
Collaborative multidisciplinary management and expertise of cT2–3 locally advanced operable esophageal squamous cell carcinoma: a report of two cases
Highlight box
Key findings
• There is need for further research and optimization to explore whether combining clinical T (cT)2 and cT3 in the esophageal squamous cell carcinoma (ESCC) staging system.
What is known and what is new?
• There are limitations of single imaging modalities for diagnosis in cT2 and cT3 ESCC.
• This manuscript provides valuable insights into the current challenges and potential solutions for accurate staging and treatment planning in ESCC.
What is the implication, and what should change now?
• Further research is needed to address the challenges in clinical staging and optimize diagnostic methods.
• The use of multidisciplinary teams should be encouraged to ensure comprehensive evaluation and treatment planning for ESCC patients.
Introduction
Background
Esophageal cancer, primarily squamous cell carcinoma, is prevalent in East Asia, with China and Japan representing the countries where the comprehensive treatment system for esophageal squamous cell carcinoma (ESCC) is evolving. According to data from the National Clinical Database in Japan, the 5-year survival rate after surgical resection for esophageal cancer has reached 59.9% (1). Parallel to updated treatment guidelines, neoadjuvant therapy combined with surgery has been implemented as a standard treatment for locally advanced operable ESCC based on studies such as JCOG1109, CROSS, and NEOCRTEC5010 (2-4). Although defining a plan for treatment of esophageal cancer requires the involvement of multidisciplinary experts, accurate diagnosis and staging have long been among the challenges perplexing clinical experts.
Rationale and knowledge gap
The 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system includes clinical tumor-node-metastasis (cTNM) staging, pathological TNM (pTNM) staging, and post-neoadjuvant therapy pTNM (ypTNM) staging. cTNM staging relies primarily on imaging data to guide treatment decision-making process. However, Rice et al. (5) have also pointed out that based on existing staging methods, cTNM serves as a proxy for treatment decision-making. The advantages and limitations of various diagnostic tests should be taken into consideration due to variations in the resolution of imaging techniques for clinical staging.
According to the 8th edition of the AJCC/UICC staging system, cT2N0–1 and cT3N0 are both defined as stage II, while cT2N2 and cT3N1 are categorized as stage III. In the 12th edition of the Japanese Esophageal Society (JES) staging system, cT2N0 and cT3rN0 both belong to stage II, while cT2N1–3M0–1a and cT3rN1–3M0–1a both belong to stage IIIA. However, most studies regarding cT staging focus on distinguishing between T1–2 and T3–4 (6,7). The differentiation between cT2 and cT3 staging, whether in cTNM or post-neoadjuvant therapy cTNM (ycTNM), warrants careful consideration.
The current methods for clinical staging include endoscopic ultrasonography (EUS), computerized tomography (CT), and positron emission tomography (PET) scanning. EUS is considered to be the most accurate modality for T staging of esophageal cancer. A study showed that endoscopic characteristics, such as cancer length and luminal stenosis degree, exhibit a correlation with T stage on EUS and could play a role in differentiating T1–2 from T3 tumors. Esophageal cancers of ≥5 cm in length or causing luminal stenosis preventing endoscope passage are more likely to be categorized as T3 or higher-stage lesions. In contrast, tumors measuring <5 cm in length have a notably higher likelihood (92%) of being staged as T1 or T2 (8). However, EUS shows great heterogeneity in the diagnosis of T staging, which may be related to the tumor location, length, operator experience, interpretation of images, and other reasons (9-12). According to a multicenter study from Germany, EUS had sensitivity, specificity, and accuracy rates for diagnosing T2 and T3 as follows (%): T2: 39/84/75; T3: 72/81/79 (13). Another study also indicated that EUS is an unreliable tool for staging esophageal cancer after neoadjuvant therapy as overstaging of T stage was frequently seen (14). A meta-analysis on the use of magnetic resonance imaging (MRI) for T staging showed that the pooled accuracy for stage T2 or lower versus stage T3 or higher had a sensitivity of 86% and a specificity of 86% (15). Another prospective study also demonstrated MRI had better diagnostic performance for staging compared to CT and EUS (16). The accuracy of CT for T staging is low (17): the accuracy, sensitivity, and specificity of CT for T2 and T3 are 66%, 61%, 68% and 63%, 67%, 56%, respectively (18). There was a study investigating combined gross tumor volume to improve the accuracy of CT in diagnosing T staging (19). However, they still cannot meet our requirements for accurate staging. A study has demonstrated that CT scans assisted by artificial intelligence (AI) can improve the accuracy of T staging diagnosis, with sensitivity and specificity reaching up to 71.7% and 90.0%, respectively, and an overall accuracy of 84% (20). These findings highlight the potential of AI-assisted radiomics in enhancing the precision of cancer diagnosis and treatment planning, and therefore, we need to further explore and optimize this method.
