
Differentiating between tuberculous and non-tuberculous pleural effusions using the pleural fluid ratio of 10× adenosine deaminase/lactate dehydrogenase
Highlight box
Key findings
• The dependent diagnostic index of 10× adenosine deaminase (ADA)/lactate dehydrogenase (LDH) can be used to distinguish tuberculous pleural effusion (TBPE) from non-TBPE.
What is known and what is new?
• We found that 10× ADA/LDH has excellent diagnostic value in differentiating TBPE from non-TBPE when the pleural ADA level is >20 U/L.
• ADA is a sensitive marker of TBPE, but its diagnostic precision is probably restricted due to the false positive and negative results. Thus, we examined the clinical value of the 10× ADA/LDH index in differentiating between TBPE and non-TBPE.
What is the implication, and what should change now?
• We found that 10× ADA/LDH had excellent diagnostic value in differentiating TBPE from non-TBPE when the pleural ADA level was >20 U/L. It is time to adjust the level of pleural ADA and 10 × Ratio of ADA/LDH.
Introduction
Tuberculosis (TB) is the leading cause of death from infections worldwide (1). About 16% of TB exists in extrapulmonary forms, of which the most frequent in adults is pleural tuberculosis (PT) (2). PT is difficult to diagnose due to the bacillus deficiency in the pleural fluid, and histological, microbiological, or molecular tests, which require the collection of pleural tissues using invasive measures, are occasionally needed to make the diagnosis (3).
Adenosine deaminase (ADA) is an effective indicator of tuberculous pleural effusion (TBPE) (4,5). The routine cut-off value for pleural fluid ADA is 40 U/L (6). The higher the level of ADA, the greater the likelihood that an individual has TB. A persistent low pleural fluid ADA level during repeated thoracentesis severely denies TB (3). However, many patients with tuberculous pleurisy have an ADA <40 U/L. In addition, the ADA level may increase to >40 U/L in several clinical situations, including in patients with parapneumonic effusion (PPE) and empyema (7). ADA levels may also be increased by lymphomas, solid tumors, and connective tissue diseases (7-11).
ADA, which is involved in purine metabolism, can catalytically convert adenosine to inosine and deoxyadenosine to deoxy inosine, producing ammonia (12). This enzyme exists in diverse cells and is especially critical in lymphoid cell differentiation in activated T cells (13). ADA has 2 isoenzymes (i.e., ADA1 and ADA2) (12). ADA1 is widespread, especially in lymphocytes and monocytes, while ADA2 mainly exists in monocytes and macrophages (14). ADA2 represents 88% of the total ADA activity in TBPE (15).
TB is a macrophage-mediated granulomatous inflammation. The proportion of macrophages and T cells in pleural fluid is significantly increased in TBPE, which results in a remarkable increase in the generation of ADA (16). The production of ADA can also increase with the accumulation of the total cell number in pleural fluids (17). In pleural effusion (PE), the detection of ADA alone cannot be used to determine whether the increase in ADA level is caused by the increased proportion of macrophages and lymphocytes in the cell components or by the increase in the total cell count. This is why a similar or even higher level of ADA can be found in other types of PEs.
ADA is only enriched in some types of cells, such as macrophages and T cells (17). Lactate dehydrogenase (LDH) usually increases in response to cell damage or cell death (18,19). Thus, we used 10× ADA/LDH to represent the degree to which mononuclear cells induce cell damage or cell death, which better indicates the level of granulomatous inflammation in pleural fluids. Specifically, this study sought to assess pleural fluid 10× ADA/LDH as a new index for distinguishing TBPE from other types of PE. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-383/rc).
Methods
Patients and data collection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of The First Hospital Affiliated to the Army Medical University [No. (B)KY2022119]. Written informed consent was not required for this retrospective study. We retrospectively recruited patients who had been hospitalized at the Department of Respiratory Medicine, The First Hospital Affiliated to the Army Medical University, between January 2018 and December 2021. All the included patients suffered from PE. The following data were collected: age, gender, smoking history, pleural LDH, ADA, etc.
