough efficiency in stroke and other neurological diseases to guide respiratory physiotherapists and others in implementing measures to help clear respiratory secretions and reduced the risk of aspiration (66).
Few studies have investigated intensity in chronic cough. One notable exception is that of Lee et al. which measured amplitude characteristics of voluntary cough sounds of patients with chronic cough, suggesting good correlation with oesophageal pressure and cough flow (67). No study to our knowledge has taken continuous measurements of cough strength or intenisty in an ambulatory setting and compared it to subjective assessments of cough-related quality of life or severity.
Cough reflex sensitivity (CRS)
A measure of the inherent tendency of an individual to cough has been of interest in cough research for some time, and standardised protocols have been developed for inhalational tussive challenges (68). Capsaicin and citric acid have been the agents most commonly used, although others, including distilled water, and recently ATP have been deployed (69). The commonly-used endpoint from incrementally increasing inhaled doses is the minimum concentration of solution required to elicit two or five coughs within 15 s (C2 and C5, respectively). Other endpoints have, however, been suggested (70,71).
A potential utility of cough challenge testing is as a biomarker of cough hypersensitivity. CRS, for example, has been shown to be higher (with a reduction in C5) during upper respiratory tract infection than during subsequent recovery in healthy individuals, correlating with self-reported cough symptoms (72). Similarly, CRS increases during COPD exacerbations compared to during periods of stable disease (73). Furthermore, cough challenge tests have given support to the concept of cough phenotypes, whereby excessive cough in different contexts or diseases may be associated with different patterns of response to a panel of tussive agents. In a study by Belvisi et al. there was a suggestion of different disease-related tussive responses to inhaled capsaicin, citric acid, and prostaglandin E2 amongst individuals with COPD, asthma, or isolated chronic cough (74). This may be driven by differences in airway receptor profile between disease groups.
A problem with CRS as it is currently measured is wide variability amongst individuals with chronic cough, and overlap with measurements from healthy subjects (75). The test has therefore so far proven elusive as a means of objectively confirming cough hypersensitivity. However, recent re-analysis of data from a previous study of capsaicin challenges in patients with RCC and healthy subjects suggested a specific threshold for C5 of 29 mmol/L as providing relatively high sensitivity and specificity (of 72% and 88%, respectively) for separating the two groups (76). This analysis needs confirmation with new data, perhaps involving variations in the methods or equipment currently employed for measuring CRS.
CRS testing has attracted a lot of interest as a potential means of investigating antitussive medication. As a means of demonstrating pharmacological efficacy against a specific airway receptor it is a powerful tool. The problem is that a reduction in the cough response to an inhaled tussive compound does not necessarily relate to improvements in either daily cough frequency or patient symptoms. This is best illustrated by research into inhibitors of the airway receptor TRPV1, a key component of the cough response to inhaled capsaicin. Despite substantially reducing capsaicin-induced cough, two-week administration of the TRPV1 inhibitor XEN-D0501 in subjects with chronic cough had no effect on either 24-h cough frequency or cough-related quality of life as measured with the LCQ (77). The complexity of cough is such that the focus on experimentally-induced cough responses to a single tussive agent has significant limitations.
Cough suppression ability
The central pathways of the cough reflex have an important role, not only in the conscious appreciation of coughing, but also in allowing a conscious (and unconscious) control of cough. In RCC, cortical inhibitory influences on the cough reflex appear to dysregulated. Decreased activity in several areas of the brain thought to be involved in cough inhibition has been demonstrated by functional magnetic resonance imaging in individuals with RCC; the dorsomedial pre-frontal cortex and anterior mid-cingulate cortex are of particular interest (78).
The ability to voluntarily suppress cough can be measured. Instructions to the subject during standard CRS testing normally includes a request to allow coughing to occur freely when the urge arises follow inhalation of tussive agent (68). However, if subjects are asked to try and prevent themselves from coughing, then measuring C5 (or another endpoint) during inhalation tussive challenge gives an indication of the ability to suppress cough. Such measures have been shown to be repeatable in healthy subjects and, furthermore, the ability to voluntarily supress capsaicin-provoked cough has been demonstrated to be much reduced in individuals with chronic refractory cough compared to healthy controls (79). Of further interest is the finding of Cho et al. that cough suppression ability appears relatively preserved in patients with excessive chronic cough associated with COPD, suggesting differing mechanism of persistent coughing in the disease compared to individuals with RCC in the absence of other lung disease (80).
Measuring cough suppression ability in chronic cough therefore suggests underlying drivers of cough in different contexts, which may help direct therapy.
