Impact of intercostal paravertebral neurectomy on post thoracotomy pain syndrome after thoracotomy in lung cancer patients: a randomized controlled trial
Introduction
Thoracotomy for different indications is a frequently performed surgical procedure worldwide (1). One of the long term postoperative complications is the occurrence of chronic pain. In this regard the international association for the study of pain defines Post Thoracotomy Pain Syndrome (PTPS) as a pain that recurs or persists along a thoracotomy incision at least two months following the surgical procedure (2). Of note PTPS is a very common condition following thoracotomy with incidence rates reportedly ranging between 30% and 50% (3-5). In addition PTPS is mainly attributed to neuropathic pain (6).
Neuropathic pain derives from a direct lesion or disease of the somatosensory system and is being described through typical symptoms (paresthesia, hypoesthesia, spontaneous or burning pain) (7). In most of the published clinical trials evaluating the PTPS the authors have suggested that primarily chronic neuropathic pain leads to PTPS and tried different techniques to avoid intraoperative injury of the nerve (harvesting intercostal muscle flap, intracostal sutures, elevation of the vascular-nerve bundle) (8-10). However the patient groups in these studies had been heterogeneous and the surgical approaches considerably varied amongst the different studies. Furthermore neuropathic pain has not been assessed specifically in most of these studies. Finally, it has not been agreed on what surgical techniques are the best for avoiding neuropathic pain.
Interestingly, in an animal model for neuropathic pain two branches of the sciatic nerve were ligated and this was aimed at inducing reproducible neuropathic pain in the foot of the rodents (11). Therefore, particularly enclosing the intercostal nerve in a suture has been suggested to induce neuropathic pain. As a consequence, the nerve should be at best spared or even resected. The principle of neurectomy as an approach to reduce chronic postoperative pain has been described for inguinal hernia surgery patients (12,13). Williams et al evaluated retrospectively the results of intercostal neurectomy for a small collective of five patients with chronic intercostal pain and neuromas after abdominal or thoracic surgery. The results depicted positive effect for the intensity of the pain after intercostal neurectomy (14). However there is no randomized controlled trial in a clearly predefined patient cohort available for paravertebral neurectomy and muscle sparing anterolateral thoracotomy for lung cancer patients.
Therefore the present study was aimed at assessing the impact of paravertebral neurectomy in a clearly predefined and homogeneous cohort of patients. If paravertebral neurectomy would improve pain intensity or quality it could become a standard procedure for thoracotomy patients.
Methods
The trial was approved by the ethic committee of the University of Witten/Herdecke (application protocol Nr. 11/2011).
Study design and patients
A parallel-group randomized controlled trial was conducted in the Lung Clinic, Hospital of Cologne, University Hospital of Witten/Herdecke, Cologne, Germany from April 2010 to February 2014. Patients planned for muscle sparing anterolateral thoracotomy and anatomical lung resection for lung cancer were eligible for the study. Patients with preexisting pain, planned chest-wall-resection, re-do-thoracotomy, chemotherapy induced polyneuropathy, preexisting pain medication, age less than 18 years or pregnancy were excluded. The day before operation written informed consent was provided by all patients. Subjects were randomly assigned to either the treatment-group, i.e., paravertebral neurectomy or to the control-group. Randomization was provided by computer that generated random numbers in sealed envelopes. The patient was blinded for the procedure until the end of the study. Pain was assessed at day 30 and 120, respectively with the methods described below.
The intraoperative drop-out criteria were as follows:
No lung cancer in frozen section and no need for anatomical resection, decortication, extrapleural or chest wall resection and epidural anesthesia failure.
The postoperative drop-out criteria were as follows:
Death or mechanical ventilation and withdrawal of informed consent.
Endpoints: The primary endpoint was neuropathic pain as defined by Leeds Assessment Score for neuropathic Symptoms and Signs (LANSS) of ≥12 points on day 120.