Inclusion and exclusion criteria
To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have a diagnosis of PE by ultrasonography, chest computed tomography, or X-ray; (II) have a diagnosis of malignant pleural effusion (MPE) by cytology or pleural biopsy; (III) have a diagnosis of TBPE based on a finding of chronic granulomatous inflammation in pleural tissues; (IV) have a diagnosis of PPE based on exudative effusions related to bacterial pneumonia, lung abscesses or bronchiectasis, and have been in remission and recovery for at least 3 months at the follow-up after antibiotic use; or (V) have a diagnosis of another type of PE of a known etiology (e.g., a parasite or rheumatoid arthritis) based on well-accepted criteria and have received the best treatment. Empyema was further diagnosed in cases of pleural frank pus. Patients were excluded from the study if they met any of the following exclusion criteria: (I) ere aged <18 years; (II) were pregnant; (III) had deficient data for the analysis; and/or (IV) had PE of unknown etiology. Written informed consent was not required for this retrospective study.
Statistical analysis
All the data were analyzed on SPSS 18.0 (SPSS Inc., Chicago, USA). The continuous data are reported as the mean ± standard deviation (SD) and were compared among 3 or more groups using a 1-way analysis of variance. The non-normally distributed variables were analyzed by non-parametric tests and were reported as the mean (upper and lower quartiles). The categorical data were analyzed using the Chi-square test. The diagnostic abilities of the indicators were assessed by computing the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), positive predictive value (PPV), negative predictive value (NPV), and Youden index. The diagnostic value of the continuous data for TBPE and the optimal cut-off point were assessed using receiver operating characteristic (ROC) curves. The significance level was set at P<0.05.
Results
Characteristics of patients
Of the 382 included patients with PE, 144 were diagnosed with TBPE, this supposes a “pre-test probability” >40%. It is quite high, 134 with MPE, 19 with PPE, 43 with empyema, 24 with transudate PE, and 18 with other types of PE of a known etiology, such as a parasite or rheumatoid arthritis. The information of the patients is listed in Table 1. The age of the TBPE group was younger than that of the MPE, PPE, and transudate groups. The proportion of females in the TBPE group was lower than that in the MPE group and higher than that in the empyema group. The proportion of smokers in the TBPE group was lower than that in the empyema group and higher than that in other types of PE.
Table 1
| Characteristic | Total | TBPE | MPE | PPE | Empyema | Transudate | Others | P |
|---|---|---|---|---|---|---|---|---|
| Number | 382 | 144 | 134 | 19 | 43 | 24 | 18 | |
| Age, years | 60.0 (50.0, 70.0) |
58.0 (42.0, 68.0) |
60.5 (53.75, 70.25) |
66.0 (52.0, 76.0) |
63.0 (54.0, 70.0) |
64.5 (54.0, 76.0) |
50.0 (43.8, 75.5) |
0.016 |
| Gender | 0.08 | |||||||
| Male | 279 | 108 | 85 | 17 | 37 | 20 | 12 | |
| Female | 103 | 36 | 49 | 2 | 6 | 4 | 6 | |
| Smoking history | <0.01 | |||||||
| Yes | 201 | 70 | 73 | 11 | 33 | 11 | 3 | |
| No | 181 | 74 | 61 | 8 | 10 | 13 | 15 | |
| Pleural fluid analysis ADA (U/L) | 13.7 (7.6, 44.5) |
34.3 (13.8, 57.0) |
8.65 (5.7, 11.3) |
11.4 (7.6, 34.7) |
124.75 (44.5, 314.0) |
5.35 (3.7, 8.5) |
16.0 (8.2, 48.7) |
<0.01 |
| ADA <20 U/L | 217 | 44 | 121 | 13 | 4 | 24 | 11 | |
| 20≤ ADA <30 U/L | 33 | 20 | 6 | 1 | 6 | 0 | 0 | |
| 30≤ ADA <40 U/L | 22 | 14 | 5 | 2 | 0 | 0 | 1 | |
| ADA ≥40 U/L | 110 | 66 | 2 | 3 | 33 | 0 | 6 | |
| LDH (U/L) | 344.9 (182.8, 873.3) |
290.0 (185.8, 573.3) |
346.05 (217.9, 679.0) |
382.7 (126.2, 1,798.8) |
8,514.1 (4,555.4, 13,820.0) |
83.2 (71.6, 113.0) |
260.1 (151.7, 2,957.0) |
<0.01 |
| Lymphocyte/neutrophil >2.53 | 119 | 102 | 89 | 13 | 23 | 14 | 6 | <0.01 |
Values are medians (upper quartile, lower quartile) or number. TBPE, tuberculous pleural effusion; MPE, malignant pleural effusion; PPE, parapneumonic effusion; ADA, adenosine deaminase; LDH, lactate dehydrogenase.
