The role of danger-associated molecular patterns (DAMPs) in trauma and infections
Severe trauma is a major cause of death among young people worldwide. Most early trauma related deaths are due to severe neurological damage or blood loss that occurs in first few hours after trauma. Those who survive the initial trauma are at an increased risk of developing nosocomial infections. The resultant severe sepsis is a major cause of late mortality. Anti-inflammatory processes activated as a result of severe trauma help to localize the inflammatory response. However, prolonged immunosuppression or defective immune responses may lead to increased susceptibility to nosocomial infections. Hotchkiss et al. have shown that in trauma patients, the adaptive immune system is suppressed and T lymphocytes are the most markedly affected cell population (1). The immune suppressed state in these patients is characterized by attenuated production of cytokines by leukocytes when stimulated by pathogen-associated molecular patterns (PAMPs) and decreased HLA-DR expression (2). Danger-associated molecular patterns (DAMPs) released from injured tissues have been shown to elicit immune responses similar to that of PAMPs and hence contribute to immune suppressed state.
PAMPs stimulate the innate immune system through their binding to pattern recognition receptors (PRRs) such as toll like receptors (TLRs). The innate immune system also recognizes endogenous molecules called DAMPs that are released by damaged or dying cells. This concept was first proposed by Polly Matzinger in his “Danger Theory”, which postulated that intracellular molecules (DAMPs) are released by injured tissues which activate immune system (3). High mobility group box 1 (HMGB1) was the first molecule to be identified as DAMP. Presently numerous nuclear, cytosolic, mitochondrial molecules have been identified as DAMPs (Table 1). Apart from their intracellular physiological role, DAMPs, when exposed to extracellular environment alert the body about the danger, stimulate an inflammatory response and promote tissue repair.
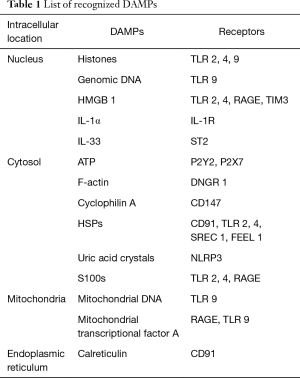
Full table
Danger-associated molecular patterns (DAMPs) in trauma
HMGB1, also called amphoterin, is a nuclear protein which interacts with the nucleosomes, transcription factors and histones. It supports transcription of various genes by interacting with transcription factors. It is secreted by macrophages and dendritic cells and acts as a mediator of inflammation. From the damaged tissues, it is released passively by diffusing out of the nucleus. It acts by binding to PRRs like TLR4 and receptor for advanced glycation end products (RAGE) (4). In a prospective cohort study of 168 severe trauma patients, Cohen et al. showed that plasma levels of HMGB1 increased within 30 min of severe trauma. The levels correlated with severity of trauma, tissue hypoperfusion, and development of coagulation abnormalities, systemic inflammatory response and hyperfibrinolysis. Plasma levels were also significantly higher in non survivors and higher levels also predicted the development of acute lung or kidney injury (5).
The S100 proteins are a family of proteins present in the cytoplasm of various cells. They consist of 24 members and are involved in regulation of protein phosphorylation, transcription factors, calcium homeostasis, enzyme activities, cell growth and differentiation. Several members of the family (S100A8, S100A9, S100A12, S100B) can be released extracellularly during tissue damage and exert proinflammatory effects by binding to TLRs and RAGE (6). Chernov et al. have shown that S100A8 and S100A9 levels were increased both in the proximal and distal nerve stumps after peripheral nerves were severed. They noted that these proteins also stimulated the release of chemokines and cytokines by activated Schwann cells leading to accumulation of immune cells in the injured nerves (7). Kabadi et al. studied the role of S100B protein in traumatic brain injury by using S100B knockout mice and S100B neutralizing antibodies. They demonstrated that both these interventions significantly reduced traumatic brain injury induced lesion volume, improved retention memory function and attenuated microglial activation indicating that S100B is important in early immune response following brain injury (8).
Heat shock proteins (HSPs) are a family of cytoplasmic proteins produced by cells during stressful conditions. They act as chaperons by stabilizing newly synthesized proteins by ensuring their correct folding and helping to refold proteins that were damaged due to cell stress. HSPs are also passively released extracellularly from necrotic and damaged cells. In a study of 67 patients with severe trauma, Pittet et al. found that HSP 72 levels were detected in serum within 30 min of injury. They have also shown that all patients with serum HSP72 levels more than 15 ng/mL survived, where as 29% of patients with low HSP72 levels died due to traumatic injuries. However, levels of HSP72 did not correlate with the incidence or severity of post injury inflammatory response or organ dysfunction (9).