Conclusions—what really matters?
There is no simple answer to the question of what really matters as the preferred approach to measuring cough. In practice, all the methods outlined above have strengths and weaknesses. Different ways of measuring cough often complement one another, and help provide a fuller picture for the patient, clinician and researcher. The particular measurement technique chosen will depend on various factors including availability, time, cost, acceptability to the patient, and not least the reason for measurement in the first place, including whether it be clinical, for the benefit of one particular patient, or for research, across a group of individuals. Ultimately, when the aim is to manage the symptom of cough in a particular individual in the clinic then the patient’s perspective is most important, favouring the subjective measures above. Conversely, objective measures are also important, and have a particular role in the research context, for example in demonstrating efficacy during drug development.
Cough frequency has now become the default primary endpoint of commercial trials of new treatments of cough, but, as discussed above, has limitations, with a particular need for more accessible means of long-duration real-time unobtrusive cough monitoring. There is discordance between objective frequency and symptoms, suggesting either a limitation in the way cough frequency is currently assessed, or the fact that the frequency of coughing is not the only feature of cough that bothers patients. Potential benefits to using patient-reported outcomes as endpoints in clinic trials are the low cost and wide availability of measurement tools, simplicity for the patient and research team, and the fact that they can measure multiple domains of subject impact of cough simultaneously. A downside of subjective measures is the relative lack of specificity for cough, potentially also capturing off-target changes in health due to other potential effects of antitussives, for example on mood.
What matters for measuring cough will depend on context. For a diagnosis of cough hypersensitivity, cough challenge testing or suppression testing may be most useful, perhaps in conjunction with specifically validated cough hypersensitivity questionnaires (81). For efficacy endpoints in clinical trials of antitussives, a composite objective and patient reported outcome might be most relevant, where the objective element not only addresses average hourly cough frequency over a 24-h period, but also includes cough bouts, other measures of the temporal distribution of cough (such as cough-free interval), captured over more prolonged periods. Non-frequency objective measures of cough such as intensity assessment might become commonplace in antitussive studies. For early detection of exacerbations of asthma, COPD, and other chronic lung diseases, cough frequency measurement would probably be the most useful variable, but would require continuous real-time monitoring, as might be possible in a smart phone app (60). Such continuous cough frequency measures, if widely available, could also be used for early detection of respiratory diseases such as lung cancer, tuberculosis, or pandemic viral infection. As a marker of the progression of diseases such as IPF objective measures are probably most appropriate, particularly cough frequency, however defined; the same is the case for monitoring the response to treatment of curable lung diseases such as tuberculosis (82).
Cough research is still currently a young and relatively niche discipline. The tools available to measure cough have been instrumental in progress to date. The resulting expansion of knowledge has made more apparent the complexity of cough. As the methods of measuring cough continue to develop and become more widely available, so understanding of cough will grow further still.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Woo-Jung Song, Kian Fan Chung) for the series “Novel Insights Into Chronic Cough” published in Journal of Thoracic Disease. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-230/coif). The series “Novel Insights Into Chronic Cough” was commissioned by the editorial office without any funding or sponsorship. SSB has received personal fees from Merck, Bayer, Shionogi, Bellus, Nerre, Gala and Trevi, research grants from Merck, fees for LCM cough monitoring analysis and LCQ. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thomson W. Popular Lectures and Addresses. Electrical Units of Measurement. In: Ratcliffe S. editor. Oxford Essential Quotations. 4th ed. Oxford University Press; 2016.
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020;55:1901136. [Crossref] [PubMed]
- Chamberlain SA, Garrod R, Douiri A, et al. The impact of chronic cough: a cross-sectional European survey. Lung 2015;193:401-8. [Crossref] [PubMed]
- Gawande A. Interview for “Being Mortal: Medicine and what Matters in the End”. [Internet]. 2017 [cited 2023 Mar 7]. Available online: https://www.cbsnews.com/news/doctor-atul-gawande-being-mortal-author-end-of-life-care/
- French CT, Irwin RS, Fletcher KE, et al. Evaluation of a cough-specific quality-of-life questionnaire. Chest 2002;121:1123-31. [Crossref] [PubMed]
- Smith J, Owen E, Earis J, et al. Cough in COPD: correlation of objective monitoring with cough challenge and subjective assessments. Chest 2006;130:379-85. [Crossref] [PubMed]
- Lechtzin N, Hilliard ME, Horton MR. Validation of the Cough Quality-of-Life Questionnaire in patients with idiopathic pulmonary fibrosis. Chest 2013;143:1745-9. [Crossref] [PubMed]
- Abdulqawi R, Dockry R, Holt K, et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2015;385:1198-205. [Crossref] [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Raj AA, Pavord DI, Birring SS. Clinical cough IV:what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol 2009;311-20. [Crossref] [PubMed]
- Akis Gonen N, Havlucu Y, Yorgancioglu A, et al. Reliability and validity of a Turkish version of Leicester cough questionnaire Eur Respir J 2015;46:PA3949. [abstract].