Comparison among groups
The approved cut-off value for pleural fluid ADA was 40 U/L. However, our results showed that only 45.8% (66/144) of the TBPE patients had an ADA level >40 U/L (Table 1). This data does not match at all with all the previous meta-analysis performed, accounting for many thousands of patients diagnosed with tuberculous pleural effusion: ADA showed in all of them a sensitivity and specificity of about 92% and 90% respectively for the diagnosis of pleural TB (20). The discrepancy in sensitivity and specificity values observed in the study sample compared to previous meta-analyses could be attributed to various factors (21). The study sample may be smaller than the ones used in previous meta-analyses, which could have an impact on the sensitivity and specificity values (22). A larger sample size generally provides more accurate and reliable results. The demographics of the study population may differ from those included in previous meta-analyses (23). Differences in age, ethnicity, geographic location, or prevalence of co-morbidities might affect the diagnostic accuracy of ADA for TBPE. Differences in laboratory techniques and equipment used to measure ADA levels might contribute to the observed discrepancies. The study design or inclusion and statistical analysis might have affected the sensitivity and specificity values. In addition, the ADA levels were also increased in the non-TBPE patients. The empyema group had a higher ADA level than the TBPE group. The TBPE group had a higher ADA level than the transudate and MPE groups, but no significant difference was found among the TBPE, PPE and other types (Figure 1).
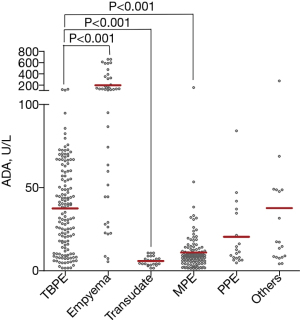
Pleural fluid LDH levels increased significantly as the total cell number in the pleural fluids increased. The highest and lowest LDH levels were found in the empyema and transudate groups, respectively. The pleural fluid LDH level in the TBPE group was lower than that in the empyema group and higher than that in the transudate group. No significant difference in the pleural fluid LDH level was found among the TBPE, MPE, other type, and PPE groups (Table 1).
Diagnostic significance of 10× ADA/LDH for TBPE
ADA was only enriched in certain types of cells, such as macrophages and T cells (17). However, only an increased ADA level caused by an increased proportion of macrophages and T cells indicates granulomatous inflammation. Thus, an increase in ADA level caused by an increase in the total cell number needed to be excluded. Surprisingly, no correlation was found between the ADA level and cell number in the TBPE patients (Figure 2A). We speculated that this may be due to the large number of inactive cells in TBPE. However, the ADA level was correlated with the LDH level in the TBPE patients (Figure 2B). LDH usually increases in response to cell damage or cell death (18). Thus, we used 10× ADA/LDH to represent the degree to which the mononuclear cells induce cell damage or cell death, which better indicates the level of granulomatous inflammation in pleural fluids.
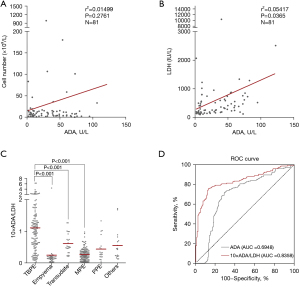
The 10× ADA/LDH level was significantly increased in the TBPE group compared to the empyema, MPE, and transudate groups (Figure 2C). We further estimated the diagnostic performance of 10× ADA/LDH for TBPE using the ROC curves and the areas under the curve (AUCs) (Figure 2D). The AUC of 10× ADA/LDH (0.8358, 95% CI: 0.7901–0.8814) was larger than that of ADA alone (0.6948, 95% CI: 0.6397–0.7499).
Cut-off level for 10× ADA/LDH
The 10× ADA/LDH increased as the ADA level increased in TBPE (Figure 3A), gradually stabilized at an ADA level >20 U/L, and began to decline at an ADA level >80 U/L. However, in non-tuberculous pleural fluids, 10× ADA/LDH did not change with the increase in the ADA level (Figure 3B). We further estimated the diagnostic performance of 10× ADA/LDH for TBPE using ROC curves and AUCs in different ADA levels (Figure 4). When the ADA level >20 U/L, 10× ADA/LDH had a larger AUC (0.9545, 95% CI: 0.9130–0.9959) compared to the ADA level >10 U/L (0.8972, 95% CI: 0.8511–0.9432), and all ADA levels (0.8358, 95% CI: 0.7901–0.8814).
