Suppressive oligonucleotides inhibit inflammation in a murine model of mechanical ventilator induced lung injury
Introduction
Mechanical ventilation (MV) is a therapeutic life-sustaining intervention widely used in combination with general anesthesia and during intensive care to support blood oxygenation in patients with diminished respiratory function. Yet MV can cause lung injury especially when high tidal volumes (Vt) and/or low positive end-expiratory pressures (PEEP) cause baro-/bio-trauma elicited by the over-distention and repeated collapse/reopening of alveoli (1). Indeed, MV can initiate or exacerbate the lung injury caused by systemic inflammation in patients with normal pulmonary function (2,3). In patients with pre-existing pulmonary diseases (e.g., lung cancer), MV is associated with a significant increase in overall mortality (59% vs. 15%) (4,5). The risk of pulmonary damage correlated with both the peak respiratory pressure and Vt applied (6).
The pathophysiology of ventilator-induced lung injury (VILI) involves the distension, deformation and strain placed on fragile alveoli by MV. These can increase endothelial permeability, reduce the production of surfactant and compromise tissue repair (7,8). Moreover, these changes trigger a reactive inflammatory response that can further damage lung tissue and reduce pulmonary function. This inflammatory response is dominated by the influx and activation of macrophages and polymorphonuclear cells (PMN) (1,9). Both cell types are major sources of pro-inflammatory cytokines. Of particular relevance is TNFα, a key mediator of inflammation and one of the earliest pro-inflammatory cytokines produced after acute lung damage. TNFα is secreted by macrophages, monocytes and granulocytes and is present at very high levels in bronchoalveolar (BAL) lavage fluid during pulmonary inflammation (10,11). TNFα triggers a signaling cascade that leads to the production of other cytokines including IL-6. IL-6 is of particular interest as a marker of severe pulmonary inflammation correlates with increased mortality in patients with pneumonia and acute respiratory distress syndrome (ARDS) (12,13). Over the past several decades, the complication and mortality rates of patients receiving MV declined reflecting the use of lower Vt (<12 mL/kg) and plateau pressures (PP <30 cmH2O) (14,15) although pulmonary inflammation persisted as a problem associated with MV (16,17). Thus, an anti-inflammatory treatment that avoids the immune suppression remains an important goal.
Our group and others have studied a class of suppressive oligodeoxynucleotide (Sup ODN) that specifically inhibits inflammatory reactions (18,19). Sup ODN block macrophage activation and down-regulate the production of pro-inflammatory and Th1 cytokines (20,21) by inhibiting activation cascades utilizing STAT1, STAT3, STAT4 and the interferon-inducible protein AIM2 (19,22-24). Treatment with Sup ODN reduced disease severity/progression in multiple animal models of inflammatory disease including pulmonary silicosis (25,26). This work examines the effect of treatment with Sup ODN on the lung inflammation induced by MV in healthy Balb/cJ mice. Results show that pulmonary inflammation characterized by the influx/activation of CD11c+/F4/80+ macrophages and CD11b+/Ly6G+ PMN is reproducibly induced by 4 h of MV. This work evaluates the hypothesis that preventive treatment with Sup ODN could attenuate this inflammation in a murine model of VILI.
Methods
Mice and reagents
Female BALB/cJ mice (Charles River, Frederick, MD, USA) were housed in a SPF facility and studied at 8–10 weeks of age. All protocols were approved by the Institutional Animal Care and Use Committee of the NCI (Frederick and Bethesda, MD) and followed the National Institutes of Health guidelines for the use and care of live mice (Bethesda, MD).
Suppressive ODN A151 (5'-TTAGGGTTAGGGTTAGGGTTAGGG-3') and control ODN (5'-TTCAAATTCAAATTCAAATTCAAA-3') were synthesized at the Core Facility of the Center for Biologics Evaluation and Research of the Food and Drug Administration (FDA) (Bethesda, MD). All ODN were free of endotoxin contamination.
MV
Animals were anesthetized with 5% isoflurane, intubated with a 20 G SurFlash i.v. catheter (TERUMO) and connected to a computer controlled small animal ventilator FlexiVent® (SCIREQ Inc., Montreal, Canada). Quasi-sinusoidal ventilation was administered using normal air, a Vt of 10 mL/kg at 150 breaths per minute, a PEEP of 3 cmH2O and maximal PP of 30 cmH2O under 2–3% isoflurane. Of note, monitoring showed that this maximum PP was never utilized as normal ventilation volumes were achieved using pressures of 15–20 cmH2O. 50 µg of suppressive or control ODN in 20 µL of PBS were administered via intra-tracheal instillation immediately prior to the initiation of MV.