Objective
Surgeons often face challenges in clinical staging to differentiate cT2 from T3/T3r tumors, as some hospitals opt for direct surgery for cT2N0 while applying neoadjuvant therapy for cT3 lesions. Here, we present a typical case of resectable locally advanced ESCC. The patient’s clinical staging was discussed in a multidisciplinary team (MDT) meeting, and neoadjuvant chemotherapy with docetaxel plus 5-fluorouracil and cisplatin (DCF) regimen was administered. Restaging was performed and the patient underwent minimally invasive esophagectomy (MIE)/robotic-assisted MIE (RAMIE) treatment. We present this article in accordance with the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1277/rc).
Case presentation
Case 1
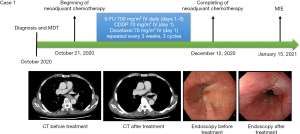
A 68-year-old Japanese male was diagnosed with middle thoracic ESCC (distance from incisor 27–32 cm) in October 2020, due to swallowing difficulties. Based on imaging CT and endoscopic examinations, the TNM staging (UICC/AJCC 8th edition) was determined as cT3N1M0 (stage III). The patient’s medical history included brain aneurysm. Following multidisciplinary discussions at the National Cancer Center Hospital, it was recommended to proceed with neoadjuvant therapy consisting of DCF for three cycles followed by combined surgical treatment. The patient started the three cycles of therapy on October 21, 2020, finished on December 12, 2020, and the re-assessment indicated a partial response (PR; ycT2N1M0). On January 15, 2021, the patient underwent thoracoscopic + laparoscopic esophagectomy + three-field lymph node dissection, and the surgical procedure and postoperative recovery were uneventful. The patient was discharged on the 11th postoperative day (Figure 1).
Case 2
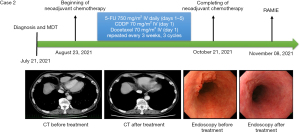
A 72-year-old Japanese male presented with progressive dysphagia and was diagnosed with ESCC (distance from incisor 35–38 cm) of the lower thoracic esophagus on July 21, 2021, based on CT imaging and endoscopic examination. The TNM staging (UICC/AJCC 8th edition) was determined as cT2N2M0 (stage III). The patient’s medical history included diabetes mellitus (DM), which was currently stable. Following multidisciplinary discussions at the National Cancer Center Hospital, it was recommended to proceed with neoadjuvant therapy consisting of DCF regimen for three cycles followed by combined surgical treatment. The patient started the three cycles of therapy on August 23, 2021, finished on October 21, 2021 and re-assessment indicated a PR (ycT2N1M0). On November 8, 2021, the patient underwent RAMIE + three-field lymph node dissection using the da Vinci Surgical System. The patient was discharged on the 12th postoperative day (Figure 2).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
International MDT (iMDT) discussion
Treatment strategy and recommendation
A multidisciplinary discussion was conducted at the National Cancer Center Hospital, involving surgeons specializing in esophageal surgery, medical oncologists, radiation oncologists, and endoscopists. The surgical team preliminarily diagnosed the two patients with potentially resectable locally advanced esophageal cancer. However, it was challenging to differentiate between cT2 and cT3 stage. Nevertheless, following the guideline, neoadjuvant chemotherapy followed by surgical treatment was advised. The specific surgical approach, either RAMIE or MIE, would be determined based on the patients’ physical condition, disease status, and da Vinci Surgical System day of surgery.
The radiation oncology department noted that the patients expressed a preference for surgical treatment, so the addition of radiation therapy was not considered. The medical oncology team recommended neoadjuvant therapy based on studies such as JCOG9907 and JCOG1109 (21,22). The choice of chemotherapy regimen would follow the current guidelines, and two-drug neoadjuvant regimen combining cisplatin at 80 mg/m2 on day 1 and fluorouracil at 800 mg/m2 on days 1–5, repeated every 3 weeks, 2 cycles, was recommended.
