
Transcatheter valve-in-valve implantation treatment with the J-valve system for tricuspid bioprosthesis deterioration: a report of two cases
Highlight box
Key findings
• The valve-in-valve (ViV) technique with J-valve allows the treatment of tricuspid structural valve degeneration in high-risk patients, avoiding cardiopulmonary bypass and conventional thoracotomy injury, and has a good short-term clinical effect.
What is known and what is new?
• For patients with tricuspid valve dysfunction, the treatment methods face challenges.
• The J-valve system with the ViV technique to treat tricuspid bioprosthetic valve deterioration is feasible.
What is the implication, and what should change now?
• Considering the few cases of tricuspid valve deterioration, a multi-center study is required.
Introduction
Tricuspid valve dysfunction is often secondary to longstanding pressure or volume overload related to left heart valve dysfunction. While treatment guidelines favor tricuspid valve repair as the preferred approach for severe tricuspid valve regurgitation, a minority of patients are not eligible for valve repair and are treated with valve replacement surgery (1,2). In this setting, bioprosthetic heart valves play an increasing role due to the continuous improvement of anti-calcification technology, the advantage of not using lifelong anticoagulants and the lower risk of valve thrombosis. According to the 2020 American heart valve disease guideline (1), opting for a bioprosthetic valve during replacement surgery at 50 years or above (class 2a indication, level of evidence B) is reasonable. Biological valve dysfunction may exist in tricuspid biological valves and require further redo valve replacement. Patients with tricuspid bioprosthetic valve deterioration often present with underlying right ventricular enlargement and dysfunction, accompanied by liver and kidney dysfunction, pleural effusion, and ascites. Therefore, surgical reintervention for bioprosthetic valve deterioration carries increased risk, with high perioperative mortality and complication rates (3). In recent years, transcatheter aortic valve implantation (TAVI) has developed rapidly (4), and the valve-in-valve (ViV) technique has become the preferred interventional treatment in most patients presenting with severe bioprosthetic structural valve degeneration (SVD) (5,6). Since tricuspid bioprosthetic valve replacement is less joint, only a few cases treated with ViV techniques have been reported (7-9). The purpose of this report was to summarize this preliminary clinical experience and 1-year follow-up. We present this article following the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1961/rc).
Case presentation
Two individuals with tricuspid bioprosthetic dysfunction were included in the outpatient of Beijing Anzhen Hospital. After the informed consent was signed by the patients and/or their family members, the operation was carried out in the hybrid operation room. Under general anesthesia in a supine position, double-lumen endotracheal intubation was performed. Generally, a 3–4 cm small incision was made at the right 5–7 intercostal at the middle axillary line, and the right lung was protected with wet gauze under single lung ventilation to expose the right atrium. 3-0 Prolene sutures were used for two purse strings at the right atrium without opening the pericardium; intravenous heparin of 0.5–0.8 mg/kg was given to maintain the anticoagulation time (ACT) between 250–300 seconds. Under fluoroscopy guidance, the right atrium was punctured, and a soft guide wire was inserted into the right ventricle through the tricuspid bioprosthesis. A pigtail catheter was inserted to measure the right ventricular pressure and subsequently exchanged for an extra stiff wire, over which the J-valve conveyor was transported into the right ventricle. The three J-valve graspers were released first and aligned one by one with three valve struts of the degenerated biological valve (Figure 1) (10). The appropriate implantation depth of the J-valve into the right atrium was adjusted as 10–20% atrial and 80–90% ventricular, and then the valve was deployed with no need for rapid pacing. Usually, the valve should be postdilated to optimize valve expansion and improve hemodynamic valve performance regarding transvalvular gradient and paravalvular leak. Intraoperative angiography and three-dimensional (3D) esophageal ultrasound were used to monitor the position and function of the implanted J-valve.
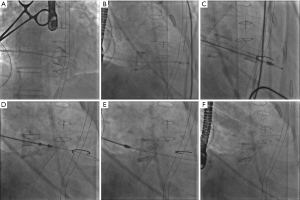
Then, the delivery sheath and supporting guide wire were removed, and a drainage tube was placed as usual. Warfarin for 6 months, targeting an international normalized ratio (INR) of 2.0–2.5, was the chosen anticoagulation regimen. After 6 months, anticoagulation was switched to oral aspirin 100 mg/day for at least 2 years. The study was conducted by the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. 2022192X). Written informed consent was obtained from the patients to publish this case report and accompanying images. Copies of the written consent are available for review by the editorial office of this journal.