Lung function measurement
Lung function was evaluated by FlexiVent® using the forced oscillation technique generated by flexiWare v5.3.4 software. Lung function was measured after administration of 0.3 mg/kg pancuronium bromide. Total lung capacity (TLC) was performed initially followed by perturbations in triplicate of sinusoidal prime waves and PV loops generated by gradual inflation of the lungs to a PP of 30 cmH2O. Newtonian resistance, static compliance, tissue damping and tissue elastance were analyzed as previously described (27).
Cell isolation and culture
Cells were collected 6 h after extubation as preliminary experiments identified that time point as being optimal for detecting impressive changes in PMN and macrophage localization. BAL fluid was collected by repeated (3×) instillation and removal of 0.5 mL PBS. Subsequently, lung tissue was obtained and processed in a gentleMACS Dissociator (Miltenyi Biotec, Auburn, CA, USA) in DNAse plus collagenases A and B (0.25 WU/mL). Single cell suspensions were cultured in RPMI 1640 with 10% FCS (Lonza, Walkersville, MD, USA), 2 mmol glutamine, 100 IU/mL penicillin and 100 µg/mL streptomycin at 37 °C for 6 h with 10 ng/mL of Brefeldin A (Golgi Plug, BD).
Flow cytometry
Cells were collected and washed in PBS 2% BSA plus 2.5 mmol EDTA, incubated with FcBlock (BD Biosciences, 1:1,000 dilution) and stained with CD45-PECy7, F4/80-PB, Ly6G-Fitc, CD11b-PerCp-Cy5.5 and CD11c-APC Ab on ice for 30’. For intra-cytoplasmic staining cells were fixed with BD Lyse/Fix Buffer, washed, permeabilized and stained using anti-TNFα-PE or anti-IL-6-PE (BD Biosciences). Data were acquired using an LSR II Sorp and analyzed by Flowjo (Tree Star Inc., Ashland, OR, USA).
Quantitative RT-PCR
Total RNA was extracted from lung tissue using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) and reverse-transcribed into cDNA using a QuantiTect Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). QRT-PCR was performed in triplicate using primer pairs for TNFα (Mm00443260_g1) and IL-6 (Mm00446190_m1, Applied Biosystem). Gene expression was analyzed using the StepOne Plus RT-PCR system (Applied Biosystems) and normalized to GAPDH.
Statistics
Comparisons between 2 groups used Student’s t-test and between 3 or more groups one-way ANOVA with Student-Newman-Keuls test was used for comparison between individual groups (GraphPad Software, LA Jolla, CA).
Results
MV induces pulmonary inflammation accompanied by changes in tissue elastance and static compliance.
Preliminary experiments were performed to identify the minimum duration of MV required to reproducibly trigger an inflammatory response in the lungs. Conditions for ventilation considered protective for humans were used: constant positive PEEP above lower inflection point combined with Vt of 10 mL/kg. Normal ventilation volumes were achieved at a PP of 15–20 cm of H2O with an allowable maximal of 30 cmH2O.
Once MV was completed, the animals were extubated and permitted to recover from anesthesia. They were evaluated 6 h later as that time point proved optimal for detecting changes in pulmonary inflammation in preliminary studies and (28).
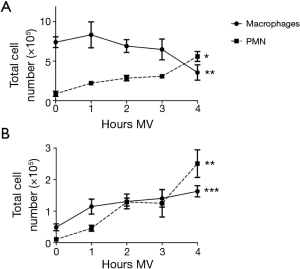
As seen in Figure 1, 4 h of MV reproducibly elicited significant inflammatory changes in the lungs of all mice tested. Inflammation was characterized by an influx of PMN (identified by surface expression of CD45, Ly6G and CD11b) into the parenchyma and BAL fluid (Figure 1). The frequency of PMN rose by ~6 fold in lung tissue and 20 fold in BAL (P<0.05 and P<0.001, respectively). The total number of macrophages (identified by expression of CD45, F4/80 and CD11c) present in BAL also increased by 3–4 fold over this period (Figure 1B, P<0.001) although their frequency in the lung parenchyma declined (Figure 1A).
