A morphologic study of the airway structure abnormalities in patients with asthma by high-resolution computed tomography
Introduction
Bronchial asthma is a common chronic disease worldwide affecting 1% to 18% of the population in different countries (1), and a recent epidemiologic study reported that the prevalence of asthma in China was 0.5% to 5% with an increasing tendency (2). Chronic airway inflammation is the fundamental characteristic and mechanism in the development of asthma (1), in which multiple inflammatory cells were recruited and activated to release various inflammatory mediators, thus resulting in airway hyperresponsiveness, bronchial constriction and airway obstruction (3,4). Moreover, it has been demonstrated that persistent airway inflammation may further induce airway remodeling, which consists of structural changes in airway wall including airway wall inflammation and edema, subepithelial fibrosis, hypertrophy/hyperplasty of the smooth muscle cells, mucus gland hyperplasia, and angiogenesis (3-7). It is believed that airway remodeling is the consequence of airway damage and repair imbalance. Meanwhile, studies also demonstrated that airway remodeling could accelerate lung function decline, which may lead to fixed airway obstruction and reduced responsiveness to inhaled corticosteroids and bronchodilators (6,7).
The diagnosis of asthma, recommended by the 2014 Global Initiative for Asthma (GINA), is initially identified by the characteristic pattern and detailed history of variable respiratory symptoms, and finally documented by the reversible expiratory airflow limitation via spirometry or pulmonary function test (PFT) (1). Although the conventional PFT could provide average measurement of lung function, it cannot identify precise location and extent of airway abnormalities (8). Furthermore, for a long time asthma is speculated to be more of a functional disease than a structural disease, which is distinct from chronic obstructive pulmonary disease (COPD), and airway structure abnormalities are indeed rarely found by chest film in asthmatics. However, in recent years, it has been recognized that airway remodeling is one of the important pathological abnormalities in patients with asthma and could lead to airway morphological changes, and an increasing number of studies have focused on the use of high-resolution computed tomography (HRCT) to evaluate the airway structure changes (8-15). It was reported that HRCT could be used to quantify static and dynamic changes in multiple independent airways as small as 1 mm (8), thus making it possible to study the subtle structure changes in the airways of asthma patients. In addition, some in vivo researches have also been carried out to assess the effect of new therapies on airway remodeling by HRCT, which are not possible using conventional spirometry and histological examinations.
Several parameters including bronchial wall thickening, bronchial dilatation, mucus impaction, emphysema, mosaic perfusion on inspiratory images, and atelectasis have been reported in measuring airway structure abnormalities by HRCT (7,9-13). It was reported that the overall incidence of abnormalities in HRCT was as high as 72% in asthma patients (14,15). However, the incidence of individual abnormality varied in different studies, and the exact relationship between airway structure changes and asthma severities did not reach a consensus. Paganin and colleagues compared 57 adult chronic asthmatics of variable severities with ten normal subjects, and found that the incidence of bronchial wall thickening, bronchial dilatation, mucus impaction, emphysema, and atelectasis was 15.7%, 64.9%, 21.1%, 17.5%, and 12.3% respectively. However, they did not find the relationship between HRCT abnormalities and disease severities (14). In the study by Harmanci and colleagues (15), the incidence of bronchial wall thickening, bronchial dilatation, mucus impaction, and emphysema was reported as 67.9%, 9.3%, 1.8%, and 7.9% respectively, but they found that bronchial wall thickening, bronchial dilation, and mucus impaction were all correlated positively with asthma severity while negatively with forced expiratory volume in one second (FEV1), which were further demonstrated by Park and Nakano (9,10). A study in China analyzed retrospectively the abnormalities of 28 asthma patients by HRCT, and the results showed a positive rate of 71.4%, of which bronchial wall thickening was detected in 15 cases (53.6%), while bronchial dilation was in 6 cases (21.4%), and mosaic pattern was in 3 cases (11.8%) (16). However, the study didn’t analyze the relationship of structural abnormalities in HRCT with clinical feature.