Central theme for iMDT
Is the clinical stage diagnosis of cT2 and cT3 important prior to initiating treatment? Does it impact patient survival? What is the recommended approach for making this diagnosis?
Japan
Surgeon: Daisuke Kurita
In the diagnosis of cT2 or cT3, there are two categories: “clear cases” and “borderline cases”. However, accurately diagnosing the “borderline cases” using the current modality is challenging. The determination of whether a case falls under T2 or T3 is primarily based on the size and volume of the tumor observed on CT scans, but the true nature of these cases remains uncertain. Moreover, preoperative chemotherapy further complicates the assessment, as it modifies the tumor characteristics, making it difficult to ascertain the accurate cT2 or cT3 diagnosis before chemotherapy through postoperative pathology.
Considering the difficulties in validating cT prior to preoperative chemotherapy using pathological methods, the most effective way to establish a common understanding is through collaborative discussions among multiple centers and across nations, involving endoscopic and CT evaluations of T2 or T3 cases.
Endoscopist: Seiichiro Abe
There are no established criteria for the endoscopic differential diagnosis between T2 and T3. We normally perform T staging for ESCC beyond cT1, and rarely perform additional EUS for T staging based on the results of JCOG1604. Because neoadjuvant chemotherapy becomes the current standard care of locally advanced ESCC in Japan, it is no longer possible to get the feedback from the histology in terms of the depth of invasion. Previously some expert endoscopists have investigated the clinical impact of T staging, but endoscopic efficacy assessment of neoadjuvant chemotherapy will be much more important rather than baseline depth diagnosis in the neoadjuvant chemotherapy era (23).
China
Surgeon: Ruixiang Zhang
The T staging of esophageal cancer is determined by the depth of tumor infiltration, in contrast to other solid tumors like lung cancer, where staging is based on tumor size or maximum diameter. This fundamental distinction presents certain challenges. While supportive measures such as CT scans, EUS, and MRI are available, their accuracy is not yet optimal. In our practical experience, current diagnostic methods do not provide a high level of accuracy in distinguishing between cT2 and cT3 stage. However, in terms of guiding treatment decisions, this differentiation is not as critical. For N0 patients, both T2 and T3 stages can be directly treated with surgery in real-world clinical practice, with postoperative treatment decisions based on pathological findings. On the other hand, for N+ patients, irrespective of T2 or T3 stage, preoperative neoadjuvant therapy is recommended according to current treatment guidelines. Hence, the precise differentiation between T2 and T3 stages is not of utmost importance in clinical management. Nevertheless, we aspire to achieve more accurate preoperative staging through the continuous advancement of various auxiliary diagnostic techniques. This will facilitate the development of precise treatment plans, ultimately maximizing the clinical benefits for patients.
Surgeon: Xufeng Guo
When it comes to preoperative differentiation between cT2 and cT3, generally speaking, cT3 is particularly challenging to distinguish from cT4a. The primary counterpart to cT2 is cT1b. It’s because cT1b and cT2 represent a watershed moment: cT1b can be treated with endoscopic submucosal dissection (ESD) as the lesion can be completely removed through endoscopy. However, cT2 cannot be removed through endoscopic means, so clinical differentiation between cT2 and cT1b is crucial.
In clinical practice, to differentiate between cT2 and cT3, our experience involves combining endoscopic examination and CT scan. If there is an ulcer observed during endoscopy, along with a relatively larger tumor cross-section on CT scan, it usually indicates cT3. On the other hand, cT2 tumors generally do not exhibit significant growth on CT imaging. Additionally, cT2 tumors are predominantly characterized by a raised appearance on endoscopy, sometimes with a relatively flat surface on top of the elevation, shallow ulcers, and detectability on esophagography. Although EUS allows visualization of the muscular and adventitial layers, and may be of benefit, its application is limited in cases where the tumor is large or causes severe stenosis, making the procedure challenging. Other techniques such as spectral CT or functional MRI may offer some assistance in distinguishing between cT2 and cT3, but there is limited experience in this area.
Regarding the differentiation between cT2 and cT3 and its relationship with survival, what matters more is the accuracy of N staging. In cases of resectable lesions of cT2 and cT3, the accuracy of cT staging does not significantly impact patient prognosis. The real difference lies in the impact on survival after lymph node metastasis. Therefore, I believe that the focus should be on improving the accuracy of N staging assessment, rather than considering cT2 and cT3 as counterparts. cT3 should be distinguished from cT4a or, more specifically, cT3r from cT3br, while cT2 should be differentiated from cT1b.