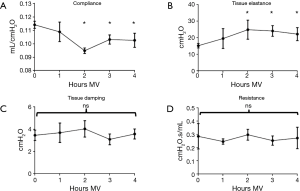
Lung function was evaluated using a FlexiVent® apparatus at baseline (0 h) and hourly after the initiation of MV in different experimental groups. Tissue elastance began to rise after 1 h and reached significantly elevated levels at 2–4 h of MV (P<0.05, Figure 2). These changes were accompanied by a decrease in static compliance over the same period of time (Figure 2) and were consistent with the effects of MV observed in other studies utilizing low ventilation volumes (29,30). By comparison, central airway resistance and lung tissue damping did not change over time. Based on results from the evaluation of pulmonary inflammation and function, all subsequent studies were conducted after 4 h of MV.
Sup ODNs reduce MV-induced pulmonary inflammation
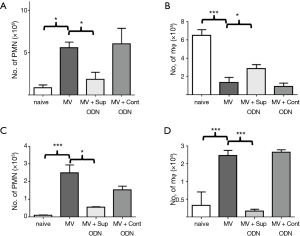
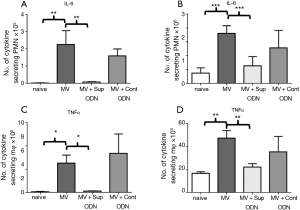
As described in the introduction, previous studies showed that Sup ODN treatment could limit the extent/duration of systemic and local inflammation (24-26,31,32). To evaluate whether this therapy could prevent the pulmonary damage associated with MV, 50 µg of Sup ODN A151 in 20 µL of PBS was instilled into the lungs of experimental mice upon intubation. This dose and route was previously found to be safe and effective in the treatment of pulmonary silicosis (25,26). Sup ODN treatment significantly reduced the inflammation induced by MV. As seen in Figure 3, administration of Sup ODN but not control ODN resulted in a 3–5 fold decrease in the number of PMN migrating into the lung parenchyma and BAL of mice after MV (P<0.05 for both parameters, Figure 3A,C). Similarly, MV-induced changes in the frequency of macrophage in BAL and lung returned towards normal in Sup ODN treated mice (P<0.05, Figure 3B,D).
Effect of Sup ODN on cytokine production and pulmonary mechanics
Another measure of MV-induced pulmonary damage is the production of the pro-inflammatory cytokines TNFα and IL-6 (11,12). Changes in these cytokines were evaluated by monitoring pulmonary mRNA levels. As expected, MV increased the amount of TNFα and IL-6 mRNA by 2–5 fold when compared to control mice (Figure 4, P<0.05).
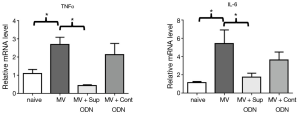
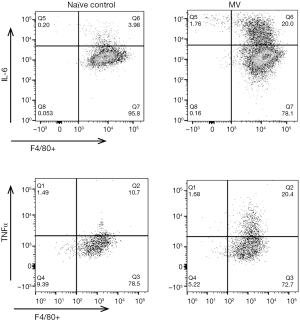
To determine whether the anti-inflammatory activity of Sup ODN extended to inhibiting cytokine production, their mRNA levels were compared in naive, MV and MV plus Sup ODN treated animals. Treatment with Sup (but not control) ODN returned cytokine mRNA levels to nearly background levels (Figure 4). To clarify whether PMN and/or macrophage were responsible for these changes, the presence of TNFα and IL-6 was evaluated by intra-cytoplasmic staining (Figure 5 and Figure S1). As seen in Figure 5, treatment with Sup ODN inhibited the activation of PMN and macrophage as reflected by a significant decline in the absolute number of cells secreting TNFα and IL-6 (P<0.05, Figure 5).
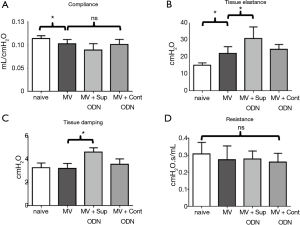
Finally, the effect of treatment on pulmonary function was evaluated by FlexiVent®. Sup ODN treatment had no effect on static compliance or resistance but significantly increased tissue damping and elastance (P<0.05, Figure 6).