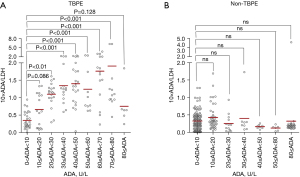
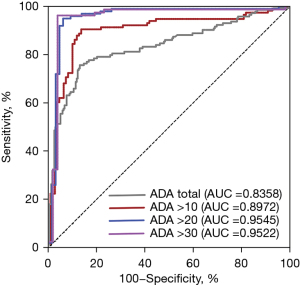
ROC curves were used to assess the optimal cut-off value of 10× ADA/LDH at different ADA levels for differentiating between TBPE and non-TBPE. The statistical results for the sensitivity, specificity, PLR, NLR, PPV, NPV, and Youden index are listed in Table 2. We found that 10× ADA/LDH had a better diagnostic performance at an ADA level >20 U/L than an ADA level >10 U/L and the total ADA level. At an ADA level >20 U/L and at cut-off level of 10× ADA/LDH >0.44, the sensitivity and specificity were 0.95 (95% CI: 0.88–0.98) and 0.94 (95% CI: 0.84–0.98), respectively. The PPV was 0.96, while the NPV at this cut-off was 0.92. The PLR was 15.44, while the NLR at this cut-off was 0.05.
Table 2
| Parameter | ADA total | 10× ADA/LDH | |||
|---|---|---|---|---|---|
| Total | ADA >10 U/L | ADA >20 U/L | ADA >30 U/L | ||
| Cut-off value | >40 U/L | >0.56 | >0.45 | >0.44 | >0.47 |
| Sensitivity (95% CI) | 0.47 (0.38–0.56) | 0.76 (0.68–0.82) | 0.91 (0.83–0.94) | 0.95 (0.88–0.98) | 0.95 (0.87–0.98) |
| Specificity (95% CI) | 0.82 (0.77–0.87) | 0.87 (0.82–0.91) | 0.86 (0.78–0.92) | 0.94 (0.84–0.98) | 0.96 (0.86–0.99) |
| PPV (95% CI) | 0.61 (0.51–0.70) | 0.78 (0.70–0.84) | 0.86 (0.80–0.93) | 0.96 (0.89–0.99) | 0.97 (0.90–1.00) |
| NPV (95% CI) | 0.73 (0.67–0.78) | 0.86 (0.80–0.90) | 0.90 (0.82–0.94) | 0.92 (0.82–0.97) | 0.93 (0.81–0.98) |
| PLR (95% CI) | 2.65 (1.90–3.71) | 5.81 (4.13–8.17) | 6.64 (4.13–10.66) | 15.44 (5.97–39.93) | 24.7 (6.34–96.23) |
| NLR (95% CI) | 0.64 (0.55–0.76) | 0.28 (0.21–0.37) | 0.11 (0.06–0.19) | 0.05 (0.02–0.13) | 0.05 (0.02–0.14) |
| Youden index | 0.29 | 0.63 | 0.77 | 0.89 | 0.91 |
ADA, adenosine deaminase; LDH, lactate dehydrogenase; TBPE, tuberculous pleural effusion; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Discussion
The molecular diagnosis of TB has advanced remarkably in the last 20 years; however, the first choice for diagnosis is still mycobacterial cultures of PE or tissues (24). Granuloma in pleural biopsy was found in 56% to 78% of TBPE cases (3). When a tissue culture is linked to a histological test, the positivity rises from 39% to 90% (25). Pleural biopsy is a favorable diagnostic approach with high efficacy, but it is invasive, complex and difficult to perform. We sought to strengthen the efficiency of TBPE identification.
TB is a macrophage-mediated granulomatous inflammation (26). ADA is enriched in some types of cells, such as macrophages and T cells (17). The production of ADA can increase with the accumulation of macrophages and T cells in pleural fluids.
The detection of ADA can indirectly indicate the degree of granulomatous inflammation in the pleural fluids, but its accuracy may be low due to false positive or negative results. In PEs, the detection of ADA alone cannot be used to determine whether the increase in the ADA level is caused by the increased proportion of macrophages and T lymphocytes in the cell components of PE or by the increase in the total cell number. Thus, it is impossible to determine whether the increase of ADA is mediated by the increase in granulomatous inflammation or the increase in inflammatory cells according to the severity of inflammation.