Surgeon: Liang Dai
Clinically, accurately distinguishing between cT2 and cT3 is extremely challenging. Despite having various tools for T staging, including EUS, contrast-enhanced CT, and contrast-enhanced MRI, accurate staging remains difficult, whether used individually or in combination. Furthermore, what is more important is that the clinical significance of accurately determining cT2 and cT3 is not so important from a surgical perspective. There is not much difference in the extent of surgical resection between cT2 and cT3 tumors before treatment. If the objective is to strategize neoadjuvant therapy based on precise differentiation between cT2 and cT3, the current evidence falls short. This is mainly because, without the capability to accurately determine T staging clinically, we cannot undertake rigorous studies to ascertain the necessity of neoadjuvant therapy for T2 or T3 patients. In our center, we place less emphasis on the accuracy of cT2 and cT3 and instead focus more on suspicious regional lymph node metastasis. We believe that this approach holds more significance for selecting neoadjuvant therapy in patients with locally advanced ESCC.
Oncologist: Yongchang Chen
First and foremost, we need to consider whether cT2 and cT3 staging will impact treatment strategy and whether these different treatment strategies will affect patient survival rates. For cT2 patients without lymph node metastasis, the initial treatment approach is controversial. Some experts suggest definitive chemoradiotherapy as the first-line treatment for squamous cell carcinoma. Other expert groups recommend upfront surgery for cT2N0, regardless of it being adenocarcinoma or squamous cell carcinoma, if the tumor is smaller than 3 cm and well-differentiated. However, if high-risk factors are present, neoadjuvant chemoradiotherapy (nCRT) is recommended. Nevertheless, the effectiveness of nCRT for cT2 thoracic esophageal cancer in terms of patient survival outcomes remains unclear.
For cT3 ESCC patients, combined treatment, particularly concurrent chemoradiotherapy, maybe a better choice. However, for patients with adenocarcinoma of the gastroesophageal junction, the optimal multidisciplinary treatment strategy is still debated due to a lack of corresponding research results. Nevertheless, regardless of whether chemoradiotherapy is the initial choice, surgery remains the cornerstone of treatment. For cT2 and cT3 patients, regardless of the treatment strategy chosen, the primary concern is the accuracy of preoperative staging. Therefore, imaging examinations such as EUS undoubtedly play a crucial role in assisting with staging.
Endoscopist: Rui Zhao
In the field of endoscopic treatment for early esophageal cancer, it is crucial to differentiate between cT1a and cT1b, as this distinction helps determine whether endoscopic treatment is feasible. However, differentiating between cT2 and cT3 has limited significance. Image-enhanced endoscopy (magnifying endoscopy) can provide a more detailed staging of early-stage T1 cancers, such as T1a-lamina propria mucosae (LPM), T1a-muscularis mucosae (MM), and T1b-submucosa (SM). However, it cannot diagnose or differentiate T2 or T3 stages. EUS can provide a relatively accurate preoperative diagnosis of cT2 and more advanced-stage cancers. Currently, neoadjuvant therapy before surgery is common practice, and after neoadjuvant therapy, the postoperative pathology often cannot accurately reflect the initial staging of the tumor. Therefore, an accurate clinical staging before treatment is necessary to guide treatment planning.
We recommend using EUS for T staging, as it is currently the most accurate method for local staging of esophageal cancer. Pre-treatment EUS is used to stage the lesion as T2 or T3, and post-treatment reassessment using EUS helps calculate the degree of tumor reduction after treatment, which aids in predicting the patient’s future survival prognosis. However, a challenge arises in distinguishing between post-treatment inflammation/fibrosis and residual tumor using EUS. Additionally, the combination of endoscopic ultrasound and endobronchial ultrasound can be used to stage mediastinal lymph nodes, and if necessary, needle aspiration can be performed to confirm suspected metastasis, providing guidance for the patient’s subsequent treatment choices.