Discussion
When MV is substituted for normal breathing, lung expansion is achieved through the application of external positive pressure. This can traumatize fragile alveoli particularly when higher inflation pressures are used (e.g., to re-open atelectatic regions of the lungs) (33). The interventions associated with the study of MV (including intubation and measurements of lung function) might increase the immune response characterized by the accumulation of inflammatory cells that release cytokines and chemokines (1,29,34). Conversely, inhaled anesthetics including the isoflurane used in this work can have anti-inflammatory and anti-apoptotic effects (35,36). Thus, the use of isoflurane might have delayed the onset or reduced the magnitude of MV-elicited changes in inflammatory cell activation/migration in the lungs.
Previous in vitro and in vivo studies demonstrated that mechanical deformation rapidly alter the phosphorylation of proteins in pulmonary parenchyma and immune cells, and that these changes could persist after MV was discontinued (37,38). In clinical settings, VILI can contribute to organ failure and increased mortality in patients with pre-existing lung disease (39,40). Efforts to avoid MV-induced lung damage currently rely on lowering Vt (<12 mL/kg of ideal body weight) and PP (<30 cmH2O) so that they are just sufficient to maintain adequate gas exchange (41,42). However, MV-induced lung damage can develop even when low Vt are used (16,43). Indeed, Vaneker et al. reported that even the low ventilation volumes used to preserve tissue integrity still induced the release of cytokines (including IL-6 and TNFα) and the migration of leukocytes into the lung (43).
The central goal of this study was to determine whether preventive treatment with Sup ODN A151 could ameliorate the pulmonary inflammation induced by MV in a murine model. A151 was selected for study because it mimics the immunosuppressive activity of telomeric mammalian DNA. A151 is composed of the same repetitive TTAGGG motifs present in telomeres. These have been shown to down-regulate the signaling pathways triggered in immune cells during the course of injurious inflammatory and autoimmune responses (19,25,26,32,44,45). Previous studies showed that Sup ODN A151 inhibited the production of pro-inflammatory cytokines and chemokines by interfering with the phosphorylation of STATs 1, 3, 4 and AIM2 (19-24). This included blocking/reducing activation of the innate immune system elicited by exposure to Toll-like receptor (TLR) ligands, and effect that persisted for up to 1 week in vivo. Of interest, evidence suggests that the immune activation induced by MV is at least partially mediated via TLRs 9 and 4 (46,47).
This work utilized the lung protective ventilator setting typically employed when MV is administered to patients. Consistent with previous reports, even these low Vt triggered an inflammatory response in mice after 4 h (43). The resultant migration/activated of macrophages and PMN into the lung and/or BAL fluid are a hallmark of many inflammatory lung diseases including VILI. Of particular note, we observed a doubling in the number of PMN in the lungs after 1 h of MV and a further increase through 4 h (Figure 1).
PMN are cellular elements of the innate immune system that help protect the lungs by phagocytosing pathogens and cell debris. PMN circulate in the blood and are attracted to inflamed tissue by chemoattractants and cytokines. Upon activation, PMN are a potent source of toxic antimicrobial peptides, proteases, oxidants and other pro-inflammatory mediators that can cause tissue damage (48,49). Considerably less is known about the activation of tissue resident macrophages through MV. They have been reported to contribute to tissue homeostasis and limit pulmonary inflammation by reducing the influx of PMN (50). In the current study, pulmonary macrophages were identified based upon their phenotype (CD45, F4/80 and CD11c). Four hours of MV stimulated and activated these cells and higher numbers were recovered from the BAL fluid (Figure 1). Preliminary studies indicated that this effect persisted for 3 days after which the number of macrophages in the lung parenchyma returned to normal. Macrophages were activated by MV as reflected by a 2–3 fold increase in the cell fraction producing TNFα and IL-6 when compared to control group (Figure 5).
Lung function was monitored by the forced oscillation technique using a FlexiVent® apparatus. Tissue elastance rose significantly while static compliance decreased after 4 h, consistent with MV having a negative impact on mechanical lung function. Airway resistance remained constant, but both, tissue damping and elastance increased in mice treated with Sup or control ODN, suggesting low-level edematous changes in distal lung tissue. As no such effects were observed when Sup ODN was instilled in the absence of prolonged MV, we hypothesize that MV worsened the effect of PBS delivery on lung function.
Our findings show that Sup ODN treatment significantly reduced the influx of inflammatory cells into the lungs and their production of pro-inflammatory cytokines, effects expected to reduce VILI (34). No clinically detectable adverse events (e.g., labored breathing, hemorrhage, infection, death) were observed in follow up periods ranging from 6–48 h, consistent with reports that Sup ODN are safe in rodents and non-human primates (18,22). Findings in this murine model support the further assessment of Sup ODN for the prevention/treatment of VILI. It is appreciated that only clinical studies will be required to determine whether Sup ODN treatment is of benefit to patients requiring prolonged MV (as in intensive care settings).
