
Surgery vs. radiotherapy in a population-based cohort of elderly patients with early-stage small-cell lung cancer: an IPTW propensity-score analysis
Highlight box
Key findings
• The elderly early-stage SCLC patients who underwent surgery had superior OS compared to those who underwent radiotherapy both before and after the IPTW adjustment. For patients aged 70–80 years, surgery was superior to radiotherapy in terms of cancer-specific survival.
What is known and what is new?
• Only limited treatment outcome data comparing surgery with radiotherapy have been available for elderly patients with early-stage SCLC, and thus the best local therapy for this population had not previously been defined.
• This retrospective study compared the use of surgery and radiotherapy in elderly patients with early-stage SCLC using data from the SEER database from 2004 to 2018. IPTW was used to balance the bias of any confounding factors that may affect local treatment choice.
What is the implication, and what should change now?
• This study provides strong evidence that surgery produces a more favorable prognosis than radiotherapy and is suitable in elderly patients with early-stage SCLC.
Introduction
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related mortality worldwide (1). Small cell lung cancer (SCLC) accounts for 15% of all lung cancers and is characterized by its rapid tumor growth, early metastatic dissemination, and poor prognosis (2). According to the latest cancer statistics, the incidence of lung cancer increases with age, and most lung cancer cases occur in adults aged ≥70 years (3). Due to the increased life expectancy of the aging population and the increasing incidence of elderly SCLC, the elderly SCLC population is increasing. In the United States, the percentage of elderly patients (aged >70 years) with SCLC increased from 23% in 1975 to 44% in 2010 (4). However, elderly patients are under-represented in clinical trials due to concerns about comorbidities and functional impairment (5).
Less than 5% of patients with SCLC have early-stage (T1–2N0) SCLC (6). Due to a lack of randomized controlled trials comparing surgical to non-surgical treatment modalities in the era of contemporary staging and treatments, the management of these patients continues to be debated. Definitive surgical resection is a potential therapeutic option, which according to population-based studies, has a 5-year survival rate of approximately 50% (7,8). Radiotherapy, including fractionated radiotherapy and stereotactic body radiotherapy (SBRT), is also available for these patients. Oncology practice guidelines recommend fractionated radiotherapy concurrent with chemotherapy or SBRT for early-stage SCLC patients who are medically inoperable or refuse surgery resection (9,10). To our knowledge, only limited treatment outcome data comparing surgery with radiotherapy have been available for elderly patients with early-stage SCLC, and thus the best local therapy for this population had not previously been defined.
In this retrospective study, we compared the use of surgery and radiotherapy in elderly patients with early-stage SCLC using data from the Surveillance, Epidemiology, and End Results (SEER) database. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-428/rc).
Methods
Data source and ethics statement
Data for the study population were retrieved from the records of the “Incidence-SEER Research Plus Data, 9 Registries, Nov 2020 Sub (2000–2018)” database. SEER*Stat software (version 8.3.9) was used to extract the cases from the database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). In this study, institutional review board approval was not required because the SEER data were anonymized and openly accessible.
Patient selection
The SEER database has adopted the American Joint Committee on Cancer (AJCC) staging system since 2004; thus, we enrolled patients with histologically confirmed SCLC between 2004 and 2018 in this study. The study cohort comprised patients with the third edition of the International Classification of Diseases for Oncology (ICD-O-3) site codes C34.0–C34.9 and ICD-O-3 histology codes 8041–8044. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have stage T1–2N0 disease in accordance with the American Joint Committee on Cancer (AJCC) 6th, 7th, or 8th edition; (II) be aged ≥70 years; and (III) have received surgery or radiotherapy as a local treatment modality. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had been diagnosed at autopsy or by death certificate; (II) had >1 malignant tumor; (III) had missing information regarding race, age at diagnosis, marital status, gender, histology, tumor location, stage, treatment (including surgery, radiotherapy, and chemotherapy), SEER cause of death, vital status, and survival month; (IV) had a survival time of <1 month; and/or (V) had been treated with neo-adjuvant radiotherapy before surgery.