During recent decades, the novel and precise technique of three-dimensional (3D) HRCT has developed as the stimulation of 3D models, which makes the measurements of structures of small airways possible and reliable, thus enabling the development of various lung models (17-20). Although 3D acquisition together with automatic analysis is time saving and is widely accepted as an optimal standard, in the study of airway morphometric characteristics in patients with asthma, the main structural features were reported to be identical with those found in HRCT alone, including bronchial wall thickness and emphysema (21-23). Furthermore, 3D is more likely to be regarded as a post-processing method, because changes visualized in two-dimension (2D) projections were demonstrated to correspond to the airway flow limitations well (24). The 3D technique has additional advantages such as clearer imaging and more accurate measurement, but conventional HRCT contains cost-effective merits including wide-spread applications and availabilities in developing countries, and are indeed of clinical implications but not outdated as reported in recent studies using HRCT to evaluate air-trapping, airway narrowing and closure, and airway wall thickness (25-27). Hence, HRCT itself, regardless of 2D or 3D, should be considered as an independent methodology in assessing diseases.
Therefore, considering the controversial conclusions in the correlation of morphologic abnormalities with clinical features and outcomes, our study aimed to further specify and evaluate the structural abnormalities of Chinese asthmatics in HRCT.
Methods
Objects
From August 2012 to February 2015, outpatients with asthma were recruited in the Asthma Center of West China Hospital, Sichuan University. The diagnosis of asthma was established according to the diagnostic criteria recommended by the Chinese Thoracic Society including typical asthmatic symptoms such as recurrent wheeze, breathlessness, chest tightness and cough when exposed to allergens, which are usually alleviated spontaneously or after medication, and reversible airflow limitation and airway hyperresponsiveness documented by positive bronchial provocation test or bronchodilator reversibility test. Patients were excluded if they met one of the following criteria: (I) age <18 years old; (II) concomitant diseases such as but not limited to COPD, bronchiectasis, cystic fibrosis, allergic bronchopulmonary aspergillosis, tuberculosis, pneumonia, or sarcoidosis; (III) unwillingness or contraindications to perform HRCT and lung function tests. Our study protocol had been approved by the Institutional Ethics Committee Board of Clinical and Biomedical Research, West China Hospital (No. 14004163), and all participants were provided written informed consent.
HRCT scanning
HRCT was performed by a scanner with 16 detector arrays (Sensation 16, Siemens Medical System, Erlangen, Germany) at a slice thickness of 1 mm and inter-slice gap of 10 mm from lung apex to the diaphragm using 1 mm of collimation and at 120 kV using a 512×512 matrix. All subjects were scanned in the supine position while holding their breath and putting their arms over the head. Images were reconstructed using a high-resolution technique, through a small field of view targeted to image only pulmonary areas, and by using a high spatial frequency (bone) algorithm.
Two independent investigators (a radiologist and a physician), both blinded to the diagnosis and clinical history, interpreted each HRCT imaging and evaluated evidences of bronchial wall thickening, bronchial dilatation, mucus impaction, emphysema, mosaic perfusion on inspiratory images and atelectasis, as well as the location, extent and types of bronchial dilatation, with an estimated median time of 6–7 minutes per patient. Identical size measurement tool and diagnostic criteria were used, and corresponding data was rechecked until the consistency test reached a Kappa of at least 75%. Bronchial wall thickening was assessed at a window level of −450 HU and a window width of 1,500 HU, while other structural abnormalities were viewed at a window level of −700 HU and a window width of 1,500 HU (28-32).
Assessment of bronchial wall thickening
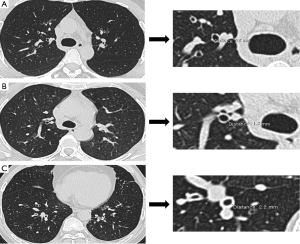
Under a magnification of ×5, bronchial wall thickening was measured manually in all bronchi (3th–5th generations) with more than 3/4 airway wall could be completely and clearly identified in a cross section in each slice of the inspiratory scans by electronic calipers. Bronchial wall thickening was further graded by severity as none (<1 mm), mild (1 to 2 mm), moderate (2 to 3 mm), and severe (≥3 mm), which are illustrated in Figure 1 (15,33).