Acknowledgements
Thanks to Dr. Springer and Michele Allen from the murine phenotyping core (Bethesda, MD, USA) for their help and support during the project.
Funding: This manuscript was supported by the Intramural Research Program of the NIH, NCI.
Footnote
Conflicts of Interest: Dr. DM Klinman and members of his lab are co-inventors on a number of patents concerning Sup ODN and their use. All rights to these patents have been assigned to the Federal government.
Ethical Statement: All protocols were approved by the Institutional Animal Care and Use Committee of the NCI (Frederick and Bethesda, MD) and followed the National Institutes of Health guidelines for the use and care of live mice (Bethesda, MD).
References
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 2004;32:1817-24. [Crossref] [PubMed]
- Wrigge H, Uhlig U, Zinserling J, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg 2004;98:775-81. table of contents. [Crossref] [PubMed]
- Andréjak C, Terzi N, Thielen S, et al. Admission of advanced lung cancer patients to intensive care unit: a retrospective study of 76 patients. BMC Cancer 2011;11:159. [Crossref] [PubMed]
- Shih CY, Hung MC, Lu HM, et al. Incidence, life expectancy and prognostic factors in cancer patients under prolonged mechanical ventilation: a nationwide analysis of 5,138 cases during 1998-2007. Crit Care 2013;17:R144. [Crossref] [PubMed]
- Jia X, Malhotra A, Saeed M, et al. Risk factors for ARDS in patients receiving mechanical ventilation for > 48 h. Chest 2008;133:853-61. [Crossref] [PubMed]
- Cohen TS, Cavanaugh KJ, Margulies SS. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J 2008;32:854-61. [Crossref] [PubMed]
- Hammerschmidt S, Kuhn H, Gessner C, et al. Stretch-induced alveolar type II cell apoptosis: role of endogenous bradykinin and PI3K-Akt signaling. Am J Respir Cell Mol Biol 2007;37:699-705. [Crossref] [PubMed]
- Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14-20. [Crossref] [PubMed]
- Patel BV, Wilson MR, O'Dea KP, et al. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol 2013;190:4274-82. [Crossref] [PubMed]
- Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:1896-903. [Crossref] [PubMed]
- Bauer TT, Montón C, Torres A, et al. Comparison of systemic cytokine levels in patients with acute respiratory distress syndrome, severe pneumonia, and controls. Thorax 2000;55:46-52. [Crossref] [PubMed]
- Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995;107:1062-73. [Crossref] [PubMed]
- Guay J, Ochroch EA. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in patients without acute lung injury. Cochrane Database Syst Rev 2015;12:CD011151. [PubMed]
- Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev 2013;2:CD003844. [PubMed]
- Wolthuis EK, Vlaar AP, Choi G, et al. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care 2009;13:R1. [Crossref] [PubMed]
- Muscedere JG, Mullen JB, Gan K, et al. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994;149:1327-34. [Crossref] [PubMed]
- Bayik D, Gursel I, Klinman DM. Structure, mechanism and therapeutic utility of immunosuppressive oligonucleotides. Pharmacol Res 2016;105:216-25. [Crossref] [PubMed]
- Gursel I, Gursel M, Yamada H, et al. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol 2003;171:1393-400. [Crossref] [PubMed]
- Pisetsky DS, Reich CF. Inhibition of murine macrophage IL-12 production by natural and synthetic DNA. Clin Immunol 2000;96:198-204. [Crossref] [PubMed]
- Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol 2004;173:5002-7. [Crossref] [PubMed]
- Klinman DM, Tross D, Klaschik S, et al. Therapeutic applications and mechanisms underlying the activity of immunosuppressive oligonucleotides. Ann N Y Acad Sci 2009;1175:80-8. [Crossref] [PubMed]
- Kaminski JJ, Schattgen SA, Tzeng TC, et al. Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J Immunol 2013;191:3876-83. [Crossref] [PubMed]
- Bode C, Wang J, Klinman DM. Suppressive oligodeoxynucleotides promote the generation of regulatory T cells by inhibiting STAT1 phosphorylation. Int Immunopharmacol 2014;23:516-22. [Crossref] [PubMed]