Radiologist: Jiahua Lv
With increasing T stage, the lymph node metastasis rate also increases, with lymph node metastasis rates of 67.9% for pathological T (pT)2 and 78% for pT3. Current guidelines recommend nCRT followed by surgery as the preferred treatment approach. However, recent studies have shown limited benefits and increased surgical complications in patients with T2N0 esophageal cancer who undergo neoadjuvant therapy (7,24). Therefore, it is important to accurately determine the cT2 and cT3 stages before treatment to predict patient prognosis effectively and guide treatment selection. However, improving the accuracy of preoperative diagnosis remains a challenge.
Early-stage esophageal cancer is difficult to detect on CT, and it cannot accurately differentiate the depth of esophageal cancer invasion, resulting in a low accuracy rate for T staging. EUS provides accurate assessment of esophageal wall layers in preoperative T staging. Although EUS has high sensitivity and specificity in diagnosing T staging, approximately 30% of esophageal cancer patients are unable to complete EUS examination due to esophageal luminal obstruction. MRI can clearly display the vascular lumen and wall characteristics without the need for contrast agents. Additionally, MRI is highly sensitive to fat signals, allowing clear visualization of the presence of periesophageal fat, which helps determine tumor invasion. Using T2-weighted high-resolution imaging, MRI has a diagnostic rate of approximately 81.0% for T staging of esophageal cancer, with a diagnostic rate of 96.0–100.0% for T3 and T4, but only 33.0–58.0% for T1 and T2.
Above all, single imaging modalities have limited value in detecting the primary lesion. The use of radiomics or multimodal imaging to improve accuracy, such as combining EUS with CT, PET/CT, and diffusion-weighted imaging (DWI) with high-resolution T2WI. Based on our previous work in radiation oncology, we have found that DWI combined with high-resolution T2WI and CT can provide accurate preoperative diagnosis of T staging. In the radiotherapy or concurrent chemoradiotherapy of esophageal cancer, DWI examination can complement the limitations of contrast-based evaluation of short-term efficacy. Monitoring changes in the apparent diffusion coefficient (ADC) values can assist in early prediction of treatment response and prognosis. DWI examination provides intuitive and accurate reference information for evaluating the efficacy of radiotherapy and concurrent chemoradiotherapy in esophageal cancer, and it has significant clinical value.
Radiology diagnosis: Haomiao Qing
Differentiating between cT2 and cT3 stages of esophageal cancer can currently influence the decision for neoadjuvant therapy and significantly impact the prediction of patient prognosis. EUS is the most commonly used examination method for T staging in esophageal cancer. It can display and measure different layers of the esophagus and achieve good accuracy in determining whether the tumor invades the adventitia. A study has also used the intrinsic muscle layer thickness and mucosal layer thickness to predict T2/T3 staging of esophageal cancer (9). However, EUS examination may be limited by stricture caused by tumor and may not be feasible in certain cases.
Among non-invasive imaging modalities, it has been found that MRI with 3.0-T or higher has excellent soft tissue resolution. DWI also assists in the differentiation of T staging. Contrast-enhanced MRI may outperform EUS and contrast-enhanced CT in differentiating between T1–2 and T3–4 tumors. When EUS is challenging or not feasible, MRI can complement the EUS findings. Contrast-enhanced CT, widely used in esophageal cancer imaging, has limited soft tissue resolution and limited effectiveness in T staging. The discriminatory effect of dynamic perfusion scanning may be better than conventional contrast-enhanced scanning, but overall performance is inferior to MRI and EUS examinations (16). PET imaging is limited by spatial resolution, making it difficult to directly determine tumor invasion of the outer layer. PET/MRI fusion imaging may be better to differentiate tumors in T2/T3 staging, with MRI playing a more significant role in fusion images.
Pathologist: Yang Liu
The pathological field typically uses the TNM staging system to assess the extent and degree of tumor invasion for ESCC patients. As the T stage increases, we are more likely to observe neural and vascular invasion in the pathology, and the likelihood of lymph node positivity also increases accordingly. In terms of prognosis, both retrospective analyses and meta-analyses have shown a close correlation between T2 and T3 staging of ESCC and patient prognosis. The T stage of the patient is an independent prognostic factor, and as the T stage increases, the prognosis of the patient gradually worsens. It is important to note that the pathological results can differentiate between pT2 and pT3 under the microscope, but there are indeed many challenges in clinical staging. Additionally, there is controversy regarding the correlation between T staging and prognosis in patients who undergo neoadjuvant therapy before surgery. Our institution’s data suggests that the tumor regression grade (TRG) grading for treatment response assessment may be a better indicator of prognosis from a pathological perspective. Therefore, a comprehensive evaluation of the prognosis of T2 and T3 stage patients should consider factors such as TRG, lymph node metastasis, and distant metastasis.