Statistical analysis
According to the local treatment approach, the enrolled patients were divided into the surgery group and radiotherapy group. The baseline characteristics between the surgery group and radiotherapy group were compared using the Student’s t-test and Pearson’s chi-square test. To account for selection bias and any potential confounding factors between the groups, we used an inverse probability of treatment weighting (IPTW) analysis to control for differences in the baseline characteristics of the patients (11). The standardized mean difference (SMD) was calculated to assess the balance of the baseline characteristics after IPTW. Unadjusted and IPTW-adjusted Kaplan-Meier analyses were performed, and the survival of the groups was compared using the log-rank test. Multivariate Cox proportional hazards models were used to evaluate the potential prognostic factors for overall survival (OS) in the non-IPTW and IPTW-adjusted cohorts. To examine the relationship between age and OS, a Cox proportional hazard regression with restricted cubic spline (RCS) was performed (12). To compare the cancer-specific survival between the groups, competing risk survival analyses using Fine and Gray’s method were performed (13). The statistical analyses were performed using R software (version 4.1.3). A two-sided P value of <0.05 was considered statistically significant.
Results
Patients’ characteristics and treatment pattern changes
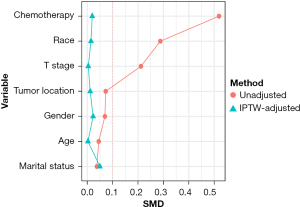
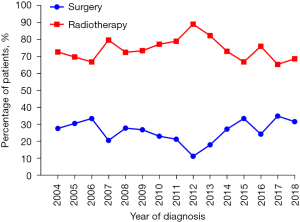
The study identified 685 patients aged ≥70 years with stage T1-2N0 SCLC in the SEER database between 2004 and 2018. Overall, 193 patients (26.6%) received surgery and 492 patients (73.4%) received radiotherapy. The patients’ baseline characteristics before and after IPTW are presented in Table 1. As Table 1 shows, non-white race (14.2% vs. 5.7%, P=0.003), stage T2 (47.2% vs. 36.8%, P=0.018), and the administration of chemotherapy (76.8% vs. 52.8%, P<0.001) were more prevalent in the radiotherapy group than the surgery group. The remaining listed clinical characteristics were comparable between the two groups. After the IPTW adjustment, the patients’ characteristics between the groups were well balanced with a SMD <0.1 (Table 1, Figure 1). The changes in the local-treatment patterns over time are displayed in Figure 2. The percentage of patients treated with radiotherapy as the local therapy increased from 68.9% in 2004 to 88.0% in 2012, and then decreased to 67.3% in 2018. A corresponding decrease in patients receiving surgery from 31.1% in 2004 to 12.0% in 2012 was observed, and in 2018, the percentage of patients receiving surgery increased back to a level similar to that in 2004.
Table 1
| Characteristics | Unadjusted cohort, n (%) | IPTW-adjusted cohort , % | |||||
|---|---|---|---|---|---|---|---|
| Surgery | Radiotherapy | P | Surgery | Radiotherapy | P | ||
| Age (years) | 0.598 | 0.976 | |||||
| Mean | 76.25 | 76.47 | 76.42 | 76.40 | |||
| SD | 4.89 | 5.02 | 4.75 | 5.03 | |||
| Gender | 0.465 | 0.806 | |||||
| Male | 82 (42.5) | 226 (45.9) | 43.8 | 44.9 | |||
| Female | 111 (57.5) | 266 (54.1) | 56.2 | 55.1 | |||
| Race | 0.003 | 0.906 | |||||
| White | 182 (94.3) | 422 (85.8) | 87.7 | 88.1 | |||
| Non-White | 11 (5.7) | 70 (14.2) | 12.3 | 11.9 | |||
| Marital status | 0.720 | 0.595 | |||||
| Unmarried | 96 (49.7) | 254 (51.6) | 54.4 | 51.9 | |||
| Married | 97 (50.3) | 238 (48.4) | 45.6 | 48.1 | |||
| Tumor location | 0.443 | 0.902 | |||||
| Upper/middle lobe | 124 (64.2) | 333 (67.7) | 67.5 | 67.0 | |||
| Lower lobe | 69 (35.8) | 159 (32.3) | 32.5 | 33.0 | |||
| T stage | 0.018 | 0.967 | |||||
| T1 | 122 (63.2) | 260 (52.8) | 55.7 | 55.9 | |||
| T2 | 71 (36.8) | 232 (47.2) | 44.3 | 44.1 | |||
| Chemotherapy | <0.001 | 0.823 | |||||
| Yes | 102 (52.8) | 378 (76.8) | 69.0 | 69.9 | |||
| No | 91 (47.2) | 114 (23.2) | 31.0 | 30.1 | |||
IPTW, inverse probability of treatment weighting; SD, standard deviation.