- Sönnerfors P, Faager G, Einarsson U. Translation of the Leicester Cough Questionnaire into Swedish, and validity and reliability in chronic obstructive pulmonary disease. Disabil Rehabil 2018;40:2662-70. [Crossref] [PubMed]
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [Crossref] [PubMed]
- McGarvey LP, Birring SS, Morice AH, et al. Efficacy and safety of gefapixant, a P2X(3) receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022;399:909-23. [Crossref] [PubMed]
- Nguyen AM, Schelfhout J, Muccino D, et al. Leicester Cough Questionnaire validation and clinically important thresholds for change in refractory or unexplained chronic cough. Ther Adv Respir Dis 2022;16:17534666221099737. [Crossref] [PubMed]
- Sumner H, Woodcock A, Kolsum U, et al. Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:943-9. [Crossref] [PubMed]
- Marsden PA, Smith JA, Kelsall AA, et al. A comparison of objective and subjective measures of cough in asthma. J Allergy Clin Immunol 2008;122:903-7. [Crossref] [PubMed]
- Murray MP, Turnbull K, MacQuarrie S, et al. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J 2009;34:125-31. [Crossref] [PubMed]
- Key AL, Holt K, Hamilton A, et al. Objective cough frequency in Idiopathic Pulmonary Fibrosis. Cough 2010;6:4. [Crossref] [PubMed]
- Yousaf N, Lee KK, Jayaraman B, et al. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough 2011;7:4. [Crossref] [PubMed]
- Wang K, Birring SS, Taylor K, et al. Montelukast for postinfectious cough in adults: a double-blind randomised placebo-controlled trial. Lancet Respir Med 2014;2:35-43. [Crossref] [PubMed]
- Martin Nguyen A, Bacci ED, Vernon M, et al. Validation of a visual analog scale for assessing cough severity in patients with chronic cough. Ther Adv Respir Dis 2021;15:17534666211049743. [Crossref] [PubMed]
- Vernon M, Kline Leidy N, Nacson A, et al. Measuring cough severity: development and pilot testing of a new seven-item cough severity patient-reported outcome measure. Ther Adv Respir Dis 2010;4:199-208. [Crossref] [PubMed]
- McGarvey LP, Carton C, Gamble LA, et al. Prevalence of psychomorbidity among patients with chronic cough. Cough 2006;2:4. [Crossref] [PubMed]
- Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest 2006;130:1839-43. [Crossref] [PubMed]
- Hirons B, Rhatigan K, Simpson A, et al. Suicidal ideation, depression and anxiety in chronic cough Thorax 2022;77:A16. [abstract].
- Morice AH. Rebuttal: cough is an expiratory sound. Lung 2008;186:S7-9. [Crossref] [PubMed]
- Hall JI, Lozano M, Estrada-Petrocelli L, et al. The present and future of cough counting tools. J Thorac Dis 2020;12:5207-23. [Crossref] [PubMed]
- Loudon RG. Smoking and cough frequency. Am Rev Respir Dis 1976;114:1033-6. [PubMed]
- Loudon RG, Spohn SK. Cough frequency and infectivity in patients with pulmonary tuberculosis. Am Rev Respir Dis 1969;99:109-11. [PubMed]
- Woolf CR, Rosenberg A. Objective assessment of cough suppressants under clinical conditions using a tape recorder system. Thorax 1964;19:125-30. [Crossref] [PubMed]
- Birring SS, Fleming T, Matos S, et al. The Leicester Cough Monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J 2008;31:1013-8. [Crossref] [PubMed]
- Birring SS, Mann VM, Matos S, et al. From the authors (response to: The Leicester Cough Monitor: a semi-automated, semi-validated cough detection system?). Eur Respir J 2008;32:530-1. [Crossref]
- Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and Speech Pathology Combination Therapy for Refractory Chronic Cough: A Randomized Controlled Trial. Chest 2016;149:639-48. [Crossref] [PubMed]
- Birring SS, Wijsenbeek MS, Agrawal S, et al. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir Med 2017;5:806-15. [Crossref] [PubMed]