The LANSS questionnaire is a validated tool for qualitative pain assessment aimed at assessing neuropathic pain. The sensitivity and the specificity were reportedly 74% and 76%, respectively (15). The questionnaire consists of 7 items which are summarized to one summary score with a scaling range between 0–24. Scores ≥12 indicate probable neuropathic pain. For the purpose of this study a validated German translation of the LANSS was used (15).
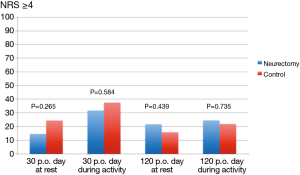
In addition, pain intensity was assessed using the numeric pain rating scale (NRS). For the purpose of the study values ≥4 were suggested to indicate relevant pain.
Surgical procedures
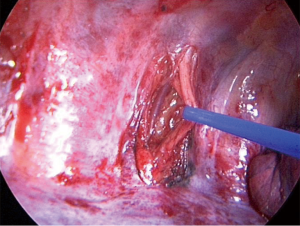
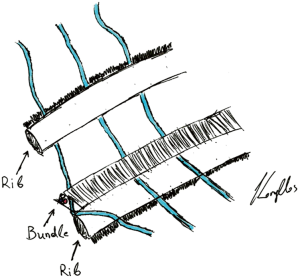
All patients received an epidural anesthesia followed by general anesthesia using a double lumen intubation. In both groups the incision was made on the level of the 4th or 5th intercostal space. The latissimus dorsi muscle was visualized and spared. The serratus anterior muscle was divided only when needed parallel to the muscle-fiber-direction and the intercostal place was opened at the lower edge of the 4th or 5th rib. The vascular-nerve bundle was carefully detached from the upper rib of the thoracotomy. A rib-retractor was placed and gradually widened taking particular caution not to break the ribs. In case of randomization to neurectomy the intercostal nerve was resected at the level of the thoracotomy just laterally from the intervertebral foramen (Figure 1). After completion of the anatomical lung resection and lymphadenectomy each patient had two chest tubes of 24 Ch. placed anteriorly and posteriorly. The intercostal space was closed using an absorbable continuous suture placed on the top of the upper rib and the intercostal muscle. In the control-group the same continuous suture was placed but without including the vascular-nerve-bundle (Figure 2).
The epidural anesthesia was terminated postoperatively according to the pain subjectively reported by the patient. Typically epidural anesthesia was replaced by oral analgesia on the third postoperative day. Analgetic medication was provided according to hospital standards using oxycodon, metamizol and/or paracetamol.
Statistics
Descriptive statistics included means, standard deviation (SDs) and medians or proportions (%) with 95% confidence intervals as appropriate. We employed parametric and non-parametric tests for between group comparisons, i.e., t-tests and Welch-tests, respectively (α =0.05), with χ2-tests for differences in proportions. All analyses were conducted using The Statistical Package for the Social Sciences (SPSS© version 20, Chicago, IL, USA).
Results
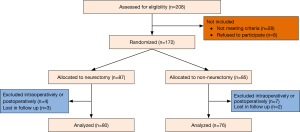
Overall, 172 patients were found eligible for randomization. Five patients had intraoperative drop-out criteria: two decortications, two epidural anesthesia failures, one chest wall resection (Figure 3). Six patients had postoperative drop-out criteria: 2 patients died following ARDS, 3 had mechanical ventilation, 1 required mechanical ventilation after postoperative bleeding and reoperation. In addition, five patients were lost for follow up. The demographic data of the remaining 156 patients who completed the study are illustrated in Table 1. There were no significant differences between the two groups with respect to the illustrated variables.