Case 1
A 42-year-old man with a history of atrial septal defect closure and tricuspid valve replacement presented with palpitations, chest tightness, abdominal distension, and a complete right bundle branch block. Years ago, he underwent tricuspid bioprosthetic valve replacement. However, the specific life expectancy of the tricuspid bioprosthesis was unknown. The Society of Thoracic Surgeons (STS) score was 2.77. Before surgery, transesophageal echocardiography (TEE) revealed that the mean transvalvular gradient was 7 mmHg, the tricuspid regurgitation was severe, the tricuspid bioprosthetic valve opening area (VOA) was 0.6 cm2 [pressure halftime (PHT) method, which might not indicate the real VOA], right atrium diameter was 64 mm, and the left ventricular ejection fraction (LVEF) was 59%. The abovementioned TEE results showed this patient with mild tricuspid bioprosthesis stenosis and severe regurgitation. Due to a hostile chest and poor preoperative cardiac status, the patient underwent transcatheter tricuspid ViV implantation with a 23 mm J-valve. The mean transvalvular gradient decreased to 5 mmHg without regurgitation, and the right atrium diameter was 52 mm after surgery with a trace paravalvular leak. At the 1-year follow-up, the mean transvalvular gradient slightly increased to 9 mmHg without regurgitation, and the LVEF was 62%.
Case 2
A 64-year-old man with a history of severe mitral valve regurgitation, tricuspid valve severe stenosis, and atrial fibrillation presented chest tightness. He received a tricuspid bioprosthetic valve 16 years ago, and the valve’s lifespan is 16 years. The STS score was 4.13. Before surgery, TEE showed his tricuspid bioprosthetic valve had severe stenosis with a mean transvalvular gradient of 17 mmHg, the right atrium diameter was 80 mm, and the mitral valve had severe regurgitation. The patient underwent transcatheter tricuspid ViV implantation with the 27 mm J-valve. The mean transvalvular gradient decreased to 2 mmHg, and the right atrium diameter decreased to 75 mm after surgery without paravalvular leak. At the 1-year follow-up, the mean transvalvular gradient was 9 mmHg, and the LVEF was 64%.
International Multidisciplinary Team (iMDT) discussion
Discussion among physicians from Beijing Anzhen Hospital
Department of Cardiac Surgery
In recent years, biological tissue-based valves have become more appealing for valve replacement surgery than mechanical heart valves because of their improved durability and the lack of need for lifelong anticoagulation. Nevertheless, bioprosthetic heart valves remain vulnerable to SVD. Moreover, these patients typically present a higher risk for reintervention due to advanced age, multiple comorbidities, and the redo setting. After the success of the TAVI, experts began to explore the ViV technology for biological SVD. Since the ViV procedure can avoid cardiopulmonary bypass, with no need for heart adhesion removal, it is an ideal choice for high-risk redo patients with tissue valve deterioration. In 2007, Wenaweser et al. first reported a successful aortic ViV procedure during the open surgery (11). In 2009, Cheung et al. reported the first mitral ViV procedure for mitral SVD (12). In 2017, the US Food and Drug Administration (FDA) officially approved the Edwards SAPIEN 3 valve for aortic and mitral ViV procedure for bioprosthetic SVD (13). Due to the few cases of tricuspid tissue valve replacement, it was not until 2011 that Van Garsse et al. first reported the successful tricuspid ViV procedure through the jugular vein (14). In 2016, McElhinney et al. (15) summarized the international registration data of 156 cases of tricuspid ViV collected at 53 international centers in 7 years, the most significant number of cases reported so far. These data showed that after ViV, the valve gradient and regurgitation improved significantly immediately following the intervention. There were four cases of perioperative right ventricular dysfunction, five cases of perioperative death, and two cases of valve displacement. After an average follow-up of 13 months, 87% of the patients had the New York Heart Association (NYHA) I/II functional class.