Assessment of bronchial dilation
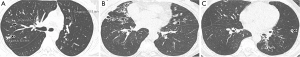
Bronchial dilation was considered if the internal diameter of any bronchi was greater than that of the adjacent pulmonary artery, the bronchial lumina (distal to bifurcation) had a lack of tapering and was greater than 2 cm, or if the peripheral airways (within 1 cm of the costal pleura) was visible (Figure 2) (15,28,31,32). Bronchial dilation were divided into three types according to the morphology of bronchi: (I) cylindric bronchial dilation, defined as the lack of bronchial tapering or the presence of smooth bronchial dilatation, and bronchial lumen greater than that of the accompanying pulmonary artery (Figure 2A,B); (II) varicose bronchial dilation, identified when irregular or beaded dilatation of the bronchus were recognized (Figure 2B,C); and (III) cystic bronchial dilation, diagnosed by presence of evident strings or clusters of cysts (15,33,34). Based on the established computed tomography (CT) criteria (35-37), the extent of bronchial dilation was scored as follows: grade 0 = no disease; grade 1 = localized bronchiectasis affecting one or part of one bronchopulmonary segment (localized); grade 2 = bronchiectasis in more than one bronchopulmonary segment (extensive); and grade 3 = generalized cystic bronchiectasis. Each of six lobes was scored and the lingular lobe was regarded as a separate lobe. We also classified bronchial dilation by severity as none (absence of bronchial dilation), mild (cylindric) and severe (varicose and cystic) bronchial dilation.
Assessment of mucus impactions
For dilated bronchi, mucus impactions were recognized as segmentally distributed densities, which were greater than the adjacent pulmonary arteries in diameter and were in beaded, gloved-finger like and nodular or oval shapes on a few consecutive scans; while for nondilated bronchi, they were presented as opacities in which normal-sized bronchial lumen was completely or partially filled forming the “tree-in-mud” pattern (15,33,38) (Figure 2C).
Assessment of emphysema
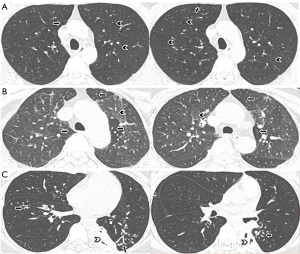
Different types of emphysema could be displayed independently or simultaneously, which included centrilobular, panlobular, paraseptal emphysema or irregular (paracicatricial) emphysema (15,31-33,38) (Figure 3A). Centrilobular emphysema was defined as focal areas of low attenuation up to 1 cm in diameter within a homogeneous background of lung parenchyma. Panlobular emphysema was considered as large and extensive areas of uniformly low attenuation associated with a reduction in size of pulmonary vessels. Paraseptal emphysema was recognized as multiple, small, contiguous subpleural air spaces which ranged from a few millimeters to 1 cm in diameter. Irregular (paracicatricial) emphysema was identified by the presence of emphysema adjacent to areas of parenchymal distortion.
Assessment of mosaic lung attenuation and atelectasis
Mosaic lung attenuation was defined as the presence of areas of variable lung attenuation on thin-section CT scans (28) (Figure 3B), while atelectasis was defined as one or more of the lung or lung capacity or air content being reduced (Figure 3C).
Pulmonary function testing
PFT was performed within the same week as the HRCT scans using plethysmography system (MastersScreen, Jäger, Germany). In accordance with the standard operation procedures recommended by the American Thoracic Society (ATS), spirometric data was recorded before inhalation of glucocorticoids or bronchodilators, and all enrolled patients were classified into three groups on the basis of predicted percentage of forced expiratory volume in one second (FEV1%) (mild, FEV1% ≥80%; moderate, 60%≤ FEV1 <80%; and severe, FEV1% <60%) along with daytime symptoms, night waking, and activity limitation due to asthma recommended by National Asthma Education and Prevention Program (NAEPP) (39).
Statistical analysis
An independent statistician, who was also blinded to the diagnosis and clinical findings of patients, conducted the statistical analysis by SPSS Statistics version 19.0 (Copyright © SPSS Inc., 1989–2007).
Dichotomous variables were reported as frequency and proportion, while continuous variables were showed as mean and standard derivation (SD). One-way analysis of variance (ANOVA) or Kruskal-Wallis Test was used to compare the age, body mass index (BMI), duration of disease, and FEV1% among different severities of asthma and bronchial dilation. Pearson Chi-Square or Fisher’s Exact Test was applied to analyze the significance of gender and different airway structural abnormalities among different groups of asthma. Clinical characteristics among different bronchial dilation severities and correlations between extent of bronchial dilation and PFT were analyzed by Games-Howell and Spearman’s correlation analyses, respectively. A P value of less than 0.05 was considered to be significant.
