Commentary on “Randomized trial of thymectomy in myasthenia gravis”
The relationship of the thymus to myasthenia gravis (MG) has been recognized at the very beginning of the last century (1,2). This relationship primarily applied to thymoma-associated MG (TAMG), in which thymectomies reportedly led to objective clinical improvement without the use of immunosuppressive drugs in the 1930-ies (3). Such TAMG cases account for approximately 10% of all MG, do not show a gender- or major histocompatibility complex (MHC) predilection, patients are elder than non-thymoma-associated MG affected individuals, and often have a specific set of anti-titin and anti-ryanodine receptor autoantibodies (4). There is experimental evidence that defective thymopoiesis (selection) of T-cells orchestrated by the neoplastic thymic epithelial cells, supported by a general cytokine network deregulation (5), are responsible for TAMG development; the neoplastic thymic epithelial cells finally being more limited (dysfunctional) to prevent export of autoreactive T-cells in the periphery. Since tumor-/thymectomy with clear resection margins is anyhow the upmost treatment strategy in thymomas (6), the indication to perform this mandatory procedure to prevent further tumor spread is obvious and independent of MG; therefore thymectomies are indisputable and almost always performed in TAMG. In addition, there is considerable body of evidence that thymectomy is not only beneficial to TAMG but also to other thymoma-associated autoimmune disorders, which, if indeed associated with thymoma, can be bona fide considered the only autoimmune diseases “curable” by a surgical procedure (7).
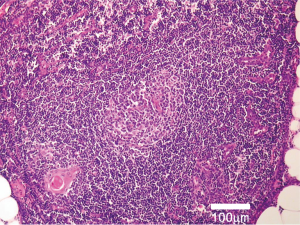
Nonetheless, a more substantial body of MG patients don’t suffer from thymoma but display non-tumorous thymic changes, particularly lymphofollicular hyperplasia or “thymitis” (8) with formation of germinal centers (Figure 1). Non-thymoma-associated MG patients are younger, show a female gender predominance of 3:1 as well as an MHC-association to HLA-B8 and HLA-DR3, and have anti-acetylcholine receptor (AChR) autoantibodies (4). Given the potential role of the thymus in such instances, thymectomy has been applied to affected patients since the 1940-ies (9,10). Yet, until August 2016 the evidence towards the efficacy of thymectomy, a potentially mutilating surgical intervention, for a non-neoplastic condition like MG was only based on retrospective data (11); the recommendation being that thymectomy may be applied to non-thymomatous patients with AChR autoantibodies. Even more, with the introduction of potent immunosuppressive drugs in the last decades it has become further difficult estimating the particular role of thymectomy in such occasions. To address this question an international randomized prospective clinical trial has been started in 2006 and the results published in the August 11th 2016 issue of the New England Journal of Medicine (12), giving an unequivocal answer: yes, thymectomy improves the clinical outcomes of non-thymomatous MG patients. In an admirably designed study, 126 non-thymomatous patients [out of 6,958 (!) screened patients] with AChR autoantibody-positive MG, aged 18 to 65, underwent randomization to thymectomy plus prednisone or prednisone alone and were followed-up over a 3-year period. The primary endpoints were time-weighted average Quantitative Myasthenia Gravis score and time-weighted average required prednisone dose. The results are unambiguous: the time-weighted average Quantitative Myasthenia Gravis scores in the thymectomy group were substantially lower (average difference 2.85 points lower) as was the weighted average required prednisone dose (average alternate-day dose 44 mg compared to 60 mg). In the subgroup analyses, these effects were independent of age at MG onset (cutoff 40 years), and both outcomes were significant for women, while only time-weighted average required prednisone dose reduction reached significance in men. In addition, the survey regarding treatment-associated symptoms, hospitalization due to MG exacerbation, azathioprine use and specific neurological assessment parameters were in favor of thymectomy over prednisone alone. The rationale for this approach can be found on page 16 of the supplementary appendix of the paper (12): out of 46 patients, who provided informed consent to have their specimens evaluated at the Institute of Pathology in Mannheim, Germany, 45 (98%) displayed histopathologic changes of overall thymic atrophy, 41 (89%) of cortical atrophy and 31 (67%) had lymphofollicular thymic hyperplasia, the latter being experimentally shown to be directly involved in MG’s pathogenesis (13). By means of the surgical procedure, the pathogenetically decisive body compartment containing these lymphoid follicles (the “fountain of evil”, Figure 1) could be removed. Importantly, since the perpetuation of the autoreactive process in MG gradually moves from the initiation organ, the thymus, to other organs (especially antibody-producing plasma cells reside in the bone marrow) with time (14), thymectomy should be considered as early as possible in the management strategy of non-thymoma-associated MG (11). The procedure should be performed under stable clinical conditions following efficient immunosuppressive measures and/or plasmapheresis (or equivalents) or intravenous immunoglobulin application, which are able to reduce perioperative mortality to <1% (11). Although no patients below the age of 18 have been included in the study of Wolfe et al., there is evidence towards the safety (and benefits) of thymectomy even down to the age of 5 (15).