Nuclear DNA (nDNA), RNA and mitochondrial DNA (mtDNA) are released into the circulation after damage of cells or necrosis of tissues. They can bind to TLRs and stimulate the production of pro-inflammatory cytokines (10). Nucleic acids have also been shown to increase the inflammatory potential of other DAMPs. In a study of 15 patients with major trauma, Zhang et al. showed that mitochondrial DAMPs were markedly elevated in the trauma patients compared to healthy volunteers (11). Simmon et al. have studied the relationship between mtDNA DAMP levels and occurrence of systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS) and mortality in patients with severe injury. Patients with SIRS and MODS had significantly higher levels of mtDNA compared to patients without SIRS or MODS. The relative risk of mortality was also significantly elevated in patients with above median mtDNA DAMP levels (12).
The interleukin 1 (IL-1) family of cytokines consists of 11 members and have both pro- and anti-inflammatory functions. IL-1 consists of two subtypes, IL-1α and IL-1β. The former is constitutively expressed in the epithelial cells, keratinocytes and fibroblasts and is released only after tissue injury. Helmy et al. have noted that the levels of IL-1α in brain extracellular fluid were 3.87 times higher than the plasma levels in patients with traumatic brain injury (13).
Histones are highly alkaline nuclear proteins that act as spools around which DNA winds and forms structural units called nucleosomes. Tissue damage leads to release of these histones to extracellular compartment and drive inflammatory response. In 132 critically injured patients, Kutcher et al. found that the histone levels were elevated on arrival to trauma ICU and started to decline by 6 hours. Patients with elevated histone levels at admission had higher injury severity score, higher incidence of multiorgan failure, acute lung injury and higher mortality. Increasing histone levels from admission to 6 hours after admission also predicted the mortality (hazards ratio =1.005) (14).
Trauma and infections
Sepsis is the leading cause of death in ICUs and its development is characterized by simultaneously occurring hyperinflammatory and immunoparalysis phases. Critical illness and severe injury are known to produce immunosuppressive state and can lead to development of nosocomial infection and sepsis in patients who are admitted into hospitals. Dysfunction of the innate immune system leading to defects in antigen presenting cells and subsequent impairment in cytokine production have been noted to contribute to immunosuppressive state in trauma patients. Decreased expression of HLA-DR, a class II major histocompatibility complex (MHC) molecule, on circulating monocytes has been shown to be a reliable indicator of immunosuppression in critically ill patients. Studies involving patients with severe trauma showed that diminished expression of monocyte HLA-DR post injury, predicted the development of sepsis during hospital stay (15,16). Cheron et al. studied 105 patients with severe trauma and found that 37 (35%) developed sepsis over 14 days after trauma. They noted that monocyte HLA-DR expression was diminished in all patients at post trauma day 1 and 2. At post trauma day 3–4, monocyte HLA-DR expression increased in patients who did not develop sepsis whereas the expression remained diminished in those who subsequently developed sepsis (15).
Impairment in the production of proinflammatory cytokine and tumor necrosis factor alpha (TNFα) was found to be a predictor of clinical outcome in multiple trauma patients. Ex vivo stimulation of immune cells, mainly macrophages, by lipopolysaccharides (LPS) leads to production of TNFα in immunocompetent individuals. Ploder et al. have demonstrated that, ex vivo stimulation of immune cells from adult trauma patients with sepsis, LPS induced TNFα secretion was significantly lower in non survivors than survivors (17). Similar results were noted in critically injured children by Muszynski et al. Here, out of 76 children with severe injury, 16 developed nosocomial infections. Ex vivo LPS induced TNFα production capacity was lower in children who developed nosocomial infections and a TNFα response of less than 520 pg/mL any time during first week after injury was associated with development of infection (18).
DAMPs released following tissue injury also contribute to the initiation and propagation of inflammation. Plasma HMGB1 levels have been shown to correlate with the degree of organ dysfunction in late phase sepsis and help in discriminating survivors from non survivors (19). Single nucleotide polymorphisms (SNPs) in the HSP70 gene were demonstrated to influence the outcomes in ICU patients with sepsis (20). Plasma levels of S100 proteins were increased in patients with severe sepsis and in healthy volunteers after administration of endotoxin, indicating that S100 proteins are involved in pathophysiology of sepsis (21). Circulating levels of nuclear DNA and mtDNA were increased at onset of septic shock and remained elevated during the period of sepsis (22,23). Their levels also correlated with the plasma levels of proinflammatory cytokines (TNFα, IL-6, IL-8, IL-1RA) and development of organ damage.