Results
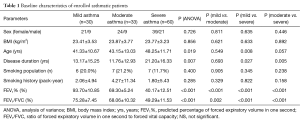
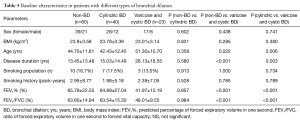
A total of 123 asthmatics were enrolled, among which 84 (68.3%) were female and 39 (31.7%) were male, and 20 patients (16.3%) were smokers or ex-smokers. On the basis of FEV1%, all enrolled patients were classified into mild (n=30), moderate (n=33) and severe (n=60) asthma, and the results showed that patients with severe asthma were significantly older (48.25±11.71, P=0.019) and had experienced longer duration of asthma (21.20±16.33, P=0.007), but there were no significant differences in smoking history and BMI among the three groups. The detailed baseline characteristics of all enrolled patients were summarized in Table 1.
Full table
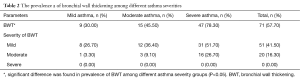
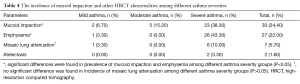
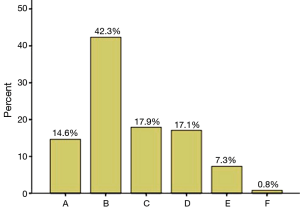
In our study, at least one HRCT structural abnormality was detected in 85.4% of the patients, and the incidence of bronchial wall thickening, bronchial dilatation, mucus impaction, emphysema, mosaic perfusion on inspiratory images, and atelectasis/Lobar collapse was 57.7%, 51.2%, 22%, 24.4%, 5.7% and 1.6%, respectively (Tables 2-4). Figure 4 shows the incidence of patients who presented different numbers of HRCT structural abnormalities, in which one, two, three, four and five abnormalities were found in 52 (42.3%), 22 (17.9%), 21 (17.1%), 9 (7.3%) and 1 (0.8%) patients, respectively. Furthermore, the incidences of bronchial wall thickening, bronchial dilation and emphysema were found to be significantly increased by asthma severity (P<0.05), while mucus impaction (26/27, 96.3%), mosaic perfusion (6/7, 85.7%) and atelectasis (2/2, 100%) were mainly found in severe asthma (Tables 2-4).
Full table

Full table
Full table
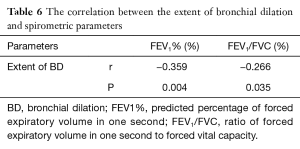
Enrolled patients were further divided into none (n=60), mild (n=40) and severe (n=33) bronchial dilation, and we found that patients with severe bronchial dilation had a longer asthma history (28.13±18.55, P<0.001, P=0.003), older age (51.30±10.70, P=0.022, P=0.006), and lower FEV1% (41.97±15.19, P<0.001, P<0.001) and ratio of forced expiratory volume to forced vital capacity (FEV1/FVC) (48.01±9.55, P<0.001, P<0.001) compared with those in none and mild bronchial dilation, and a negative correlation was found between the extent of bronchial dilation and FEV1% and FEV1/FVC (r=−0.359, P=0.004; r=−0.266, P=0.035, respectively) (Tables 5,6). However, there were no significant differences in BMI or smoking history among the three groups, neither in age, FEV1% or FEV1/FVC between the latter two groups.
Full table
Full table
The involved regions of bronchial dilation in the lungs were given in Table 7, which demonstrated that bronchial dilation was predominant in the right upper lobe, followed by right middle lobe, left lingual, and left lower lobe, and cylindric bronchial dilation mostly involved right middle lobe, while cystic bronchial dilation mainly occurred in left lower lobe.

Full table
Discussion
Our study found a relatively high incidence of structural abnormalities in Chinese patients with asthma, and the incidences of bronchial wall thickening, bronchial dilation and emphysema were more common in severe asthma. Patients with severe bronchial dilation had a longer asthma history, older age and lower FEV1 compared with none and mild types, and a negative correlation was found between the extent of bronchial dilation and pulmonary functions.
Increasing evidence has demonstrated that asthma is an airway disorder with both structural and functional abnormalities, although the structural changes are obscure and not visible on routine chest X-rays. HRCT is a more sensitive tool, which can provide much morphologic information of the lungs and has been widely used in asthma study in recent years. A large variety of structural abnormalities in patients with asthma have been reported in HRCT, which mainly include bronchial wall thickening, bronchial dilatation, mucus impaction, air trapping on expiratory CT scans, small centrilobular opacities emphysema, and mosaic perfusion (14). However, there is no clear consensus about the ongoing role of HRCT in routine management of asthma patients or in clinical research in this area.