Hong Kong (China)
Surgeon: Ian Yu-Hong Wong
Evidence from the CROSS trial and JCOG 9907 trial has shown that patients with advanced esophageal cancer would benefit from preoperative treatment. If one strictly follows the inclusion criteria of the clinical trials on preoperative chemotherapy or chemoradiotherapy, the clinical stage of cT2 or cT3 would not make any difference in the treatment decision. Technically, it is also difficult to differentiate between these two stages, whether using radiological, endoscopic, or endosonographic means. Therefore, in real-world scenarios, decisions are often made by a consensus within the MDT consisting of a panel of surgeons, oncologists, radiologists, and pathologists. Many of these decisions are based on the experience and impression of the expert panel and mostly rely on the “bulkiness” of the primary tumor from CT scan findings. Similarly, it is also very difficult to differentiate between cT3 or cT4 disease, which may affect the choice of treatment between neoadjuvant therapy, a definitive chemoradiotherapy approach, or even with the aim of conversion surgery. Therefore, real-world data often deviates or disappoints compared to trial results, making genuine survival outside the trial setting important (25,26). In our institute, we aim to perform routine endoscopy, PET-CT scan, and EUS for all patients to provide more objective information to our MDT panel for a fair judgment.
Korea
Surgeon: Seong Yong Park
Differentiation between cT2 and cT3 is not meaningful when patients present with clinically suspected metastatic lymph nodes. In cases of cN+, the standard treatment is neoadjuvant chemoradiation or chemotherapy followed by surgery. For cN− patients, the differentiation between cT2 and cT3 can be important in determining treatment plans. Controversies exist in cT2N0 cases, with both upfront surgery and neoadjuvant therapy followed by surgery being acceptable options so far. However, the current National Comprehensive Cancer Network guidelines recommend neoadjuvant therapy for cT3N0 patients.
Currently, EUS is the most accurate tool for differentiating the cT stage. A systematic review of 27 studies evaluating its performance found EUS to be highly effective in distinguishing T1 or T2 cancers from T3 or T4 cancers, with a performance index of 0.89 for esophageal cancers and 0.91 for esophagogastric junction cancers (27). Despite the high performance of EUS in differentiating the cT stage, approximately 10% of patients may still not receive optimal treatment due to the inaccuracy of clinical staging. Further studies are needed to address this issue.
Europe
Surgeon: Bas Wijnhoven
In my opinion, the clinical diagnosis of cT2 and cT3 prior to initiating treatment is not of utmost importance. The treatment approach remains the same in both situations: neoadjuvant chemoradiation followed by surgery or (within a current trial) active surveillance. cT stage can be somewhat unreliable, and while it may have an impact on patient survival, the pathological tumor stage holds greater significance. Based on my experience, determining the cT stage is done through EUS, and if EUS is unavailable or has not been conducted, a CT scan becomes crucial.
Surgeon: Xing Gao
The significance of determining whether ESCC is classified as cT2 or cT3 lies in the need for more precise categorization of each patient and the administration of evidence-based treatments to similar groups of patients. In other words, in previous treatment modalities, whether a tumor is classified as cT2 or cT3 is important for treatment selection. However, under the current neoadjuvant treatment paradigm, this significance is not as prominent. In the era of neoadjuvant therapy, the importance of cT1b–2 becomes crucial again. For example, with the emergence of new treatments such as the ability to perform deeper ESD, the distinction between cT2 and cT3 becomes significant once more. Therefore, it is necessary to discuss patient classification within an MDT setting.
For T staging, we rely on EUS examination followed by confirmation with CT. If EUS examination is not available, CT alone cannot differentiate between cT2 and cT3, and it should not delay patient treatment. When considering N0 status, the patient would face a decision between nCRT or direct surgery, which calls for shared decision making between the medical team and the patient.
Surgeon: Berend J. van der Wilk
cT2 ESCC comprises all cancers reaching the proper muscle layer and cT3 reaches the adventitia. It is known that this pretreatment TNM classification system is quite unreliable, especially for nodal staging and for early cT2 squamous cell carcinoma. Furthermore, we know that nearly 60% of patients with cT2–3N0 patients have nodal disease (28). Finally, preoperative CRT seems inferior to surgery alone in patients with cT2 esophageal cancer (29).