Compared with the aortic and mitral valves, the tricuspid valve was called a forgotten valve. Thus, patients with severe tricuspid valve diseases find it difficult to reverse the right heart physiology even if they underwent tricuspid valve surgery. Under the exposure to pathophysiological hemodynamics, patients who have a previous history of tricuspid valve surgery, including tricuspid valve plastic and tricuspid valve replacement, are more challenging to manage during perioperative phage, and the effect of redo surgery is unsure in the past. However, the transcatheter ViV technique offers a less invasive and effective option for patients with failed tricuspid bioprosthetic valves. Although transcatheter ViV can provide good hemodynamics and symptom improvement to patients, there are some problems (suboptimal hemodynamics, valve thrombosis, prothesis-patients mismatch, SVD, and so on) that we need to fix.
As for the valve type and surgical pathway selections, several choices have been described (7,16-18). However, in these two cases, we chose to use the J-valve system (Suzhou Jiecheng Company, Suzhou, China), which was demonstrated as an excellent transcatheter heart valve to treat patients with aortic and mitral valve diseases in many large heart centers (6,10,17), to treat patients with tricuspid bioprosthetic degeneration. The unique design of the J-valve, with three graspers connected with the stent and the three-valve struts of the biological valve, is aligned to facilitate the positioning and reduce the risk of displacement. The operation only needs to be completed through an atrium puncture site with no need for rapid pacing. The core operation is less than 10 minutes. It makes the redo of cardiac surgery more minimally invasive and simple. In our study, the first generation of the J-valve delivery system was 32F, and the smaller size of the second-generation delivery system is still in the clinical trial stage. Therefore, the right atrial puncture path was selected for all cases in this study. The effects of these cases were ideal; both patients had good hemodynamics and symptom improvement. Although the transvalvular gradient in 1-year follow-up slightly increased, it was a common phenomenon after transcatheter ViV treatment (19), and this phenomenon might relate to many factors, such as heart remodeling, without postoperative guideline-directed medical treatment (GDMT), poor preoperative heart function, and so on. Referring to the tricuspid valve with normal physiology, the average gradient is generally less than 5 mmHg (20). Therefore, the ideal gradient after implantation is less than 5 mmHg. Van Garsse et al. first reported that after the tricuspid ViV procedure, the gradient decreased from 10.5 to 2 mmHg (14). However, in the real world, the average gradient of many well-functioning tricuspid valves after surgical valve replacement is also about 5–10 mmHg (21). Most researchers believe the average mean gradient should be controlled at less than 6 mmHg (20,22). In our group, the mean gradient after tricuspid ViV of two patients was below 10 mmHg, and there was no significant change after follow-up for 1 year. The patient’s clinical symptoms improved significantly, and the size of the right atrium also decreased, indicating that the patient still benefited significantly.
Different from the more common aortic valve or mitral valve interventional therapy, the mechanism and treatment of right ventricular dysfunction involved is still a clinically tricky problem. Many fields need to be clarified in the evaluation of right ventricular function and medicine selection strategy. In theory, after the interventional tricuspid ViV procedure, especially after the correction of tricuspid regurgitation, the original reflux blood into the right atrium is immediately incorporated into the right ventricle and pumped into the pulmonary artery system. Therefore, the preload of the right ventricle and pulmonary circulation will increase significantly, the pulmonary artery pressure will increase, and the afterload of the right heart will also increase. The four cases summarized by Scarsini et al. (16) were followed up for 2.5 years. The average mean gradient decreased, and tricuspid regurgitation was stable as trace, tricuspid annular plane systolic excursion (TAPSE) was consistent with that before the operation, the average right atrial pressure decreased from 21 to 8.5 mmHg, and the cardiac output index and stroke output increased. Therefore, the overall benefit to patients was obvious.
Several issues on the treatment of these patients were further discussed as follows
Question 1: What are the current treatment options for failing bioprosthetic valves in the tricuspid position? How to choose the treatment method?
Expert opinion 1: Dr. Christophe Dubois
Prevention is better than cure. Tricuspid valve repair remains the preferred technique that should be performed whenever possible for the treatment of native tricuspid valve regurgitation, hereby limiting the risk for patients to develop bioprosthetic SVD. For patients undergoing tricuspid valve replacement, more recent bioprostheses present novel tissue treatment solutions primarily targeting a reduction in leaflet calcification leading to improved valve durability. For patients developing severe SVD of the bioprosthesis in the tricuspid position, reintervention with valve replacement remains the only viable option. Usually, isolated surgical tricuspid valve replacement is not an attractive option, given its bad reputation with poor early and long-term outcomes. Therefore, less invasive transcatheter techniques using heterotopic implantation of valved stents developed for aortic or pulmonary valve disease offer lower-risk solutions for complex patients, often presenting with right ventricular dysfunction and hepatic or renal failure.