Bronchial wall thickening is a common abnormality in HRCT in asthmatics, but the prevalence varies in different studies (7,14,40,41). Up to date, there are no recognized diagnostic criteria for bronchial wall thickening (7). Paganin (14), Lynch (41) and Carr (40) reported that the prevalence of bronchial wall thickening was 16%, 92% and 62.5% respectively, and the latter two also concluded that the prevalence of bronchial wall thickening was significantly higher in asthmatics than in healthy subjects. Furthermore, Carr (40) discovered that the prevalence of bronchial wall thickening was higher in severe asthmatics than in mild and moderate asthmatics. Harmanci and colleagues (15) found that bronchial wall thickening was present in bronchi not less than 1 mm, and the prevalence of bronchial wall thickening was 67.9%. They also found a positive correlation of bronchial wall thickening with disease severity in asthma and an inverse correlation with FEV1. Our study adopted Harmanci’s criterion and found that the incidence of bronchial wall thickening was 57.7%, which was much higher in severe asthma. The differences in reported prevalence of bronchial wall thickening may be due to different methods, the number of studied bronchi, the status of the patients, and the set of the window level. The pathogenesis of bronchial wall thickening may be airway remodeling, namely subepithelial fibrosis, hypertrophy of the smooth muscle cell, mucus gland hyperplasia, and angiogenesis. Otherwise some reversible elements such as chronic airway inflammation and edema may play roles (15,33). Whether or not the structural changes such as subepithelial fibrosis, hypertrophy of the smooth muscle cell are reversible is still controversial (6,14,15,33).
Although radiation dose is an important problem, HRCT is treated as a noninvasive diagnosis tool to accurately detect bronchial dilation. Lynch and colleagues (41) found that 77% of asthmatic subjects had at least one bronchus larger than the accompanying pulmonary artery. The widely accepted diagnostic criteria for bronchial dilation are internal diameter greater than adjacent pulmonary artery or lack of tapering or visibility of peripheral airways. According to these criteria, the prevalence of bronchial dilatation in asthmatic patients has been reported to be 3% to 66.7% (40,42). Bronchial dilation can also occur in healthy subjects, but the prevalence has been reported to be much lower than that in asthmatic subjects (28,30,41,43). Cylindrical bronchial dilation was reported to be the most common morphologic pattern identified on CT scans of asthmatics, mainly involved in segmental and distal bronchi, especially in the lower lobes (14,15,43). Varicose and cystic bronchial dilation were rare, and they were observed mainly in the patients with the most severe asthma, while cylindrical bronchiectasis could be found in nearly all asthmatic patients (14,15). Some investigators reported that bronchial dilation was correlated with disease severity in asthma, FEV1, and duration of asthma (15,30,44,45), and contributed to disease severity and difficulty of asthma control (42) but other studies haven’t found this correlation (14,28,40,41). Bronchial dilation is an irreversible change due to bronchial wall weakening and increased pressure in airways. Airway inflammation could undermine the integrity of the bronchial wall, and airflow limitation and air trapping could increase the pressure in airways. A balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) may control the remodeling of extracellular matrix, and excessive MMPs expression may be associated with destruction or dilatation of airways in patients with asthma (30). One study reported that sputum elastase and metalloproteinase-9/tissue-inhibitor metalloproteinase-1 ratio were associated with the magnitude of abnormalities in the airways (including bronchial wall thickening and bronchial dilatation) on HRCT (46). In the study by Takemura and colleagues (30), 23 asthmatic subjects and 2 control subjects presented at least one dilated bronchus on HRCT sans, but they did not find the imbalance of MMP-9 and TIMP-1 to be related to bronchiectasis in asthma. They suspected that the negative result might result from the influence of inhaled corticosteroid therapy on the levels of MMP-9 and TIMP-1. Another explanation was that pulmonary artery diameter may be affected by blood volume or local hypoxia, thus affecting the identification of bronchiectasis. The exact causal relationship between bronchiectasis and asthma has not been fully understood. In the clinical setting, there are indeed some patients who are definitely diagnosed as bronchiectasis but manifest airway hyperresponsiveness or even typical wheeze. In this study, the enrolled asthma patients didn’t manifest typical symptoms of bronchiectasis such as sputum expectoration, hemoptysis, and recurrent airway infection. Therefore we considered that bronchial dilation/bronchiectasis of these subjects might be ascribed to airway inflammation and consequent tissue destruction caused by asthma itself, namely a complication of asthma. Considering the fact that bronchiectasis was first listed as a complication of COPD in the GOLD document renewed in 2014, it is not a wild speculation that bronchiectasis may be a comorbidity of asthma. Whether or not the bronchiectasis should be included in the diagnosis of asthma is another question.