We found that the ADA level was correlated with the LDH level in TBPE. The LDH level usually indicates cell damage or cell death (18,27,28). Thus, we used 10× ADA/LDH to represent the degree to which mononuclear cells induce cell damage or cell death, which better indicates the level of granulomatous inflammation in pleural fluids. We found that 10× ADA/LDH was higher in the TBPE group than the other groups and increased more significantly than the ADA level. Notably, due to the significantly increased cell count in the empyema group, the ADA level was significantly lower in the TBPE group than the empyema group. However, 10× ADA/LDH in the TBPE group significantly surpassed that of the empyema group. These results suggest that 10× ADA/LDH indicates the severity of granulomatous inflammation in PEs.
In addition, the 10× ADA/LDH gradually increased with the increase in ADA level in TBPE, and decreased at an ADA level >80 U/L. These results suggest the severity of granulomatous inflammation in TBPE will gradually increase with the increase of ADA level. When the ADA level is >80 U/L, immune exhaustion may occur, resulting in a decrease in granulomatous inflammation. In addition, ADA >80 (and mostly >120) are more frequent related to empyemas and lymphomas, in which LDH levels could be proportionally higher. In this sense, the ratio ADA/LDH might gradually decrease in this scenario. However, 10× ADA/LDH in the non-TBPE group did not significantly increase, and seemingly gradually decreased, but the difference was not significant. These results provide further evidence that 10× ADA/LDH indicates the severity of granulomatous inflammation in PE and may have diagnostic significance in TBPE.
We also examined the diagnostic value of 10× ADA/LDH at different ADA levels of PE to differentiate TBPE from non-TBPE. At different ADA levels, 10× ADA/LDH had a larger AUC than ADA alone. These results suggest that 10× ADA/LDH has a better diagnostic value at differentiating TBPE from non-TBPE than that of the ADA level alone.
We also examined the cut-off value for 10× ADA/LDH using ROC curves. At an ADA level >20 U/L and at a cut-off level of 10× ADA/LDH >0.44, the sensitivity and specificity were 0.95 (95% CI: 0.88–0.98) and 0.94 (95% CI: 0.84–0.98), respectively, which were higher than those of ADA alone. Additionally, at an ADA level >20 U/L, the sensitivity and specificity were higher than the total ADA and an ADA level >10 U/L and were similar to those at an ADA level >30 U/L. Thus, 10× ADA/LDH had excellent diagnostic value in differentiating TBPE from non-TBPE when the pleural ADA level was >20 U/L.
Limitations
There are some shortcomings in this study. Firstly, this is a retrospective study and a portion of prospective follow-up should be conducted to more accurately confirm our conclusions. Secondly, this is a single center study that may result in differences in results due to regional differences, and multicenter studies should be conducted to confirm our results.
Conclusions
We found that 10× ADA/LDH had excellent diagnostic value in differentiating TBPE from non-TBPE when the pleural ADA level was >20 U/L. These findings may guide future clinical decisions.
Acknowledgments
Funding: This work was funded by the Army Medical University Foundation of China (XZ-2019-505-075).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-383/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-383/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-383/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-383/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of The First Hospital Affiliated to the Army Medical University [No. (B)KY2022119]. Written informed consent was not required for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Furin J, Cox H, Pai M. Tuberculosis. Lancet 2019;393:1642-56. [Crossref] [PubMed]
- Yan Z, Wen JX, Wang H, et al. Diagnostic accuracy of pleural fluid lactate dehydrogenase to adenosine deaminase ratio for tuberculous pleural effusion: an analysis of two cohorts. BMC Pulm Med 2022;22:428. [Crossref] [PubMed]
- Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med 2016;22:367-77. [Crossref] [PubMed]
- Zhang X, Meng Q, Miao R, et al. The diagnostic value of T cell spot test and adenosine deaminase in pleural effusion for tuberculous pleurisy: A systematic review and meta-analysis. Tuberculosis (Edinb) 2022;135:102223. [Crossref] [PubMed]