- Sato T, Shimosato T, Alvord WG, et al. Suppressive oligodeoxynucleotides inhibit silica-induced pulmonary inflammation. J Immunol 2008;180:7648-54. [Crossref] [PubMed]
- Bode C, Kinjo T, Alvord WG, et al. Suppressive oligodeoxynucleotides reduce lung cancer susceptibility in mice with silicosis. Carcinogenesis 2014;35:1078-83. [Crossref] [PubMed]
- Patel BV, Wilson MR, Takata M. Resolution of acute lung injury and inflammation: a translational mouse model. Eur Respir J 2012;39:1162-70. [Crossref] [PubMed]
- Reutershan J, Basit A, Galkina EV, et al. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2005;289:L807-15. [Crossref] [PubMed]
- Hauber HP, Karp D, Goldmann T, et al. Effect of low tidal volume ventilation on lung function and inflammation in mice. BMC Pulm Med 2010;10:21. [Crossref] [PubMed]
- Allen G, Lundblad LK, Parsons P, et al. Transient mechanical benefits of a deep inflation in the injured mouse lung. J Appl Physiol (1985) 2002;93:1709-15. [Crossref] [PubMed]
- Yamada H, Gursel I, Takeshita F, et al. Effect of suppressive DNA on CpG-induced immune activation. J Immunol 2002;169:5590-4. [Crossref] [PubMed]
- Dong L, Ito S, Ishii KJ, et al. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB x NZW mice. Arthritis Rheum 2005;52:651-8. [Crossref] [PubMed]
- Cavanaugh KJ Jr, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 2001;25:584-91. [Crossref] [PubMed]
- Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care 2003;7:233-41. [Crossref] [PubMed]
- Reutershan J, Chang D, Hayes JK, et al. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology 2006;104:511-7. [Crossref] [PubMed]
- Pang YL, Chen BS, Li SP, et al. The preconditioning pulmonary protective effect of volatile isoflurane in acute lung injury is mediated by activation of endogenous iNOS. J Anesth 2012;26:822-8. [Crossref] [PubMed]
- Kawano T, Mori S, Cybulsky M, et al. Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J Appl Physiol (1985) 1987;62:27-33. [PubMed]
- Woods SJ, Waite AA, O'Dea KP, et al. Kinetic profiling of in vivo lung cellular inflammatory responses to mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 2015;308:L912-21. [Crossref] [PubMed]
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012;122:2731-40. [Crossref] [PubMed]
- Villar J, Blanco J, Añón JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011;37:1932-41. [Crossref] [PubMed]
- Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651-9. [Crossref] [PubMed]
- Ferguson ND. Low tidal volumes for all? JAMA 2012;308:1689-90. [Crossref] [PubMed]
- Vaneker M, Halbertsma FJ, van Egmond J, et al. Mechanical ventilation in healthy mice induces reversible pulmonary and systemic cytokine elevation with preserved alveolar integrity: an in vivo model using clinical relevant ventilation settings. Anesthesiology 2007;107:419-26. [Crossref] [PubMed]
- Ikeuchi H, Kinjo T, Klinman DM. Effect of suppressive oligodeoxynucleotides on the development of inflammation-induced papillomas. Cancer Prev Res (Phila) 2011;4:752-7. [Crossref] [PubMed]
- Bode C, Yang XP, Kiu H, et al. Suppressive oligodeoxynucleotides promote the development of Th17 cells. PLoS One 2013;8:e67991. [Crossref] [PubMed]
- Villar J, Cabrera N, Casula M, et al. Mechanical ventilation modulates Toll-like receptor signaling pathway in a sepsis-induced lung injury model. Intensive Care Med 2010;36:1049-57. [Crossref] [PubMed]
- Dai H, Pan L, Lin F, et al. Mechanical ventilation modulates Toll-like receptors 2, 4, and 9 on alveolar macrophages in a ventilator-induced lung injury model. J Thorac Dis 2015;7:616-24. [PubMed]
- Imanaka H, Shimaoka M, Matsuura N, et al. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth Analg 2001;92:428-36. [Crossref] [PubMed]
- Nakos G, Tsangaris H, Liokatis S, et al. Ventilator-associated pneumonia and atelectasis: evaluation through bronchoalveolar lavage fluid analysis. Intensive Care Med 2003;29:555-63. [Crossref] [PubMed]
- Beck-Schimmer B, Schwendener R, Pasch T, et al. Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir Res 2005;6:61. [Crossref] [PubMed]