Mucus impactions have been found mainly in patients with severe asthma, but rarely found in mild and moderate asthma (14,15,47), and the incidence varied from 1.8% to 21.1% (14,15,33,36,38,47). Mucus impactions are reversible and could thoroughly disappear after therapy (14,33). In our study, the prevalence of mucus impactions was 22%, which was higher than that reported previously. We consider that it may be attributed to more severe asthma patients in our study and most patients not receiving regular therapy.
In previous studies, the prevalence of emphysematous changes in asthma ranged from 15% to 39% (15), while it was reported to be 24.4% in our study. We found that most of the asthmatics presented centrilobular emphysema, which was consistent with other studies (14,15,41). Emphysema was found to be closely related to smoking history, though it is also presented in nonsmoking asthmatics (41). Paganin (44) found that emphysema was significantly associated with the severity of disease. Park (9) revealed that asthmatics with emphysema tend to have a longer duration with asthma and severer airway obstruction. However, Harmanci (15) didn’t find such a relationship. Generally, emphysema is an irreversible change (14,15,31,33). Paganin (14) speculated that emphysema in asthmatics revealed by CT scan might not be explained by parenchymal destruction such as COPD. Extensive peribronchial fibrosis might be a culprit of cicatricial emphysema.
Mosaic pattern of attenuation with patchy areas of increased and decreased attenuation is nonspecific and may be seen on HRCT scans in various infiltrating lung, airway, or vascular diseases or even healthy subjects (48). Prevalence of mosaic pattern of attenuation in asthmatics ranged from 3% to 18% (9,28,47). In the current study, mosaic pattern of attenuation was presented only in 7 asthmatics (5.7%), of which 6 were severe asthma. Park and colleagues (9) found that asthmatics presented with mosaic pattern of attenuation had a longer duration with asthma and severer airway obstruction, but Park (28) didn’t find the correlation between the presence of mosaic pattern of attenuation and FEV1%. It is believed that mosaic patterns results from reflective vasoconstriction secondary to hypoventilation of alveoli distal to airway obstruction and blood flow redistribution to adjacent normal areas of lung (38,48).
Limitations for our study included: (I) lack of data of medication duration and adherence especially the inhaled corticosteroids before enrollment, which may result in potential bias in selecting patients; (II) lack of inflammatory profile information, which may limit the implication and interpretation of our study results in different asthma phenotypes; (III) lack of HRCT radiation dose record even though a median radiation dose of CTDI of 7.87 mGyL and DLP of 281 mGycm being provided in our informed consent, which may lead to further restrictions on application of HRCT in patients with asthma; and (IV) manual examination of bronchial wall thickness rather than using automated computer software, as well as lack of control subjects for comparison, which may affect the accuracy of our study data because tangential cuts of airways and measurement of airway wall in a perpendicular to tubular plane could not be guaranteed.
Conclusions
In summary, our study provided further evidence that asthma is not only a functional but also a structural disease. The incidences of structural abnormalities detected by HRCT are fairly high in Chinese asthma populations, especially the bronchial wall thickening and bronchial dilation, which are significantly increased in severe asthma, and are potential risk factors of pulmonary function decline in asthmatics.
Acknowledgements
We thank Professor Dongtao Lin (College of Foreign Languages, Sichuan University), who is specialized in biomedical writing and editing, for copyediting this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our study protocol had been approved by the Institutional Ethics Committee Board of Clinical and Biomedical Research, West China Hospital (NO. 14004163), and all participants were provided written informed consent.
References
- Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA), 2014. Available online: www.ginasthma.org, accessed on August 12, 2014.
- Ge JB, Xu YJ. Internal Medicine. Beijing: People’s Medical Publishing House, 2013.
- Shaw RJ, Djukanovic R, Tashkin DP, et al. The role of small airways in lung disease. Respir Med 2002;96:67-80. [Crossref] [PubMed]
- Lazaar AL, Panettieri RA Jr. Is airway remodeling clinically relevant in asthma? Am J Med 2003;115:652-9. [Crossref] [PubMed]
- Busse W, Elias J, Sheppard D, et al. Airway remodeling and repair. Am J Respir Crit Care Med 1999;160:1035-42. [Crossref] [PubMed]
- King GG, Farah CS. Targeting airway remodelling in asthma: the how, what and where? Respirology 2012;17:585-7. [Crossref] [PubMed]
- Webb WR, Müller NL, Naidich DP. editors. High-resolution CT of the lung. Philadelphia: Lippincott Williams & Wilkins, 2014.