Earlier, our study group investigated a new classification system depending on regressional changes in the resection specimen. We proved that pretreatment pathological prep-N staging was more accurate than conventional N-staging (30,31).
In conclusion, it is important to stage cT2 or cT3 prior to initiating treatment since it can have clinical consequences. It is still hard, however, to reliably distinct these two types of tumors. Furthermore, nodal staging remains difficult in these patients.
USA
Surgeon: Kyle G. Mitchell
At MD Anderson Cancer Center, all medically operable patients with locoregionally advanced ESCC are treated with trimodality therapy. Patients who are marginal candidates for surgery are considered for bimodality therapy (definitive chemoradiation) with selective surgery (or “watch and wait”) approach on an individualized basis (32,33).
A critical node in the decision tree is confirmation of cT2 disease, as accurate clinical staging of cT2N0 tumors is notoriously difficult (24). We will regularly pursue endoscopic mucosal resection as a confirmatory diagnostic maneuver prior to proceeding with therapy for patients with cT2N0 lesions (34). It is worth noting that smaller tumors (<3.5 cm in length) are less likely to be upstaged and may be considered for surgery alone. Patients with cT3N0–1 ESCC would be considered for multimodality therapy at our institution.
Conclusions
Differentiating between cT2 and cT3 esophageal cancer poses significant challenges and the value for clinical decision making remains to be determined. Accurate clinical staging, also for nodal staging, remains difficult and requires improvement. The treatment approach for cT2 and cT3 tumors is often similar, with neoadjuvant therapy followed by surgery being the recommended approach. However, there is a need to not only enhance the accuracy of nodal staging but also differentiate between cT2 and cT3 tumors. Further studies are warranted to explore whether combining these two stages in the staging system is necessary in future research and to assess its impact on treatment decisions and patient outcomes.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1277/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1277/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1277/coif). I.Y.H.W. serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2023 to March 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Watanabe M, Toh Y, Ishihara R, et al. Comprehensive registry of esophageal cancer in Japan, 2015. Esophagus 2023;20:1-28. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg 2021;156:721-9. [Crossref] [PubMed]
- Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol 2022;40:238.
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- Qu J, Zhang H, Wang Z, et al. Comparison between free-breathing radial VIBE on 3-T MRI and endoscopic ultrasound for preoperative T staging of resectable oesophageal cancer, with histopathological correlation. Eur Radiol 2018;28:780-7. [Crossref] [PubMed]
- Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: a review of the Society of Thoracic Surgeons database. Ann Thorac Surg 2013;96:382-90. [Crossref] [PubMed]
- Bhutani MS, Barde CJ, Markert RJ, et al. Length of esophageal cancer and degree of luminal stenosis during upper endoscopy predict T stage by endoscopic ultrasound. Endoscopy 2002;34:461-3. [Crossref] [PubMed]
- Yu Y, Wei X, Chen X, et al. The T stage of esophageal cancer can be effectively predicted by muscularis propria thickness and muscularis propria + mucosa thickness under ultrasonic gastroscopy. Thorac Cancer 2023;14:127-34. [Crossref] [PubMed]
- Yang J, Luo GY, Liang RB, et al. Efficacy of Endoscopic Ultrasonography for Determining Clinical T Category for Esophageal Squamous Cell Carcinoma: Data From 1434 Surgical Cases. Ann Surg Oncol 2018;25:2075-82. [Crossref] [PubMed]
- Kutup A, Link BC, Schurr PG, et al. Quality control of endoscopic ultrasound in preoperative staging of esophageal cancer. Endoscopy 2007;39:715-9. [Crossref] [PubMed]
- Klamt AL, Neyeloff JL, Santos LM, et al. Echoendoscopy in Preoperative Evaluation of Esophageal Adenocarcinoma and Gastroesophageal Junction: Systematic Review and Meta-analysis. Ultrasound Med Biol 2021;47:1657-69. [Crossref] [PubMed]
- Meister T, Heinzow HS, Osterkamp R, et al. Miniprobe endoscopic ultrasound accurately stages esophageal cancer and guides therapeutic decisions in the era of neoadjuvant therapy: results of a multicenter cohort analysis. Surg Endosc 2013;27:2813-9. [Crossref] [PubMed]
- Heinzow HS, Seifert H, Tsepetonidis S, et al. Endoscopic ultrasound in staging esophageal cancer after neoadjuvant chemotherapy--results of a multicenter cohort analysis. J Gastrointest Surg 2013;17:1050-7. [Crossref] [PubMed]
- Lee SL, Yadav P, Starekova J, et al. Diagnostic Performance of MRI for Esophageal Carcinoma: A Systematic Review and Meta-Analysis. Radiology 2021;299:583-94. [Crossref] [PubMed]
- Guo J, Wang Z, Qin J, et al. A prospective analysis of the diagnostic accuracy of 3 T MRI, CT and endoscopic ultrasound for preoperative T staging of potentially resectable esophageal cancer. Cancer Imaging 2020;20:64. [Crossref] [PubMed]
- Bunting D, Bracey T, Fox B, et al. Loco-regional staging accuracy in oesophageal cancer-How good are we in the modern era? Eur J Radiol 2017;97:71-5. [Crossref] [PubMed]
- Sultan R, Haider Z, Chawla TU. Diagnostic accuracy of CT scan in staging resectable esophageal cancer. J Pak Med Assoc 2016;66:90-2.
