A mortality score for acute respiratory distress syndrome: predicting the future without a crystal ball
Introduction
In a recent paper published in Critical Care Medicine, Villar et al. (1) proposed a scoring system that could be easily calculated at the bedside to predict mortality in patients with acute respiratory distress syndrome (ARDS) 24 hours after diagnosis. As in-hospital mortality ranges from 34.9% to 46.1% across the spectrum of mild to severe ARDS, respectively, the potential for improvement in management of patients with this syndrome remains high (2). Based on 62 recorded variables, age, oxygenation (PaO2/FiO2), and the plateau airway pressure score (APPS) were chosen to establish a 9-point stratification 24 hours after ARDS diagnosis, followed by categorization into low, intermediate, and high risk of death with predictive validity. The APPS classified ARDS into three severity subgroups (<5, 5–7, and >7 points), and significantly higher mortality was observed in those patients with an APPS greater than 7 (>80%) compared to those with an APPS of less than 5 (<14%).
To date, no validated scoring system has been available to predict mortality rates in ARDS. The strengths of the Villar et al. study include their standardization of mechanical ventilation parameters and the use of simple, low-cost variables that can be promptly gathered at the bedside (1). Among these variables, age is the only non-modifiable risk factor. Age is associated with increasing disease severity in patients admitted to intensive care units, and has also been used in general illness severity scores, such as APACHE, simplified acute physiology score (SAPS), and MPM (3). Conversely, oxygenation is potentially modifiable at the bedside. One previous study showed that, when positive end-expiratory pressure (PEEP) was adjusted to the standard low level (5 cm H2O), patients were reclassified between ARDS categories (4). Even with the Berlin criteria stating that oxygenation can be evaluated with a PEEP level equal or higher than 5 cm H2O, the reduction to PEEP =5 cm H2O provided a clear cut-off for differentiation between the three categories of ARDS severity. Nevertheless, oxygenation has been used to predict therapeutic response after prone positioning (5), recruitment maneuvers (6), high levels of PEEP (7), and administration of neuromuscular blocking agents (8), which appeared to be more effective in severe ARDS patients. The 7-day oxygenation index change has limited utility in predicting mortality in individual patients with ARDS, but it discriminates between efficacious and non-efficacious ARDS therapies very well (9). Respiratory system mechanics parameters can add valuable information regarding patient severity at ICU admission, as well as for prognostic purposes, as these variables are monitored during the patient’s ICU stay.
Prediction scores were developed to assess ARDS prognosis and risk of death (1,10-12). In 1988, Murray et al. proposed the lung injury score (LIS) (12), based on oxygenation, chest radiograph findings, PEEP, and static respiratory system compliance, to predict clinical therapies in ARDS trials (13,14). However, so far, the LIS has not been validated as an accurate predictor of ARDS severity (15). In the era of the Berlin definition, a large, multicenter study with 550 ARDS patients compared the predictive validity of LIS for mortality in mild, moderate, and severe stages of ARDS (15). Predictive validity to identify mortality according to ARDS severity was found to be limited [area under the receiver operating characteristic (ROC) curve =0.58 vs. 0.60, respectively; P=0.49]. A multicenter prospective cohort study in the ICU setting that included 646 patients with ARDS showed that death after hospital discharge was more related to underlying comorbidity and age than to ARDS severity, and that comorbidities could better predict long-term ARDS outcome (16). In 1998, Monchi et al. evaluated the ability of different severity scores-such as the SAPS and SAPS II, organ system failure (OSF), and LIS-to predict ARDS outcome, and concluded that the SAPS II was better to predict ARDS severity, while ARDS mortality was better related to the triggering risk factors of ARDS (direct or indirect lung injury associated with ARDS within the first 24 h) (11). In a sample of 1,999 patients with ARDS, Cooke et al. compared a score based on variables selected specifically for patients with lung injury, such as ventilator variables, arterial blood gases, severity