- Brown RH, Mitzner W. Understanding airway pathophysiology with computed tomograpy. J Appl Physiol (1985) 2003;95:854-62. [PubMed]
- Park JW, Hong YK, Kim CW, et al. High-resolution computed tomography in patients with bronchial asthma: correlation with clinical features, pulmonary functions and bronchial hyperresponsiveness. J Investig Allergol Clin Immunol 1997;7:186-92. [PubMed]
- Nakano Y, Van Tho N, et al. Radiological approach to asthma and COPD--the role of computed tomography. Allergol Int 2009;58:323-31. [Crossref] [PubMed]
- Niimi A, Matsumoto H, Takemura M, et al. Clinical assessment of airway remodeling in asthma: utility of computed tomography. Clin Rev Allergy Immunol 2004;27:45-58. [Crossref] [PubMed]
- Sciurba FC. Physiologic similarities and differences between COPD and asthma. Chest 2004;126:117S-124S; discussion 159S-161S.
- de Jong PA, Müller NL, Paré PD, et al. Computed tomographic imaging of the airways: relationship to structure and function. Eur Respir J 2005;26:140-52. [Crossref] [PubMed]
- Paganin F, Trussard V, Seneterre E, et al. Chest radiography and high resolution computed tomography of the lungs in asthma. Am Rev Respir Dis 1992;146:1084-7. [Crossref] [PubMed]
- Harmanci E, Kebapci M, Metintas M, et al. High-resolution computed tomography findings are correlated with disease severity in asthma. Respiration 2002;69:420-6. [Crossref] [PubMed]
- Chen QH, Liu PG, Wu GG, et al. HRCT of the Lungs in Bronchial Asthma. China JMIT 2000;16:557-9.
- Kitaoka H, Takaki R, Suki B. A three-dimensional model of the human airway tree. J Appl Physiol 1985;1999:2207-17. [PubMed]
- Schmidt A, Zidowitz S, Kriete A, et al. A digital reference model of the human bronchial tree. Comput Med Imaging Graph 2004;28:203-11. [Crossref] [PubMed]
- Montesantos S, Fleming JS, Tossici-Bolt L. A spatial model of the human airway tree: the hybrid conceptual model. J Aerosol Med Pulm Drug Deliv 2010;23:59-68. [Crossref] [PubMed]
- Newell JD Jr, Sieren J, Hoffman EA. Development of quantitative computed tomography lung protocols. J Thorac Imaging 2013;28:266-71. [Crossref] [PubMed]
- Montesantos S, Katz I, Fleming J, et al. Airway morphology from high resolution computed tomography in healthy subjects and patients with moderate persistent asthma. Anat Rec (Hoboken) 2013;296:852-66. [Crossref] [PubMed]
- Xie M, Wang W, Dou S, et al. Quantitative computed tomography measurements of emphysema for diagnosing asthma-chronic obstructive pulmonary disease overlap syndrome. Int J Chron Obstruct Pulmon Dis 2016;11:953-61. [Crossref] [PubMed]
- Martinez CH, Chen YH, Westgate PM, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax 2012;67:399-406. [Crossref] [PubMed]
- Patyk M, Obojski A, Gojny Ł, et al. Airway Evaluation with Multidetector Computed Tomography Post-Processing Methods in Asthmatic Patients. Adv Exp Med Biol 2016;934:41-7. [Crossref] [PubMed]
- Usmani OS, Singh D, Spinola M, et al. The prevalence of small airways disease in adult asthma: A systematic literature review. Respir Med 2016;116:19-27. [Crossref] [PubMed]
- Dame Carroll JR, Magnussen JS, Berend N, et al. Greater parallel heterogeneity of airway narrowing and airway closure in asthma measured by high-resolution CT. Thorax 2015;70:1163-70. [Crossref] [PubMed]
- Liu L, Li G, Sun Y, et al. Airway wall thickness of allergic asthma caused by weed pollen or house dust mite assessed by computed tomography. Respir Med 2015;109:339-46. [Crossref] [PubMed]
- Park CS, Müller NL, Worthy SA, et al. Airway obstruction in asthmatic and healthy individuals: inspiratory and expiratory thin-section CT findings. Radiology 1997;203:361-7. [Crossref] [PubMed]
- Ooi GC, Khong PL, Chan-Yeung M, et al. High-resolution CT quantification of bronchiectasis: clinical and functional correlation. Radiology 2002;225:663-72. [Crossref] [PubMed]