Expert opinion 2: Dr. Rodrigo Bagur
It is worldwide recognized that the go-to device for ViV or valve-in-ring (ViRing) is the Edwards SAPIEN device.
Question 2: What are the advantages and disadvantages of transcatheter tricuspid valve replacement and redo tricuspid valve replacement?
Expert opinion 1: Dr. Christophe Dubois
Patients requiring reintervention on a tricuspid valve bioprosthesis often present a high-risk phenotype according to their advanced age, redo status, and multiple comorbidities, in part related to the poor hemodynamic status inherent to advanced right heart failure. These patients are particularly at risk for surgery requiring cardiopulmonary bypass. In contrast, transcatheter ViV implantation in the tricuspid position is a short and exclusively transvenous procedure, provided systems are used that can be manipulated from the femoral or jugular vein (as opposed to the transatrial cases presented above). The transvenous approach offers direct access to the tricuspid valve, with the failing bioprosthesis serving as a partially radio-opaque docking station for the freshly implanted transcatheter valve. Most short-frame balloon-expandable TAVI valves qualify for ViV implantation in the tricuspid space, with Sapien (Edwards Lifesciences, Irvine, CA, USA) being most frequently used in this indication. The procedure can be performed under local anesthesia or conscious sedation. While meticulous patient screening remains essential for these procedures, most patients with SVD of the tricuspid bioprosthesis qualify for this intervention. In contrast to ViV procedures in the mitral space, there is no risk for outflow tract obstruction on the right side of the heart, and obviously no need for transseptal puncture. Provided an extra-stiff wire rail can be installed in the right ventricle or preferably deep in the pulmonary artery, a controlled positioning and valve expansion can be accomplished without the need for rapid pacing. In contrast to tricuspid valve surgery or transcatheter native valve or ViRing procedures, there is no increased risk for conduction disturbances nor need for permanent pacemaker implantation, since the new valve does not interfere with surrounding structures as it remains engaged in the failing bioprosthesis. Finally, ViV implantation can even be performed in patients with a pacemaker lead that traverses the failing bioprosthesis. In such cases, the pacemaker lead is jailed between both bioprosthetic valve frames, and lead integrity and function should be carefully monitored.
Expert opinion 2: Dr. Rodrigo Bagur
The advantages have been pointed out, namely, a mini-invasive procedure, no-pump requirement, and using either the femoral or jugular veins, even under conscious sedation.
Question 3: What are the hemodynamic characteristics and anticoagulant strategies of biological valve degeneration?
Expert opinion 1: Dr. Christophe Dubois
Assessment of bioprosthetic valve durability is difficult in the absence of a clear definition of bioprosthetic valve dysfunction in the tricuspid position. As for bioprostheses in mitral position, assessment of morphological valve deterioration and functional changes contributes to an estimation of the severity of SVD (among others: increase in mean gradient of 5/10 mmHg indicating moderate/severe hemodynamic deterioration; increase in intraprosthetic regurgitation ≥1/≥2 grades indicating moderate/severe hemodynamic deterioration).
For ViV procedures in bioprostheses in the tricuspid position, again, in the absence of clear recommendations, it seems appropriate to follow antithrombotic regimens that are proposed after surgical bioprosthesis implantation. According to European Society of Cardiology/European Association of Cardio-Thoracic Surgery guidelines (23), oral anticoagulation with vitamin K antagonists seems appropriate for 3 to 6 months after bioprosthetic valve implantation in mitral and tricuspid position (target INR 2.5), after which no further therapy is required in patients without other indication for oral anticoagulation. However, most patients with severe tricuspid and/or mitral valve disease have a history of atrial fibrillation and qualify for continued oral anticoagulation, preferably with direct oral anticoagulants.
Expert opinion 2: Dr. Rodrigo Bagur
There are no solid data supporting anticoagulation therapy; however, considering the slow flow and stasis, there is always the concern for valve thrombosis, even more so in smaller valves such as 23 mm SAPIEN, thereby making one prone to prescribe long-term anticoagulation. Again, there is not a strong level of evidence.