Survival outcomes
The median follow-up time was 90 months (interquartile range, 32–123 months). As Figure 3A shows, the median OS time was significantly higher in the surgery group than the radiotherapy group (32 vs. 20 months, P=0.002). The OS rates at 5 years were 30.6% with surgery and 17.6% with radiotherapy. In the multivariate Cox regression model of the unadjusted cohort, increased age (P=0.001), stage T2 (P=0.047), radiotherapy (P<0.001), and no chemotherapy (P=0.034) were associated with unfavorable OS (Table 2). After the IPTW adjustment, surgery was still associated with improved survival (Figure 3B); the median OS time of the surgery group was 32 months and that of the radiotherapy group was 20 months. The 5-year OS rate of the surgery group was 30.6% and that of the radiotherapy group was 17.6% (P<0.001). In the IPTW-adjusted cohort, the multivariate analysis showed that a decreased age (P<0.001), stage T1 (P=0.038), and surgery (P<0.001) were associated with superior OS (Table 2).
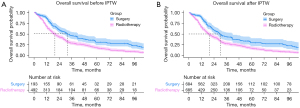
Table 2
| Variables | Multivariate analysis of the unadjusted cohort | Multivariate analysis of the IPTW-adjusted cohort | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | 1.03 (1.01–1.05) | 0.001 | 1.04 (1.02–1.06) | <0.001 | |
| Gender | |||||
| Male | 1 (Reference) | 1 (Reference) | |||
| Female | 0.87 (0.72–1.06) | 0.169 | 0.84 (0.66–1.06) | 0.135 | |
| Race | |||||
| White | 1 (Reference) | 1 (Reference) | |||
| Non-White | 0.83 (0.63–1.09) | 0.187 | 0.68 (0.41–1.13) | 0.139 | |
| Marital status | |||||
| Unmarried | 1 (Reference) | 1 (Reference) | |||
| Married | 1.04 (0.86–1.27) | 0.669 | 1.05 (0.83–1.31) | 0.704 | |
| Tumor location | |||||
| Upper/middle lobe | 1 (Reference) | 1 (Reference) | |||
| Lower lobe | 0.85 (0.70–1.02) | 0.088 | 0.90 (0.72–1.12) | 0.335 | |
| T stage | |||||
| T1 | 1 (Reference) | 1 (Reference) | |||
| T2 | 1.20 (1.00–1.44) | 0.047 | 1.25 (1.01–1.55) | 0.038 | |
| Local treatment | |||||
| Surgery | 1 (Reference) | 1 (Reference) | |||
| Radiotherapy | 1.75 (1.40–2.18) | <0.001 | 1.68 (1.35–2.10) | <0.001 | |
| Chemotherapy | |||||
| Yes | 1 (Reference) | 1 (Reference) | |||
| No | 1.27 (1.02–1.59) | 0.034 | 1.24 (0.96–1.60) | 0.100 | |
IPTW, inverse probability of treatment weighting; HR, hazard ratio; CI, confidence interval.
Relationship between age and OS
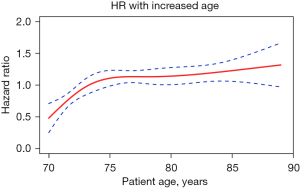
The RCS plot (Figure 4) shows the relationship between age as a continuous variable and OS. As Figure 4 shows, as age increased, the mortality rate increased in a age-dependent pattern in the patients aged 70–75 years. However, after the age of 75, the risk of death plateaued, and then slightly increased after the age of 85 years.
Cause-specific mortality by competing risk analyses
At last contact, 183 patients (26.7%) had been censored, and 502 patients (73.3%) had died. Among these 502 patients, 140 (20.4%) had died from SCLC, 356 (52.0%) from other causes, and 6 (0.9%) for unknown reasons. The competing risk survival results are presented in Figure 5. In the whole cohort (Figure 5A), the patients in the surgery group had a lower 5-year cumulative incidence estimate of cause-specific mortality than those in the radiotherapy group (53.6% vs. 61.0%, P=0.01). There was no significant difference in the 5-year cumulative incidence rates of non-cancer deaths between the surgery and radiotherapy groups (15.9% vs. 21.5%, P=0.15).