- Shimoda M, Hirata A, Tanaka Y, et al. Characteristics of pleural effusion with a high adenosine deaminase level: a case-control study. BMC Pulm Med 2022;22:359. [Crossref] [PubMed]
- Na F, Wang Y, Yang H, et al. Performance of adenosine deaminase in detecting paediatric pleural tuberculosis: a systematic review and meta-analysis. Ann Med 2022;54:3129-35. [Crossref] [PubMed]
- Wang J, Liu J, Xie X, et al. The pleural fluid lactate dehydrogenase/adenosine deaminase ratio differentiates between tuberculous and parapneumonic pleural effusions. BMC Pulm Med 2017;17:168. [Crossref] [PubMed]
- Verma A, Abisheganaden J, Light RW. Identifying Malignant Pleural Effusion by A Cancer Ratio (Serum LDH: Pleural Fluid ADA Ratio). Lung 2016;194:147-53. [Crossref] [PubMed]
- Verma A, Dagaonkar RS, Marshall D, et al. Differentiating Malignant from Tubercular Pleural Effusion by Cancer Ratio Plus (Cancer Ratio: Pleural Lymphocyte Count). Can Respir J 2016;2016:7348239. [Crossref] [PubMed]
- Kim CH, Oh HG, Lee SY, et al. Differential diagnosis between lymphoma-associated malignant pleural effusion and tuberculous pleural effusion. Ann Transl Med 2019;7:373. [Crossref] [PubMed]
- Lee J, Park JE, Choi SH, et al. Laboratory and radiological discrimination between tuberculous and malignant pleural effusions with high adenosine deaminase levels. Korean J Intern Med 2022;37:137-45. [Crossref] [PubMed]
- Zeng T, Ling B, Hu X, et al. The Value of Adenosine Deaminase 2 in the Detection of Tuberculous Pleural Effusion: A Meta-Analysis and Systematic Review. Can Respir J 2022;2022:7078652. [Crossref] [PubMed]
- Passos DF, Bernardes VM, da Silva JLG, et al. Adenosine signaling and adenosine deaminase regulation of immune responses: impact on the immunopathogenesis of HIV infection. Purinergic Signal 2018;14:309-20. [Crossref] [PubMed]
- Meyts I, Aksentijevich I. Deficiency of Adenosine Deaminase 2 (DADA2): Updates on the Phenotype, Genetics, Pathogenesis, and Treatment. J Clin Immunol 2018;38:569-78. [Crossref] [PubMed]
- Antonangelo L, Faria CS, Sales RK. Tuberculous pleural effusion: diagnosis & management. Expert Rev Respir Med 2019;13:747-59. [Crossref] [PubMed]
- Klimiuk J, Safianowska A, Chazan R, et al. Development and Evaluation of the New Predictive Models in Tuberculous Pleuritis. Adv Exp Med Biol 2015;873:53-63. [Crossref] [PubMed]
- Kim CH, Lee J. Mycoplasma pneumoniae Pleural Effusion in Adults. J Clin Med 2022;11:1281. [Crossref] [PubMed]
- Zarić B, Kuruc V, Milovančev A, et al. Differential diagnosis of tuberculous and malignant pleural effusions: what is the role of adenosine deaminase? Lung 2008;186:233-40. [Crossref] [PubMed]
- GuTBPEa GS. The Lactate and the Lactate Dehydrogenase in Inflammatory Diseases and Major Risk Factors in COVID-19 Patients. Inflammation 2022;45:2091-123. [Crossref] [PubMed]
- Palma RM, Bielsa S, Esquerda A, et al. Diagnostic Accuracy of Pleural Fluid Adenosine Deaminase for Diagnosing Tuberculosis. Meta-analysis of Spanish Studies. Arch Bronconeumol 2019;55:23-30. (Engl Ed). [Crossref] [PubMed]
- Greco S, Girardi E, Masciangelo R, et al. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis 2003;7:777-86. [PubMed]
- Liang QL, Shi HZ, Wang K, et al. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med 2008;102:744-54. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Sehgal IS, et al. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PLoS One 2019;14:e0213728. [Crossref] [PubMed]
- Freyer D, Harms C. Kinetic Lactate Dehydrogenase Assay for Detection of Cell Damage in Primary Neuronal Cell Cultures. Bio Protoc 2017;7:e2308. [Crossref] [PubMed]
- Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology 2019;24:962-71. [Crossref] [PubMed]
- Zhang M, Li D, Hu ZD, et al. The diagnostic utility of pleural markers for tuberculosis pleural effusion. Ann Transl Med 2020;8:607. [Crossref] [PubMed]
- Nguyen TKT, Niaz Z, d'Aigle J, et al. Lactoferrin reduces mycobacterial M1-type inflammation induced with trehalose 6,6'-dimycolate and facilitates the entry of fluoroquinolone into granulomas. Biochem Cell Biol 2021;99:73-80. [Crossref] [PubMed]
- Klein R, Nagy O, Tóthová C, et al. Clinical and Diagnostic Significance of Lactate Dehydrogenase and Its Isoenzymes in Animals. Vet Med Int 2020;2020:5346483. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