Conclusions
It is feasible to use the J-valve for transcatheter ViV implantation. However, more cases are needed to assess the safety and effectiveness of this operation. Considering the few cases of tricuspid valve deterioration, a multi-center study is required.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1961/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1961/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1961/coif). C.D. receives consulting fees as TAVI Proctor for Edwards LifeSciences, and payment or honoraria for speakers bureaus from Corcym. R.B. receives consulting fees from MEDTRONIC as a consultant. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted by the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. 2022192X). Written informed consent was obtained from the patients to publish this case report and accompanying images. Copies of the written consent are available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Writing Committee Members. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:e25-197. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Majmundar M, Kumar A, Doshi R, et al. Early outcomes of transcatheter versus surgical aortic valve implantation in patients with bicuspid aortic valve stenosis. EuroIntervention 2022;18:23-32. [Crossref] [PubMed]
- Alvarez HA, Joner M, Xhepa E, et al. Single centre experience in transcatheter mitral valve implantation: valve in valve, valve in ring and valve in MAC. Eur Heart J 2022;43:ehac544.1632.
- Liu K, Shen J, Wu K, et al. Transapical mitral valve-in-valve implantation for failed bioprosthetic valve using the J-valve system with locator device: early and mid-term outcomes. Ann Transl Med 2022;10:21. [Crossref] [PubMed]
- Dannenberg V, Donà C, Koschutnik M, et al. Transcatheter treatment by valve-in-valve and valve-in-ring implantation for prosthetic tricuspid valve dysfunction. Wien Klin Wochenschr 2021;133:780-5. [Crossref] [PubMed]
- Chandavimol M, Ngernsritrakul T, Meemook K, et al. Transcatheter tricuspid valve-in-valve implantation for degenerative surgical bio-prosthesis using SAPIEN 3: A case series. Clin Case Rep 2021;9:e05029. [Crossref] [PubMed]
- Gewillig M, Dubois C. Percutaneous re-revalvulation of the tricuspid valve. Catheter Cardiovasc Interv 2011;77:692-5. [Crossref] [PubMed]
- Wei L, Liu H, Zhu L, et al. A New Transcatheter Aortic Valve Replacement System for Predominant Aortic Regurgitation Implantation of the J-Valve and Early Outcome. JACC Cardiovasc Interv 2015;8:1831-41. [Crossref] [PubMed]
- Wenaweser P, Buellesfeld L, Gerckens U, et al. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using the Corevalve Revalving system. Catheter Cardiovasc Interv 2007;70:760-4. [Crossref] [PubMed]
- Cheung A, Webb JG, Wong DR, et al. Transapical transcatheter mitral valve-in-valve implantation in a human. Ann Thorac Surg 2009;87:e18-20. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Van Garsse LA, Ter Bekke RM, van Ommen VG. Percutaneous transcatheter valve-in-valve implantation in stenosed tricuspid valve bioprosthesis. Circulation 2011;123:e219-21. [Crossref] [PubMed]
- McElhinney DB, Cabalka AK, Aboulhosn JA, et al. Transcatheter Tricuspid Valve-in-Valve Implantation for the Treatment of Dysfunctional Surgical Bioprosthetic Valves: An International, Multicenter Registry Study. Circulation 2016;133:1582-93. [Crossref] [PubMed]
- Scarsini R, Lunardi M, Pesarini G, et al. Long-term follow-up after trans-catheter tricuspid valve-in-valve replacement with balloon-expandable aortic valves. Int J Cardiol 2017;235:141-6. [Crossref] [PubMed]
- Ye J. Transcatheter aortic valve replacement for isolated aortic regurgitation is coming! J Thorac Cardiovasc Surg 2018;156:117-8. [Crossref] [PubMed]
- Viotto G, Paim L, Souza R, et al. Early outcomes of transcatheter tricuspid valve-in-valve implantation: a case series. Interact Cardiovasc Thorac Surg 2019;29:59-63. [Crossref] [PubMed]
- Haji-Zeinali AM, Etesamifard N, Mohammadi Z, et al. Transcatheter tricuspid valve-in-valve implantation with bioprosthetic balloon expandable valve. Gen Thorac Cardiovasc Surg 2022;70:947-53. [Crossref] [PubMed]
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1-23; quiz 101-2. [Crossref] [PubMed]
- Connolly HM, Miller FA Jr, Taylor CL, et al. Doppler hemodynamic profiles of 82 clinically and echocardiographically normal tricuspid valve prostheses. Circulation 1993;88:2722-7. [Crossref] [PubMed]
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975-1014; quiz 1082-4. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]