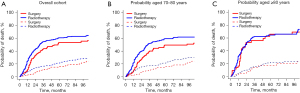
As patients aged ≥80 years are typically considered very elderly patients, we selected 80 years as the cut-off point and divided the patients into the 70–80-year-old age group and ≥80-year-old age group. For the patients aged 70–80 years (Figure 5B), the 5-year cumulative incidence estimate of cause-specific mortality of the surgery group (49.5%) was significantly lower than that of the radiotherapy group (59.5%) (P=0.008). For patients aged ≥80 years (Figure 5C), no significant difference was observed in the 5-year cumulative incidence rates of cancer-related death between the surgery and radiotherapy groups (66.3% vs. 64.9%, P=0.66).
Discussion
The optimal management of elderly patients with early-stage (T1–2N0M0) SCLC continues to be debated. In the era of modern staging and treatments, no randomized controlled trials have compared surgery to radiation to determine the best local treatment approach for such aging populations. Previous retrospective studies of large databases have shown the benefit of surgery in the management of T1–2N0 SCLC and reported a 5-year rate survival ranging from 50–60% (8,14-17). Current oncology practice guidelines (9,10) recommend surgical resection for tumor-node-metastasis (TNM) stage T1–2N0 SCLC. The evidence available on fractionated radiotherapy in early-stage SCLC is limited. A secondary analysis of the CONVERT trial (18) revealed that concurrent chemoradiotherapy (CRT) provided the stage I–II SCLC patients (who represented 16.9% of the enrolled patients) with a long-term OS rate (a 5-year OS rate of 49%) comparable to that of those who received surgery with acceptable toxicities, but individuals aged ≥70 years represented only 15% of all the enrolled patients. A subsequent age-subgroup analysis of the CONVERT trial suggested comparable survival and toxicity between older patients (aged ≥70 years) and younger patients receiving concurrent CRT with modern radiotherapy techniques (19).
A few retrospective studies have demonstrated that SBRT might be a promising approach for early-stage SCLC patients (20,21). The multi-institutional cohort study by Verma et al. (20) reported a 3-year OS rate of 34.0% with low rates of toxicity in inoperable stage I (T1–2N0) SCLC patients treated with SBRT. As a result, oncology guidelines now include radiotherapy as an option for the treatment of T1–2N0 SCLC patients who are inoperable or refuse to receive surgery. However, the oncology guidelines cannot be directly extended to elderly patients aged ≥70 years due to the lack of randomized and retrospective studies in elderly patients, and concerns related to their treatment tolerance and limited life expectancy.
To our knowledge, this was the first retrospective study to compare local-treatment patterns among patients aged ≥70 years diagnosed with stage T1–2N0 SCLC in the era of modern staging and treatments. The use of surgery fluctuated among elderly patients with T1–2N0 SCLC from 2004 to 2018 and was associated with superior OS compared to radiotherapy. The benefit of surgery in terms of OS remained significant in multivariate models and an IPTW-adjusted cohort after controlling for potential known confounders. The competing risk analyses showed that surgery had a consistent cancer-specific survival advantage over radiotherapy. However, the survival benefit of surgery compared to radiotherapy disappeared in patients aged ≥80 years with T1–2N0 SCLC in the subgroup analyses. These results suggested that local-treatment protocols for octogenarians with T1–2N0 SCLC should be further investigated, as there may be a subset of patients who will not benefit from surgery.
Despite evidence supporting a favorable prognosis in the surgery group of all-age early-stage (T1–2N0) SCLC patients, analyses of the National Cancer Database (NCDB) in 2004–2013 revealed an underutilization of surgical resection; only 22.7% patients received surgery despite having no identified contraindications to surgery (22). Previous studies have shown that age is inversely associated with the resection rate for early-stage SCLC (15,22,23). Jazieh et al. found that elderly patients were less likely to undergo surgery due to comorbidities and a lower performance status (23). Our study revealed that among the relatively fit elderly early-stage SCLC patients who received local treatment, only 26.6% underwent surgical resection, which was approximately one third of the number of patients who received radiotherapy. In terms of the trends of using surgery and radiation over time, no significant change was observed in the patients diagnosed in 2004 and those diagnosed in 2018. However, a fluctuation was observed in the rate of radiotherapy with an increase from 68.9% in 2004 to a peak of 88.0% in 2012, followed by a decline to 67.3% in 2018. The increased use of radiotherapy in 2004–2012 was inconsistent with the trend toward radiotherapy technique advances and the wider acceptance of SBRT for early-stage SCLC. A population-based study of NCDB found that the use of SBRT increased from 2004 (0.4% of all stage I SCLC patients diagnosed that year) to 2013 (6.4%) (24). The reason for the decline in the use of radiotherapy in 2013–2018 was unclear. Institutional practice changes affected by the guidelines recommending surgery for operable early-stage SCLC may have contributed to the increased use of surgery in 2013–2018. It has been acknowledged by physicians that age alone should not be a contraindication for curative surgical resection.