- Chamberlain Mitchell SA, Garrod R, Clark L, et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax 2017;72:129-36. [Crossref] [PubMed]
- Sinha A, Lee KK, Rafferty GF, et al. Predictors of objective cough frequency in pulmonary sarcoidosis. Eur Respir J 2016;47:1461-71. [Crossref] [PubMed]
- Turner RD, Birring SS, Darmalingam M, et al. Daily cough frequency in tuberculosis and association with household infection. Int J Tuberc Lung Dis 2018;22:863-70. [Crossref] [PubMed]
- Smith JA, Holt K, Dockry R, et al. Performance of a digital signal processing algorithm for the accurate quantification of cough frequency. Eur Respir J 2021;58:2004271. [Crossref] [PubMed]
- Smith J, Allman D, Badri H, et al. The Neurokinin-1 Receptor Antagonist Orvepitant Is a Novel Antitussive Therapy for Chronic Refractory Cough: Results From a Phase 2 Pilot Study (VOLCANO-1). Chest 2020;157:111-8. [Crossref] [PubMed]
- Protocol Information. EudraCT Number 2017-003108-27 [Internet]. [cited 2023 Jan 30]. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-003108-27/GB#A
- Lodhi S, Smith JA, Satia I, et al. Cough rhythms in asthma: Potential implication for management. J Allergy Clin Immunol Pract 2019;7:2024-7. [Crossref] [PubMed]
- Smith JA, Harle A, Dockry R, et al. Aprepitant for Cough in Lung Cancer. A Randomized Placebo-controlled Trial and Mechanistic Insights. Am J Respir Crit Care Med 2021;203:737-45. [Crossref] [PubMed]
- Galvosas M, Gabaldón-Figueira JC, Keen EM, et al. Performance evaluation of the smartphone-based AI cough monitoring app - Hyfe Cough Tracker against solicited respiratory sounds F1000Research 2022;11:730. [version 1; peer review: 1 approved with reservations, 1 not approved]. [Crossref]
- Do W, Russell R, Wheeler C, et al. Performance of cough monitoring by Albus Home, a contactless and automated system for nocturnal respiratory monitoring at home. ERJ Open Res 2022;8:00265-2022. [Crossref] [PubMed]
- Kuhn M, Nalbant E, Kohlbrenner D, et al. Validation of a small cough detector. ERJ Open Res 2023;9:00279-2022. [Crossref] [PubMed]
- Crooks MG, den Brinker AC, Thackray-Nocera S, et al. Domiciliary Cough Monitoring for the Prediction of COPD Exacerbations. Lung 2021;199:131-7. [Crossref] [PubMed]
- Gabaldón-Figueira JC, Keen E, Giménez G, et al. Acoustic surveillance of cough for detecting respiratory disease using artificial intelligence. ERJ Open Res 2022;8:00053-2022. [Crossref] [PubMed]
- Fontana GA, Widdicombe J. What is cough and what should be measured? Pulm Pharmacol Ther 2007;20:307-12. [Crossref] [PubMed]
- Turner RD, Bothamley GH. How to count coughs? Counting by ear, the effect of visual data and the evaluation of an automated cough monitor. Respir Med 2014;108:1808-15. [Crossref] [PubMed]
- Proaño A, Bravard MA, Tracey BH, et al. Protocol for studying cough frequency in people with pulmonary tuberculosis. BMJ Open 2016;6:e010365. [Crossref] [PubMed]
- Kelsall A, Decalmer S, Webster D, et al. How to quantify coughing: correlations with quality of life in chronic cough. Eur Respir J 2008;32:175-9. [Crossref] [PubMed]
- Turner RD. A description of cough in tuberculosis and other respiratory conditions. PhD Thesis. Queen Mary University of London; 2016.
- Dockry R, Holt K, Smith J, et al. S17 A relevant definition of cough bouts Thorax 2022;77:A14-5. [abstract].
- Lee KK, Birring SS. Cough and sleep. Lung 2010;188:S91-4. [Crossref] [PubMed]
- Smith JA, Kitt MM, Morice AH, et al. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med 2020;8:775-85. [Crossref] [PubMed]
- Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology 2010;139:754-62. [Crossref] [PubMed]
- Al-Sheklly B, Ford J, Foden P, et al. P1 Is the distribution of cough just as important as cough frequency? Thorax 2018;73:A96. [abstract].