- Takemura M, Niimi A, Minakuchi M, et al. Bronchial dilatation in asthma: relation to clinical and sputum indices. Chest 2004;125:1352-8. [Crossref] [PubMed]
- Lee YM, Park JS, Hwang JH, et al. High-resolution CT findings in patients with near-fatal asthma: comparison of patients with mild-to-severe asthma and normal control subjects and changes in airway abnormalities following steroid treatment. Chest 2004;126:1840-8. [Crossref] [PubMed]
- Park SW, Park JS, Lee YM, et al. Differences in radiological/HRCT findings in eosinophilic bronchitis and asthma: implication for bronchial responsiveness. Thorax 2006;61:41-7. [Crossref] [PubMed]
- Kurt E, Ozkan R, Orman A, et al. Irreversiblity of remodeled features on high-resolution computerized tomography scans of asthmatic patients on conventional therapy: a 6-year longitudinal study. J Asthma 2009;46:300-7. [Crossref] [PubMed]
- Lynch DA, Newell J, Hale V, et al. Correlation of CT findings with clinical evaluations in 261 patients with symptomatic bronchiectasis. AJR Am J Roentgenol 1999;173:53-8. [Crossref] [PubMed]
- Guilemany JM, Angrill J, Alobid I, et al. United airways: the impact of chronic rhinosinusitis and nasal polyps in bronchiectasic patient's quality of life. Allergy 2009;64:1524-9. [Crossref] [PubMed]
- Simpson JL, Milne DG, Gibson PG. Neutrophilic asthma has different radiographic features to COPD and smokers. Respir Med 2009;103:881-7. [Crossref] [PubMed]
- Habesoglu MA, Tercan F, Ozkan U, et al. Effect of radiological extent and severity of bronchiectasis on pulmonary function. Multidiscip Respir Med 2011;6:284-90. [Crossref] [PubMed]
- Yilmaz S, Ekici A, Ekici M, et al. High-resolution computed tomography findings in elderly patients with asthma. Eur J Radiol 2006;59:238-43. [Crossref] [PubMed]
- New NHLBI guidelines for the diagnosis and management of asthma. National Heart, Lung and Blood Institute. Lippincott Health Promot Lett 1997;2:1-8-9. [PubMed]
- Carr DH, Hibon S, Rubens M, et al. Peripheral airways obstruction on high-resolution computed tomography in chronic severe asthma. Respir Med 1998;92:448-53. [Crossref] [PubMed]
- Lynch DA, Newell JD, Tschomper BA, et al. Uncomplicated asthma in adults: comparison of CT appearance of the lungs in asthmatic and healthy subjects. Radiology 1993;188:829-33. [Crossref] [PubMed]
- Oguzulgen IK, Kervan F, Ozis T, et al. The impact of bronchiectasis in clinical presentation of asthma. South Med J 2007;100:468-71. [Crossref] [PubMed]
- Grenier P, Mourey-Gerosa I, Benali K, et al. Abnormalities of the airways and lung parenchyma in asthmatics: CT observations in 50 patients and inter- and intraobserver variability. Eur Radiol 1996;6:199-206. [Crossref] [PubMed]
- Paganin F, Séneterre E, Chanez P, et al. Computed tomography of the lungs in asthma: influence of disease severity and etiology. Am J Respir Crit Care Med 1996;153:110-4. [Crossref] [PubMed]
- Walker C, Gupta S, Hartley R, et al. Computed tomography scans in severe asthma: utility and clinical implications. Curr Opin Pulm Med 2012;18:42-7. [Crossref] [PubMed]
- Vignola AM, Paganin F, Capieu L, et al. Airway remodelling assessed by sputum and high-resolution computed tomography in asthma and COPD. Eur Respir J 2004;24:910-7. [Crossref] [PubMed]
- Jensen SP, Lynch DA, Brown KK, et al. High-resolution CT features of severe asthma and bronchiolitis obliterans. Clin Radiol 2002;57:1078-85. [Crossref] [PubMed]
- Worthy SA, Müller NL, Hartman TE, et al. Mosaic attenuation pattern on thin-section CT scans of the lung: differentiation among infiltrative lung, airway, and vascular diseases as a cause. Radiology 1997;205:465-70. [Crossref] [PubMed]