As expected, the survival of lung cancer patients decreases as age increases (25). In our study, the 5-year OS rates were 30.6% with surgery and 17.6% with radiotherapy, which were far lower than the 5-year survival rate (of approximately 50%) reported in previous research on surgery or radiotherapy for early-stage SCLC (7,8,18). Our study also found that increases in age were related to increases in the mortality rate in a age-dependent pattern until the age of 75, and after the age of 75, the mortality rate plateaued, only to slightly increase in patients aged ≥85 years. The increase in mortality with age in the elderly might be related to several factors, including an increased risk of comorbidity-related mortality, death due to treatment toxicities, or death from cancer progression due to reduced treatment intensity. It is reasonable to speculate that there is an inverse relationship between the proportion of patients receiving curative local treatment and age. Schild et al. (26) reported that only 26% of octogenarians (aged ≥80 years) with SCLC received local therapy. Only elderly patients who underwent local therapy were enrolled in our study. The small sample size of patients aged ≥75 years in our study may explain the inconsistent relationship between mortality and age for patients aged ≥75 years old compared to those aged 70–75 years.
As SCLC is an exceptionally chemo-responsive disease with a strong predilection for early metastasis, the current NCCN guidelines recommend either adjuvant chemotherapy after resection/SBRT or concurrent chemotherapy with radiotherapy for early-stage SCLC (9). Our study found that 70.1% of the elderly patients with early-stage SCLC received chemotherapy. The multivariable analysis revealed that the administration of chemotherapy was correlated with superior survival. In terms of chemotherapy for elderly patients with limited-stage SCLC, a population-based study in the Netherlands found that patients who received chemotherapy had significantly superior survival, which is consistent with our results. In terms of chemotherapy compliance, Janssen-Heijnen et al. reported that as many as 70% of elderly patients experienced toxicity, which led to the early termination of chemotherapy in over 50% of the patients (27). A CONVERT and Intergroup 0096 subgroup analysis showed similar survival between age groups for limited-stage SCLC who received concurrent CRT, but more treatment-related mortality and worse toxicity were observed in the elderly patients than their younger counterparts (19,28). These studies highlight that age alone should not preclude considerations of chemotherapy. A validated functional assessment tool, such as the comprehensive geriatric assessment (CGA), can be used to identify fit elderly patients who are able to receive chemotherapy and should be introduced into clinical practice (29,30). Our findings suggest that elderly patients should be considered for chemotherapy, and chemotherapy regimens should deviate according to each individualized patient’s performance or medical comorbidities.
Our results are particularly relevant because there is a lack of robust evidence to guide local-treatment patterns in elderly early-stage SCLC patients. However, this study had several limitations. First, this retrospective study is subject to unmeasured confounders. We attempted to minimize this bias through the IPTW adjustment, but this cannot fully account for unmeasured bias. Second, detailed information about radiotherapy, including the radiotherapy technique and fractionation, was not available in the SEER database. As a result, we could not compare the effect of fractionated radiotherapy with SBRT. Third, we did not have data on measures of comorbidity and functional status. Disparities in performance status may have partially contributed to the inferior prognosis in the radiotherapy group. Further, data on the treatment-related toxicities and quality of life were not available in the SEER database, which are essential factors that should be taken into account when making treatment decisions.
Conclusions
In conclusion, our findings suggest that elderly patients with T1–2N0M0 SCLC who are candidates for surgery should be strongly considered for surgery with adjuvant chemotherapy, which appeared to confer a prolonged OS compared to radiation as the local treatment before and after the IPTW adjustment. In addition, surgery was superior to radiotherapy in terms of cancer-specific survival in the patients aged 70–80 years. Beyond a patient’s chronologic age, their individual risks and preferences, should be considered when making treatment decisions for elderly patients with T1–2N0M0 SCLC. Prospective studies need to be conducted to confirm our findings.