Despite the concept-changing answer of the current study of Wolfe et al. (12), requiring recommendation modification towards that thymectomy should (instead of “may”) be applied to non-thymomatous patients with AChR autoantibodies, there are still open questions: (I) is extended transsternal thymectomy (sternotomy!) always needed or could less-invasive thymectomy approaches be considered as effective as the former? (II) Is a similar approach indicated in seronegative or muscle-specific tyrosine kinase (MuSK)-positive MG patients? (III) Is there justification for an analogous procedure in children aged <5 years? Some retrospective data imply the potential answers… Evidence respecting the main concerns of the 1st question, namely that less invasive surgical techniques may leave ectopic thymic tissue having a negative effect on outcomes, is at least 2 decades old (16,17), while reports from newer series applying modern surgical techniques show comparable results between less and more invasive approaches (18,19). Considering the 2nd question, smaller series indicate that even thymectomy at earlier age than 5 does not impair immunological functions and that there is an approximately 60% chance of MG response to thymectomy in this age group (20). Respecting the 3rd question, seronegative MG patients show comparable response rates after thymectomy as their AChR-seropositive counterparts, thus an analogous “may” recommendation has been established for them (4,21), while MuSK-positive patients seem not to respond to thymectomy and this procedure is not recommended in such instances (22).
In conclusion, by mid-2016 Wolfe and colleagues (12) provided prospective evidence that extended transsternal thymectomy is clearly indicated for the therapy of anti-AChR-autoantibody positive postpubertal MG patients, and there is retrospective data suggesting that this procedure may be beneficial in children as well as in seronegative (but not in MuSK-positive) individuals. Finally, prospective trials comparing less invasive resection techniques in that consideration are urgently needed.
Acknowledgements
None.
Footnote
Provenance: This is an invited Commentary commissioned by the Section Editor Gang Shen, MMSC (The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Weigert C. Pathologisch-anatomischer Beitrag zur Erb’schen Krankheit (Myasthenia gravis). Neurologisches Centralblatt 1901;20:597-601.
- Hughes T. The early history of myasthenia gravis. Neuromuscul Disord 2005;15:878-86. [Crossref] [PubMed]
- Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2002;2:797-804. [Crossref] [PubMed]
- Melzer N, Ruck T, Fuhr P, et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol 2016;263:1473-94. [Crossref] [PubMed]
- Buckley C, Newsom-Davis J, Willcox N, et al. Do titin and cytokine antibodies in MG patients predict thymoma or thymoma recurrence? Neurology 2001;57:1579-82. [Crossref] [PubMed]
- Ried M, Marx A, Götz A, et al. State of the art: diagnostic tools and innovative therapies for treatment of advanced thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2016;49:1545-52. [Crossref] [PubMed]
- Shelly S, Agmon-Levin N, Altman A, et al. Thymoma and autoimmunity. Cell Mol Immunol 2011;8:199-202. [Crossref] [PubMed]
- Marx A, Pfister F, Schalke B, et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev 2013;12:875-84. [Crossref] [PubMed]
- Blalock A, Harvey AM, Ford PR, et al. Treatment of myasthenia gravis by removal of the thymus gland. A preliminary report. J Am Med Assoc 1941;117:1529-33. [Crossref]
- Harvey AM, Lilienthal JL, Talbot SA. Observations on the nature of myasthenia gravis. the effect of thymectomy on neuro-muscular transmission. J Clin Invest 1942;21:579-88. [Crossref] [PubMed]
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:7-15. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Willcox N, Leite MI, Kadota Y, et al. Autoimmunizing mechanisms in thymoma and thymus. Ann N Y Acad Sci 2008;1132:163-73. [Crossref] [PubMed]
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015;14:1023-36. [Crossref] [PubMed]
- Liew WK, Kang PB. Update on juvenile myasthenia gravis. Curr Opin Pediatr 2013;25:694-700. [Crossref] [PubMed]
- Jaretzki A 3rd, Penn AS, Younger DS, et al. "Maximal" thymectomy for myasthenia gravis. Results. J Thorac Cardiovasc Surg 1988;95:747-57. [PubMed]
- Masaoka A, Monden Y, Seike Y, et al. Reoperation after transcervical thymectomy for myasthenia gravis. Neurology 1982;32:83-5. [Crossref] [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [Crossref] [PubMed]
- Ismail M, Swierzy M, Rückert RI, et al. Robotic thymectomy for myasthenia gravis. Thorac Surg Clin 2014;24:189-95. vi-vii. [Crossref] [PubMed]
- Tracy MM, McRae W, Millichap JG. Graded response to thymectomy in children with myasthenia gravis. J Child Neurol 2009;24:454-9. [Crossref] [PubMed]
- Guillermo GR, Téllez-Zenteno JF, Weder-Cisneros N, et al. Response of thymectomy: clinical and pathological characteristics among seronegative and seropositive myasthenia gravis patients. Acta Neurol Scand 2004;109:217-21. [Crossref] [PubMed]
- Evoli A, Tonali PA, Padua L, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003;126:2304-11. [Crossref] [PubMed]