- Li H, Chen TW, Zhang XM, et al. Computed tomography scan as a tool to predict tumor T category in resectable esophageal squamous cell carcinoma. Ann Thorac Surg 2013;95:1749-55. [Crossref] [PubMed]
- Takeuchi M, Seto T, Hashimoto M, et al. Performance of a deep learning-based identification system for esophageal cancer from CT images. Esophagus 2021;18:612-20. [Crossref] [PubMed]
- Kato K, Igaki H, Ito Y, et al. Next study (JCOG1109): A three-arm randomized phase III study comparing preoperative CDDP+ 5-FU (CF) versus docetaxel+ CF versus CF-radiation followed by esophagectomy with D2-3 lymphadenectomy for locally advanced esophageal squamous cell cancer. J Clin Oncol 2013;31:TPS4152. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Kadota T, Abe S, Yoda Y, et al. Clinical outcomes according to the modified endoscopic criteria for neoadjuvant chemotherapy in resectable esophageal squamous cell carcinoma. Dig Endosc 2020;32:337-45. [Crossref] [PubMed]
- Predictors of staging accuracy, pathologic nodal involvement, and overall survival for cT2N0 carcinoma of the esophagus. J Thorac Cardiovasc Surg 2019;157:1264-1272.e6. [Crossref] [PubMed]
- Wong IYH, Lam KO, Zhang RQ, et al. Neoadjuvant Chemoradiotherapy Using Cisplatin and 5-Fluorouracil (PF) Versus Carboplatin and Paclitaxel (CROSS Regimen) for Esophageal Squamous Cell Carcinoma (ESCC): A Propensity Score-matched Study. Ann Surg 2020;272:779-85. [Crossref] [PubMed]
- Wong IYH, Lam KO, Chan W, et al. Real-world Scenario: CROSS Regimen as Preoperative Therapy for Oesophageal Squamous Cell Carcinoma. J Gastrointest Surg 2020;24:1937-47. [Crossref] [PubMed]
- Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001;49:534-9. [Crossref] [PubMed]
- Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg 2011;92:491-6; discussion 496-8. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Shapiro J, Biermann K, van Klaveren D, et al. Prognostic Value of Pretreatment Pathological Tumor Extent in Patients Treated With Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal or Junctional Cancer. Ann Surg 2017;265:356-62. [Crossref] [PubMed]
- Brinkmann S, Noordman BJ, Hölscher AH, et al. External Validation of Pretreatment Pathological Tumor Extent in Patients with Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer. Ann Surg Oncol 2020;27:1250-8. [Crossref] [PubMed]
- Mitchell KG, Nelson DB, Corsini EM, et al. Morbidity following salvage esophagectomy for squamous cell carcinoma: the MD Anderson experience. Dis Esophagus 2020;33:doz067. [Crossref] [PubMed]
- Zhou N, Mitchell KG, Corsini EM, et al. Analysis of trimodal and bimodal therapy in a selective-surgery paradigm for locally advanced oesophageal squamous cell carcinoma. Br J Surg 2021;108:1207-15. [Crossref] [PubMed]
- Nelson DB, Mitchell KG, Weston BR, et al. Should endoscopic mucosal resection be attempted for cT2N0 esophageal cancer? Dis Esophagus 2019;32:1-6. [Crossref] [PubMed]