of chest radiograph findings, and timing of acute lung injury onset, versus severity-of-illness scores (SAPS II, APACHE II, and APACHE III) (10). For a definition cohort, the area under the ROC curve for the multivariable model was superior to that of APACHE III (P<0.001); however, no difference was observed in the external validation cohort (P=0.64) (10). The lung injury prediction score (LIPS) seems to be a promising tool for ARDS outcome prediction. In a multicenter study, 5,584 patients met the criteria for ARDS after hospital admission and were screened for low or high risk according to presence of alcohol abuse, hypoalbuminemia, tachypnea, oxygen supplementation, chemotherapy, obesity, and diabetes mellitus. A LIPS score greater than 4 had 69% sensitivity and 78% specificity for identifying patients who would develop ARDS after admission (17). Conversely, Damluji et al. reported imprecise mortality prediction among patients with low, intermediate, and high risk of ARDS (18), probably due to an overly broad patient cohort. Based on these inconclusive data, a combination of the LIPS prediction score with a biomarker that reflects ARDS pathogenesis seems to be a promising alternative. In this context, the combination of LIPS score and angiopoietin-2, an endothelial growth factor that is a potent regulator of vascular permeability and a key mediator of mortality in ARDS patients, increased the area under the ROC curve to 0.84 (vs. 0.74, P=0.05). The early acute lung injury (EALI) score is another tool that has been proposed for identifying patients at risk for ARDS (19). An EALI score ≥2 had a sensitivity of 89%, specificity of 75%, and positive predictive value of 53% for this purpose; however, this tool still requires further validation in an external cohort or use in a clinical trial for ARDS prevention (Table 1).
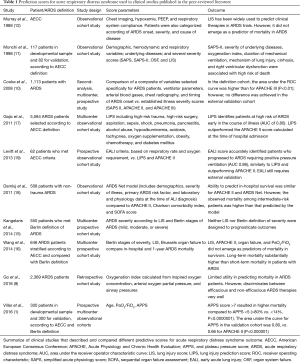
Full table
Definitions are an essential component of medical progress, and need to be continuously refined. In this line, any outcome score for ARDS requires that patients be: diagnosed on the basis of the Berlin definition, a more reliable definition that may facilitate case recognition and stratification, moving patients closer to individualized or ARDS-specific medicine (20); ventilated with low tidal volumes, a protective strategy associated with improved survival and the most important variable associated with reduction of ventilator-induce lung injury (VILI) (21); and included early (24 h after ARDS diagnosis) for outcome score calculation, which may facilitate interventions and treatments in the course of ARDS. In short, better identification of patients with ARDS is key for appropriate management and characterization of patient status.
The Villar et al. study presents some important limitations that must be addressed for better interpretation of their results. The authors did not take into account respiratory system compliance and driving pressure, since they share collinearity with tidal volume, plateau pressure, and PEEP (for the former) and plateau pressure and PEEP (for the latter). However, the variables included in their analysis can also present collinearity and thus must be carefully evaluated. The absolute value of plateau pressure, although easily measured at the bedside, also depends on tidal volume and PEEP level and thus shares collinearity with these parameters. Recent studies reported that driving pressure represents a good marker that can unify the forces that act in the ARDS-affected lung. Specifically, in one study, driving pressure >15 cm H2O was associated with higher mortality rate in ARDS patients (22). Although driving pressure is indeed collinear with other variables, careful control of measurement technique may minimize this association.
The recently completed LUNG-SAFE study offers the most global assessment of ARDS prevalence and care patterns to date. Bellani et al. (2) showed, in 459 ICUs from 50 countries, that less than two-thirds of patients with ARDS received a tidal volume of 8 mL/kg predicted body weight or less, plateau pressure was measured in only 40.1% of patients, and 82.6% received a PEEP of <12 cm H2O. Based on these findings, greater attention to individualized ARDS therapeutics, establishing when and for whom treatment may be selected and which specific subgroups may respond differently to interventions, is needed urgently. Future clinical trials will need to consider enrichment strategies and incorporate long-term functional outcomes.