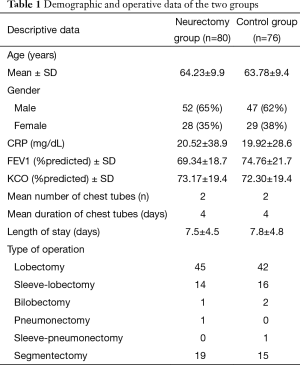
Full table
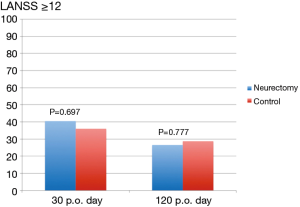
The results of the pain scores according to LANSS and NRS are illustrated in Table 2 and in Figures 4,5. There were no statistical significant difference between the two groups concerning neuropathic pain and NRS-pain scores (equal or more than 4).

Full table
Discussion
This is the first randomized controlled trial investigating paravertebral neurectomy and its impact on neuropathic pain. The main finding of the present study was that paravertebral neurectomy in patients with anterolateral muscle sparing thoracotomy and lung cancer did not result in a reduction of neuropathic pain. In this regard, the present study clearly shows that both quality and intensity of pain were comparable between patients receiving paravertebral neurectomy and those serving as a control group.
In general, the current findings are in contrast to the findings of Cerfolio et al. who applied two different techniques of avoiding neural injuring of the intercostal nerve (8,9). In the first of these two trials intracostal sutures were used for closure of the thoracotomy (9). In the second trial the harvesting of an intercostal muscle flap (vascular-nerve bundle included) was used as a mean to reduce intraoperative nerve damage (8). In both studies the intervention led to a reduction of the postoperative pain. However, this was only true for pain intensity as neuropathic pain has not been specifically addressed by specific questionnaires. In addition, the difference between the treatment and the control group had been reportedly low respectively. Contrarily, in the present study neuropathic pain was specifically addressed by a questionnaire which has been specifically developed for neuropathic pain assessment. For these reasons a detailed comparison between the present study and the cited ones is hindered.
It is the strength of the present study that a rather homogeneous cohort has been investigated. All patients had lung cancer and anatomic resection has been performed in all patients, since wedge resections have led to study exclusion. Moreover, the surgical approach was similar in all subjects. This is different to previous studies as most studies had different surgical approaches (8-10). However it is also true that the current results are only valid for lung cancer patients receiving anatomical resections via anterolateral muscle sparing thoracotomy. As a consequence, it remains to be elucidated if paravertebral neurectomy has beneficial effects in patients with different surgical approaches.
The reasons for why paravertebral neurectomy do not provide clinical improvements in regard to neuropathic pain remain unclear. From the current data it might be speculated that the nerve itself is not the main actor driving postoperative pain, since neurectomy in the present trial did not reduce pain quantity and quality at all. Other factors are highly suggested to at least contribute to the phenomenon of postsurgical neuropathic pain. This is evidenced by previous findings. In this regard, age, sex and other psychological or social factors have been shown to also play a significant role in postoperative chronic pain rates (16-18).
Overall, the rate of neuropathic pain was rather low in our study (26–28%) compared to previous studies with neuropathic rates reported to range between 40–50% (3,17,19). Again, studies were quite heterogeneous concerning patient characteristics and surgical approaches. Therefore, a direct comparison is difficult. Nevertheless, the rather low rate of neuropathic pain following surgery in the control group of our study could perhaps also explain why a difference between the treatment group and the control group was not established. However, the fact that 156 patients had been finally investigated following a randomized controlled design indicates that a selection bias is unlikely.
The present study has some limitations. First, different surgeons were involved in the surgical procedures, and surgeons were not blinded and not randomized to specific procedures. However, it is unlikely that this has affected the study results as paravertebral neurectomy is technically a very simple procedure. Another issue is that the present study exclusively focused on neuropathic pain as only this pain character is addressed by the LANSS-Score. However the complex phenomenon of the PTPS could possibly not only include neuropathic pain, but also different types of pain, which has been not assessed by our study. Therefore, it remains unclear if paravertebral neurectomy does positively impact on different pain characters. Nevertheless, in most cases the chronic pain of PTPS is suggested to be similar to neuropathic pain (6). In addition, pain intensity was also assessed by the NRS and was comparable between the two groups. Therefore, the current data show that complex pain assessment by NRS and LANSS-Scores did not reside in group differences following paravertebral neurectomy.