- Rudd M, Song WJ, Small PM. The Statistics of Counting Coughs: Easy as 1, 2, 3? Lung 2022;200:531-7. [Crossref] [PubMed]
- Gabaldón-Figueira JC, Keen E, Rudd M, et al. Longitudinal passive cough monitoring and its implications for detecting changes in clinical status. ERJ Open Res 2022;8:00001-2022. [Crossref] [PubMed]
- Loudon RG, Brown LC, Hurst SK. Cough frequency in a group of males. Arch Environ Health 1965;11:372-4. [Crossref]
- Loudon RG, Brown LC. Cough frequency in patients with respiratory disease. Am Rev Respir Dis 1967;96:1137-43. [PubMed]
- Smith J, Ballantyne E, Kerr M, et al. Late Breaking Abstract - The neurokinin-1 receptor antagonist orvepitant improves chronic cough symptoms: results from a Phase 2b trial Eur Respir J 2019;54:PA600. [abstract].
- Vernon M, Leidy NK, Nacson A, et al. Measuring cough severity: Perspectives from the literature and from patients with chronic cough. Cough 2009;5:5. [Crossref] [PubMed]
- Cho PSP, Birring SS, Fletcher HV, et al. Methods of Cough Assessment. J Allergy Clin Immunol Pract 2019;7:1715-23. [Crossref] [PubMed]
- Suárez AA, Pessolano FA, Monteiro SG, et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am J Phys Med Rehabil 2002;81:506-11. [Crossref] [PubMed]
- Lee KK, Matos S, Ward K, et al. Sound: a non-invasive measure of cough intensity. BMJ Open Respir Res 2017;4:e000178. [Crossref] [PubMed]
- Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007;29:1256-76. [Crossref] [PubMed]
- Morice AH, Kitt MM, Ford AP, et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J 2019;54:1900439. [Crossref] [PubMed]
- Hilton EC, Baverel PG, Woodcock A, et al. Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J Allergy Clin Immunol 2013;132:847-55.e1-5.
- Turner RD, Birring SS, Bothamley GH. What is the optimum endpoint for the capsaicin cough challenge? Am J Respir Crit Care Med 2016;193:A5169. [abstract].
- O'Connell F, Thomas VE, Studham JM, et al. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 1996;90:279-86. [Crossref] [PubMed]
- Cho PSP, Fletcher HV, Turner RD, et al. The Relationship Between Cough Reflex Sensitivity and Exacerbation Frequency in Chronic Obstructive Pulmonary Disease. Lung 2020;198:617-28. [Crossref] [PubMed]
- Belvisi MG, Birrell MA, Khalid S, et al. Neurophenotypes in Airway Diseases. Insights from Translational Cough Studies. Am J Respir Crit Care Med 2016;193:1364-72. [Crossref] [PubMed]
- Prudon B, Birring SS, Vara DD, et al. Cough and glottic-stop reflex sensitivity in health and disease. Chest 2005;127:550-7. [Crossref] [PubMed]
- Koskela HO, Nurmi HM, Birring SS. Utility of Cough Provocation Tests in Chronic Cough and Respiratory Diseases: A Comprehensive Review and Introduction of New Reference Ranges for the Capsaicin Test. Allergy Asthma Immunol Res 2021;13:833-49. [Crossref] [PubMed]
- Belvisi MG, Birrell MA, Wortley MA, et al. XEN-D0501, a Novel Transient Receptor Potential Vanilloid 1 Antagonist, Does Not Reduce Cough in Patients with Refractory Cough. Am J Respir Crit Care Med 2017;196:1255-63. [Crossref] [PubMed]
- Ando A, Smallwood D, McMahon M, et al. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax 2016;71:323-9. [Crossref] [PubMed]
- Cho PSP, Fletcher HV, Turner RD, et al. Impaired cough suppression in chronic refractory cough. Eur Respir J 2019;53:1802203. [Crossref] [PubMed]
- Cho PSP, Fletcher HV, Patel IS, et al. Cough hypersensitivity and suppression in COPD. Eur Respir J 2021;57:2003569. [Crossref] [PubMed]
- Won HK, Kang SY, Kang Y, et al. Cough-Related Laryngeal Sensations and Triggers in Adults With Chronic Cough: Symptom Profile and Impact. Allergy Asthma Immunol Res 2019;11:622-31. [Crossref] [PubMed]
- Proaño A, Bravard MA, López JW, et al. Dynamics of Cough Frequency in Adults Undergoing Treatment for Pulmonary Tuberculosis. Clin Infect Dis 2017;64:1174-81. [Crossref] [PubMed]