More recently, Timmermans et al. (24) have studied 166 adult trauma patients for DAMPs release, HLA-DR gene expression and ex vivo LPS stimulated cytokine production following trauma. Plasma levels of nDNA, HSP70, but not mtDNA, were significantly increased immediately after trauma and remained elevated for 10 days post trauma. HLA-DR expression was attenuated after trauma and ex vivo LPS stimulation revealed decreased TNFα and IL-6 production with increased IL-10 production. Thirty three (20%) of the patients developed infections within 28 days post trauma and among these patients, plasma mtDNA and nDNA, but not HSP70, levels were higher than those patients who did not develop infections.
Trauma is associated with an early inflammatory response followed by a predominantly anti-inflammatory response and a state of immune suppression. DAMPs, which are released immediately following major trauma, contribute significantly in the development of these inflammatory responses. Exaggerated immune suppression resulting from these mechanisms is associated with development of nosocomial infections and thus contributes to increased mortality among patients with severe trauma.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138-50. [Crossref] [PubMed]
- Muehlstedt SG, Lyte M, Rodriguez JL. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock 2002;17:443-50. [Crossref] [PubMed]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994;12:991-1045. [Crossref] [PubMed]
- Klune JR, Dhupar R, Cardinal J, et al. HMGB1: endogenous danger signaling. Mol Med 2008;14:476-84. [Crossref] [PubMed]
- Cohen MJ, Brohi K, Calfee CS, et al. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care 2009;13:R174. [Crossref] [PubMed]
- Foell D, Wittkowski H, Vogl T, et al. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 2007;81:28-37. [Crossref] [PubMed]
- Chernov AV, Dolkas J, Hoang K, et al. The calcium-binding proteins S100A8 and S100A9 initiate the early inflammatory program in injured peripheral nerves. J Biol Chem 2015;290:11771-84. [Crossref] [PubMed]
- Kabadi SV, Stoica BA, Zimmer DB, et al. S100B inhibition reduces behavioral and pathologic changes in experimental traumatic brain injury. J Cereb Blood Flow Metab 2015;35:2010-20. [Crossref] [PubMed]
- Pittet JF, Lee H, Morabito D, et al. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma 2002;52:611-7; discussion 617. [Crossref] [PubMed]
- Jounai N, Kobiyama K, Takeshita F, et al. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front Cell Infect Microbiol 2013;2:168. [Crossref] [PubMed]
- Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010;464:104-7. [Crossref] [PubMed]
- Simmons JD, Lee YL, Mulekar S, et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 2013;258:591-6; discussion 596-8. [PubMed]
- Helmy A, Carpenter KL, Menon DK, et al. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab 2011;31:658-70. [Crossref] [PubMed]
- Kutcher ME, Xu J, Vilardi RF, et al. Extracellular histone release in response to traumatic injury: implications for a compensatory role of activated protein C. J Trauma Acute Care Surg 2012;73:1389-94. [Crossref] [PubMed]
- Cheron A, Floccard B, Allaouchiche B, et al. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after major trauma. Crit Care 2010;14:R208. [Crossref] [PubMed]
- Gouel-Chéron A, Allaouchiche B, Guignant C, et al. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS One 2012;7:e33095. [Crossref] [PubMed]
- Ploder M, Pelinka L, Schmuckenschlager C, et al. Lipopolysaccharide-induced tumor necrosis factor alpha production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock 2006;25:129-34. [PubMed]
- Muszynski JA, Nofziger R, Greathouse K, et al. Innate immune function predicts the development of nosocomial infection in critically injured children. Shock 2014;42:313-21. [Crossref] [PubMed]
- Gibot S, Massin F, Cravoisy A, et al. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med 2007;33:1347-53. [Crossref] [PubMed]
- Ramakrishna K, Pugazhendhi S, Kabeerdoss J, et al. Association between heat shock protein 70 gene polymorphisms and clinical outcomes in intensive care unit patients with sepsis. Indian J Crit Care Med 2014;18:205-11. [Crossref] [PubMed]
- van Zoelen MA, Vogl T, Foell D, et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am J Respir Crit Care Med 2009;180:1098-106. [Crossref] [PubMed]
- Timmermans K, Kox M, Scheffer GJ, et al. Plasma nuclear and mitochondrial DNA levels, and markers of inflammation, shock, and organ damage in patients with septic shock. Shock 2016;45:607-12. [Crossref] [PubMed]
- Yamanouchi S, Kudo D, Yamada M, et al. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care 2013;28:1027-31. [Crossref] [PubMed]
- Timmermans K, Kox M, Vaneker M, et al. Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med 2016;42:551-61. [Crossref] [PubMed]