In conclusion, the present randomized controlled trial shows that paravertebral neurectomy does not reduce at 120-day pain intensity and quality in regard to neuropathic pain in patients with lung cancer undergoing anatomical resections via anterolateral muscle sparing thoracotomy. Therefore routine paravertebral neurectomy cannot be recommended in these patients. Whether paravertebral neurectomy is beneficial in different disease groups and or different surgical approaches remains to be elucidated.
Acknowledgements
None.
Footnote
Conflicts of Interest: Parts of study results have been presented at ATS Congress 2014 San Francisco.
Ethical Statement: The above study was conducted according to the latest standards of the declaration of Helsinki and was approved through the ethic committee of the University of Witten/Herdecke (application protocol Nr. 11/2011) and written informed consent was obtained from all patients.
References
- Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol 2016;33:160-71. [Crossref] [PubMed]
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1-226. [PubMed]
- Kinney MA, Hooten WM, Cassivi SD, et al. Chronic postthoracotomy pain and health-related quality of life. Ann Thorac Surg 2012;93:1242-7. [Crossref] [PubMed]
- Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand 1999;43:563-7. [Crossref] [PubMed]
- Song JG, Shin JW, Lee EH, et al. Incidence of post-thoracotomy pain: a comparison between total intravenous anaesthesia and inhalation anaesthesia. Eur J Cardiothorac Surg 2012;41:1078-82. [Crossref] [PubMed]
- Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain 2008;137:473-7. [Crossref] [PubMed]
- Nau C. Pathophysiology of chronic postoperative pain. Anasthesiol Intensivmed Notfallmed Schmerzther 2010;45:480-6. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Patel B, et al. Intercostal muscle flap reduces the pain of thoracotomy: a prospective randomized trial. J Thorac Cardiovasc Surg 2005;130:987-93. [Crossref] [PubMed]
- Cerfolio RJ, Price TN, Bryant AS, et al. Intracostal sutures decrease the pain of thoracotomy. Ann Thorac Surg 2003;76:407-11; discussion 411-2. [Crossref] [PubMed]
- Lee JI, Kim GW, Park KY. Intercostal bundle-splitting thoracotomy reduces chronic post-thoracotomy pain. Thorac Cardiovasc Surg 2007;55:401-2. [Crossref] [PubMed]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33:87-107. [Crossref] [PubMed]
- Aasvang EK, Kehlet H. The effect of mesh removal and selective neurectomy on persistent postherniotomy pain. Ann Surg 2009;249:327-34. [Crossref] [PubMed]
- Alfieri S, Di Miceli D, Doglietto GB. Prophylactic ilioinguinal neurectomy in open inguinal hernia repair. Ann Surg 2007;245:663. [Crossref] [PubMed]
- Williams EH, Williams CG, Rosson GD, et al. Neurectomy for treatment of intercostal neuralgia. Ann Thorac Surg 2008;85:1766-70. [Crossref] [PubMed]
- Bennett MI, Smith BH, Torrance N, et al. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 2005;6:149-58. [Crossref] [PubMed]
- Wildgaard K, Ravn J, Nikolajsen L, et al. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand 2011;55:60-8. [Crossref] [PubMed]
- Pluijms WA, Steegers MA, Verhagen AF, et al. Chronic post-thoracotomy pain: a retrospective study. Acta Anaesthesiol Scand 2006;50:804-8. [Crossref] [PubMed]
- Peters ML, Sommer M, de Rijke JM, et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg 2007;245:487-94. [Crossref] [PubMed]
- Guastella V, Mick G, Soriano C, et al. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain 2011;152:74-81. [Crossref] [PubMed]