Acknowledgments
Funding: This study was funded by the Beijing Municipal Science and Technology Commission (No. Z191100006619116), the Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2020A05), and the CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-022).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-428/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-428/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-428/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Abdel-Rahman O. Changing epidemiology of elderly small cell lung cancer patients over the last 40 years; a SEER database analysis. Clin Respir J 2018;12:1093-9. [Crossref] [PubMed]
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. [Crossref] [PubMed]
- Varlotto JM, Recht A, Flickinger JC, et al. Lobectomy leads to optimal survival in early-stage small cell lung cancer: a retrospective analysis. J Thorac Cardiovasc Surg 2011;142:538-46. [Crossref] [PubMed]
- Schreiber D, Rineer J, Weedon J, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer 2010;116:1350-7. [Crossref] [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [Crossref] [PubMed]
- NCCN. NCCN Guidelines: Small Cell Lung Cancer, Version 1. 2022. Available online: https://www.nccn.org/
- Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi99-105. [Crossref] [PubMed]
- Narduzzi S, Golini MN, Porta D, et al. Inverse probability weighting (IPW) for evaluating and "correcting" selection bias. Epidemiol Prev 2014;38:335-41. [PubMed]
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037-57. [Crossref] [PubMed]
- de Glas NA, Kiderlen M, Vandenbroucke JP, et al. Performing Survival Analyses in the Presence of Competing Risks: A Clinical Example in Older Breast Cancer Patients. J Natl Cancer Inst 2016;108:djv366. [Crossref] [PubMed]
- Cheng X, Zeng W, Liu Y, et al. Impact of Lymph Node Dissection on Survival and Tumor Recurrence for Patients with Resected cT1-2N0 Small Cell Lung Cancer. Ann Surg Oncol 2022;29:7512-25. [Crossref] [PubMed]
- Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol 2015;10:316-23. [Crossref] [PubMed]
- Casiraghi M, Sedda G, Del Signore E, et al. Surgery for small cell lung cancer: When and how. Lung Cancer 2021;152:71-7. [Crossref] [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Salem A, Mistry H, Hatton M, et al. Association of Chemoradiotherapy With Outcomes Among Patients With Stage I to II vs Stage III Small Cell Lung Cancer: Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 2019;5:e185335. [Crossref] [PubMed]
- Christodoulou M, Blackhall F, Mistry H, et al. Compliance and Outcome of Elderly Patients Treated in the Concurrent Once-Daily Versus Twice-Daily Radiotherapy (CONVERT) Trial. J Thorac Oncol 2019;14:63-71. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Allen PK, et al. Multi-Institutional Experience of Stereotactic Ablative Radiation Therapy for Stage I Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;97:362-71. [Crossref] [PubMed]
- Safavi AH, Mak DY, Boldt RG, et al. Stereotactic ablative radiotherapy in T1-2N0M0 small cell lung cancer: A systematic review and meta-analysis. Lung Cancer 2021;160:179-86. [Crossref] [PubMed]
- Wakeam E, Varghese TK Jr, Leighl NB, et al. Trends, practice patterns and underuse of surgery in the treatment of early stage small cell lung cancer. Lung Cancer 2017;109:117-23. [Crossref] [PubMed]
- Jazieh AR, Kyasa MJ, Sethuraman G, et al. Disparities in surgical resection of early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2002;123:1173-6. [Crossref] [PubMed]
- Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer 2017;103:11-6. [Crossref] [PubMed]
- Tas F, Ciftci R, Kilic L, et al. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett 2013;6:1507-13. [Crossref] [PubMed]
- Schild SE, Zhao L, Wampfler JA, et al. Small-cell Lung Cancer in Very Elderly (≥80 Years) Patients. Clin Lung Cancer 2019;20:313-21. [Crossref] [PubMed]
- Janssen-Heijnen ML, Maas HA, Koning CC, et al. Tolerance and benefits of treatment for elderly patients with limited small-cell lung cancer. J Geriatr Oncol 2014;5:71-7. [Crossref] [PubMed]
- Yuen AR, Zou G, Turrisi AT, et al. Similar outcome of elderly patients in intergroup trial 0096: Cisplatin, etoposide, and thoracic radiotherapy administered once or twice daily in limited stage small cell lung carcinoma. Cancer 2000;89:1953-60. [Crossref] [PubMed]
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824-31. [Crossref] [PubMed]
- Rier HN, Meinardi MC, van Rosmalen J, et al. Association Between Geriatric Assessment and Post-Chemotherapy Functional Status in Older Patients with Cancer. Oncologist 2022;27:e878-88. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