Conclusions
Prognostic scores, such as APACHE III, SAPS 3 and MPM III0, can provide estimates of the probability of death for individual patients in the ICU. However, there are controversies regarding the use of these scores for predicting outcome in patients with ARDS. Some studies have developed scores to predict mortality at bedside in this patient population (10,11,23,24); however, all had limitations such as the use of non-protective mechanical ventilation strategies and small sample sizes. Villar et al. designed an outcome score (APPS) that combines variables that are readily and routinely obtained at bedside 24 h after ARDS diagnosis (age, PaO2/FiO2, and plateau pressure). They reported that the APPS can be useful for predicting patients at high risk of fatal outcome, selecting patients with ARDS in clinical studies, and guiding ventilator management. Certainly, further multicenter studies should be performed for external validation of this score in other clinical management settings and countries.
Acknowledgements
The authors would like to express their gratitude to Mr. Filippe Vasconcellos for his assistance in editing the manuscript.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Villar J, Ambrós A, Soler JA, et al. Age, PaO2/FIO2, and Plateau Pressure Score: A Proposal for a Simple Outcome Score in Patients With the Acute Respiratory Distress Syndrome. Crit Care Med 2016;44:1361-9. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care 2010;14:207. [Crossref] [PubMed]
- Caironi P, Carlesso E, Cressoni M, et al. Lung recruitability is better estimated according to the Berlin definition of acute respiratory distress syndrome at standard 5 cm H2O rather than higher positive end-expiratory pressure: a retrospective cohort study. Crit Care Med 2015;43:781-90. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC. Prone positioning in the acute respiratory distress syndrome. N Engl J Med 2013;369:980-1. [PubMed]
- Suzumura EA, Figueiró M, Normilio-Silva K, et al. Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med 2014;40:1227-40. [Crossref] [PubMed]
- Dasenbrook EC, Needham DM, Brower RG, et al. Higher PEEP in patients with acute lung injury: a systematic review and meta-analysis. Respir Care 2011;56:568-75. [Crossref] [PubMed]
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013;17:R43. [Crossref] [PubMed]
- Go L, Budinger GR, Kwasny MJ, et al. Failure to Improve the Oxygenation Index Is a Useful Predictor of Therapy Failure in Acute Respiratory Distress Syndrome Clinical Trials. Crit Care Med 2016;44:e40-4. [Crossref] [PubMed]
- Cooke CR, Kahn JM, Caldwell E, et al. Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit Care Med 2008;36:1412-2. [Crossref] [PubMed]
- Monchi M, Bellenfant F, Cariou A, et al. Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med 1998;158:1076-81. [Crossref] [PubMed]
- Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720-3. [Crossref] [PubMed]
- Peek GJ, Clemens F, Elbourne D, et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res 2006;6:163. [Crossref] [PubMed]
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007;131:954-63. [Crossref] [PubMed]
- Kangelaris KN, Calfee CS, May AK, et al. Is there still a role for the lung injury score in the era of the Berlin definition ARDS? Ann Intensive Care 2014;4:4. [Crossref] [PubMed]
- Wang CY, Calfee CS, Paul DW, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 2014;40:388-96. [Crossref] [PubMed]
- Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 2011;183:462-70. [Crossref] [PubMed]
- Damluji A, Colantuoni E, Mendez-Tellez PA, et al. Short-term mortality prediction for acute lung injury patients: external validation of the Acute Respiratory Distress Syndrome Network prediction model. Crit Care Med 2011;39:1023-8. [Crossref] [PubMed]
- Levitt JE, Calfee CS, Goldstein BA, et al. Early acute lung injury: criteria for identifying lung injury prior to the need for positive pressure ventilation*. Crit Care Med 2013;41:1929-37. [Crossref] [PubMed]
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012;38:1573-82. [Crossref] [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Cooke CR, Shah CV, Gallop R, et al. A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med 2009;37:1913-20. [Crossref] [PubMed]
- Heffner JE, Brown LK, Barbieri CA, et al. Prospective validation of an acute respiratory distress syndrome predictive score. Am J Respir Crit Care Med 1995;152:1518-26. [Crossref] [PubMed]


